Bifunctional Derivative of p,p'-Dichlorochalcone. Part II. Synthesis of a Novel Compound 2-[2-Carboxymethylthio-2-(4-chlorophenyl)ethyl]-2-(4-chlorophenyl)-4-thiazolidinone
Abstract
:Introduction
Results and Discussion
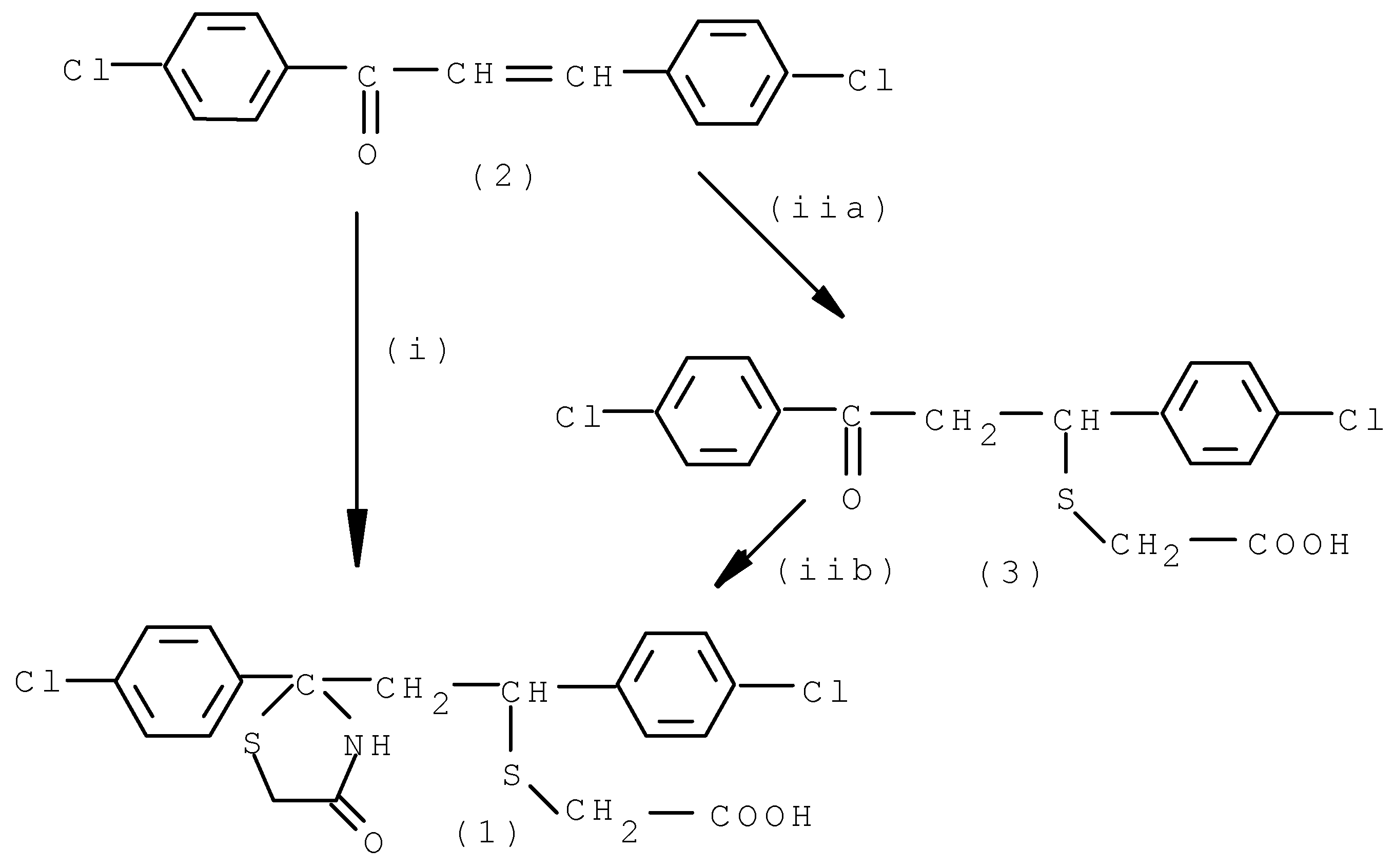
- (i)
- - a mixture of chalcone (2), thioglycollic acid and ammonium carbonate (molar ratio (1:2.5:5) in dry benzene was refluxed for 35 h. Purification of the products by column chromatography over silicagel followed by crystallisation afforded compound (1) as colourless globules in good yield (65%).
- (ii-a)
- - a mixture of chalcone (2) and thioglycollic acid (molar ratio 1:1.25) in dry benzene was refluxed for 2h; the reaction mixture on crystallisation gave colourless needles of thioether (3) in 77% yield.
- (ii-b)
- - the thioether (3) in dry benzene was then refluxed with thioglycollic acid and ammonium carbonate (molar ratio 1:1.25:5) for 32 h and the products on purification as above furnished compound (1) (64%).
Experimental
2-[2-Carboxymethylthio-2-(4-chlorophenyl)ethyl]-2-(4-chlorophenyl)-4-thiazolidinone(1)
Acknowledgements
References and Notes
- McLamore, W.M.; Celmer, W.D.; Bogert, V.V.; Pennington, F.C.; Solomons, I.A. J. Am. Chem. Soc. 1952, 74, 2946. Sobin, B.A. J. Am. Chem. Soc. 1952, 74, 2947. McLamore, W.M.; Celmer, W.D.; Bogert, V.V.; Pennington, F.C.; Sobin, B.A.; Solomons, I.A. J. Am. Chem. Soc. 1953, 75, 105. Pennington, F. C.; Celmer, W. D.; McLamore, W.M.; Bogert, V.V.; Solomons, I.A. J. Am. Chem. Soc. 1953, 75, 109.
- Tanabe, Y.; Yamamoto, H.; Murakami, M.; Yanagi, K.; Kubota, Y.; Okumura, H.; Sanemitsu, Y.; Suzukamo, G. J. Chem. Soc. Perkin Trans I 1995, 7, 935. [CrossRef]
- Khatoon, F.; Pachauri, R.; Ansari, W.H. J. Indian Chem. Soc. 1997, 74 1997, 74, 417.
- National Cancer Institute, Bethesda, Maryland (Development therapeutic program in vitro NSC No. 703776).
- Basic Terminology of Stereochemistry (IUPAC Recommendations 1996), http://www.chem.qmw.ac.uk/iupac/stereo/.
- The relative configuration of (1) is depicted according to a proposal of Maehr H. [7].
- Maehr, H. J. Chem. Ed. 1985, 62, 114. [CrossRef]
- Karplus, M. J. Chem. Phys. 1959, 30, 11.
- Davey, W.; Gwilt, J. R. J. Chem. Soc. 1957, 1008.
- Vogel, A.I. A Text Book of Practical Organic Chemistry, 4th editionLongman London, 1978. [Google Scholar]
- Sample Availability: Available from the authors.
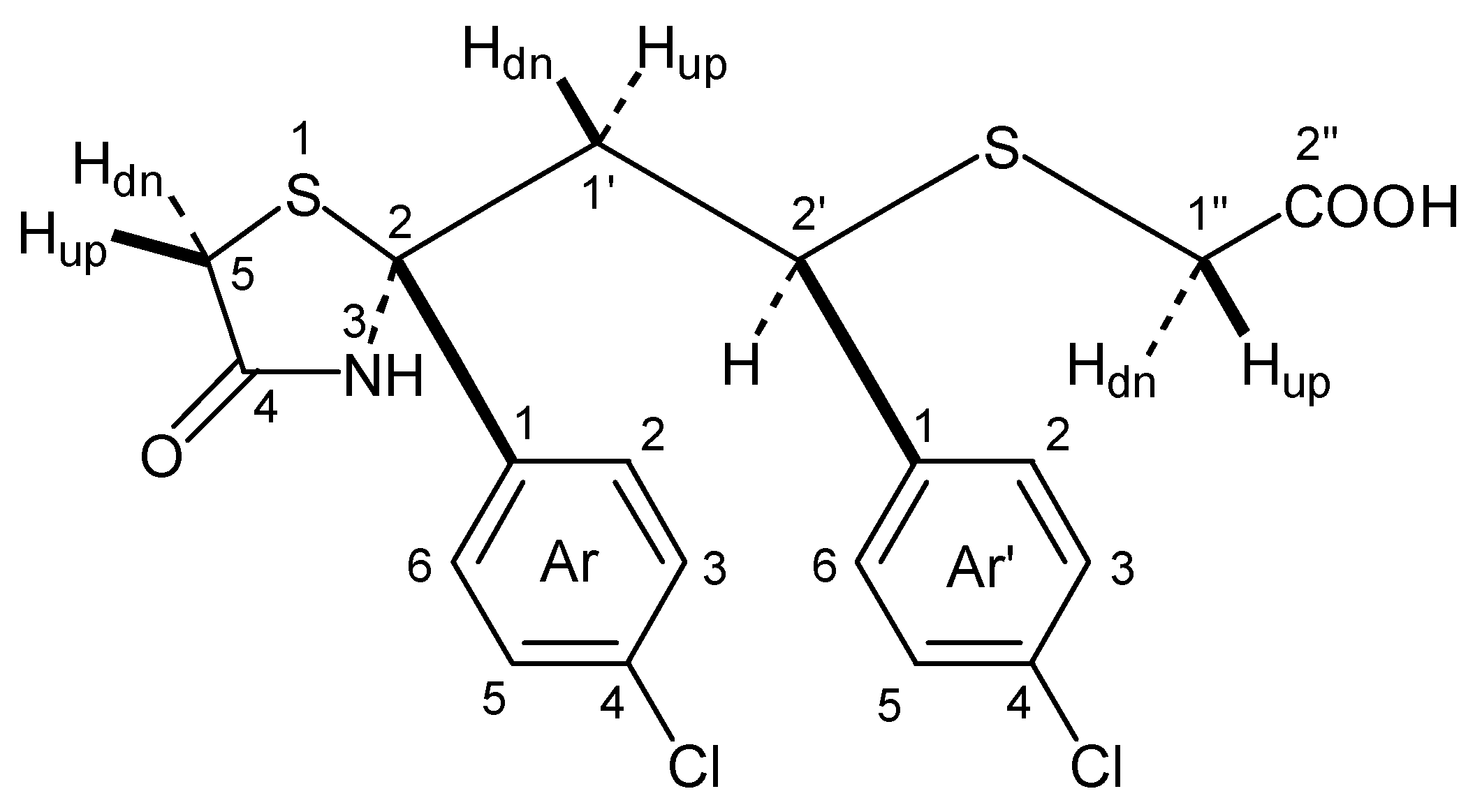
| H-nr | δ (ppm) | Integration | Multiplicity | J (Hz) | NOE |
|---|---|---|---|---|---|
| 5up | 3.42 | 1H | d | J5up,5dn 15.56 | |
| 5dn | 3.59 | 1H | d | J5up,5dn 15.56 | |
| NH | 9.20 | 1H | s | - | Ar-2,6; 2 ' |
| 1'up | 2.66 | 1H | dd | J1'up,1'dn 14.65; J1'up,2' 3.21 | |
| 1'dn | 2.82 | 1H | dd | J1'up,1'dn 14.65; J1'dn,2' 9.46 | |
| 2' | 4.14 | 1H | dd | J1'up,2' 3.21; J1'dn,2' 9.46 | |
| 1''up | 2.85 | 1H | d | J1''up,1''dn 15.26 | |
| 1''dn | 3.04 | 1H | d | J1''up,1''dn 15.26 | |
| Ar-2,6 | 7.29 | 2H | d | JAr-2,6,Ar-3,5 8.85 | 1'up, 1'dn, 2', NH |
| Ar-3,5 | 7.23 | 2H | d | JAr-2,6,Ar-3,5 8.85 | |
| Ar'-2,6 | 7.13 | 2H | d | JAr'-2,6,Ar'-3,5 8.55 | 1'dn, 2', 1''up |
| Ar'-3,5 | 7.20 | 2H | d | JAr'-2,6,Ar'-3,5 8.55 |
| C-nr | δ INEPT(ppm) | Longe-range HETCOR correlation with |
|---|---|---|
| 2 | 69.30 C | H-Ar-2,6; H-1'up; H-2'; NH |
| 4 | 172.45 C | H-5up; H-5dn |
| 5 | 32.91 CH2 | NH |
| 1' | 49.08 CH2 | |
| 2' | 44.52 CH | H-Ar'-2,6; H-1''up; H-1''dn |
| 1'' | 32.91 CH2 | |
| 2'' | 170.70 C | H-1''up; H-1''dn |
| Ar-1 | 143.41 C | H-Ar-3,5 |
| Ar-2,6 | 126.79 CH | H-Ar-2,6 |
| Ar-3,5 | 127.78a CH | H-Ar-3,5 |
| Ar-4 | 131.84 C | H-Ar-2,6 |
| Ar'-1 | 139.93 C | H-Ar'-3,5; H-1' |
| Ar'-2,6 | 129.87 CH | H-Ar-2,6; H-2' |
| Ar'-3,5 | 127.86a CH | H-Ar'-3,5 |
| Ar'-4 | 131.28 C | H-Ar'-2,6 |
© 1999 by the authors. Reproduction of this article, by any means, is permitted for noncommercial purposes.
Share and Cite
Mukhtar, S.; Rahman, M.V.P.; Ansari, W.H.; Lemière, G.; De Groot, A.; Dommisse, R. Bifunctional Derivative of p,p'-Dichlorochalcone. Part II. Synthesis of a Novel Compound 2-[2-Carboxymethylthio-2-(4-chlorophenyl)ethyl]-2-(4-chlorophenyl)-4-thiazolidinone. Molecules 1999, 4, 232-237. https://doi.org/10.3390/40700232
Mukhtar S, Rahman MVP, Ansari WH, Lemière G, De Groot A, Dommisse R. Bifunctional Derivative of p,p'-Dichlorochalcone. Part II. Synthesis of a Novel Compound 2-[2-Carboxymethylthio-2-(4-chlorophenyl)ethyl]-2-(4-chlorophenyl)-4-thiazolidinone. Molecules. 1999; 4(7):232-237. https://doi.org/10.3390/40700232
Chicago/Turabian StyleMukhtar, Sayeed, Mujeebur V.P. Rahman, Wajid Husain Ansari, Guy Lemière, Alex De Groot, and Roger Dommisse. 1999. "Bifunctional Derivative of p,p'-Dichlorochalcone. Part II. Synthesis of a Novel Compound 2-[2-Carboxymethylthio-2-(4-chlorophenyl)ethyl]-2-(4-chlorophenyl)-4-thiazolidinone" Molecules 4, no. 7: 232-237. https://doi.org/10.3390/40700232




