Introduction
2,4,6,8,10,12-Hexabenzyl-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.0
5,9.0
3,11]dodecane (hexabenzyl-hexaazaisowurtzitane,
1) is an interesting polyaza “cage” compound synthesized recently [
1]. In an effort to study the chemical behaviour of
1, we have explored a series of debenzylation method to remove the
N-benzyl groups in
1 [
2]. Reductive acetyldebenzylation of
1 (H
2, 20%Pd(OH)
2/C, Ac
2O) gives 2,6,8,12-tetraacetyl-4,10-dibenzyl-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.0
5,9.0
3,11]dodecane[
2,
3], which could be further transformed to hexanitrohexaazaisowurtzitane [
2]. In this communication we wish to report an unexpected carbon-carbon oxidative cleavage of
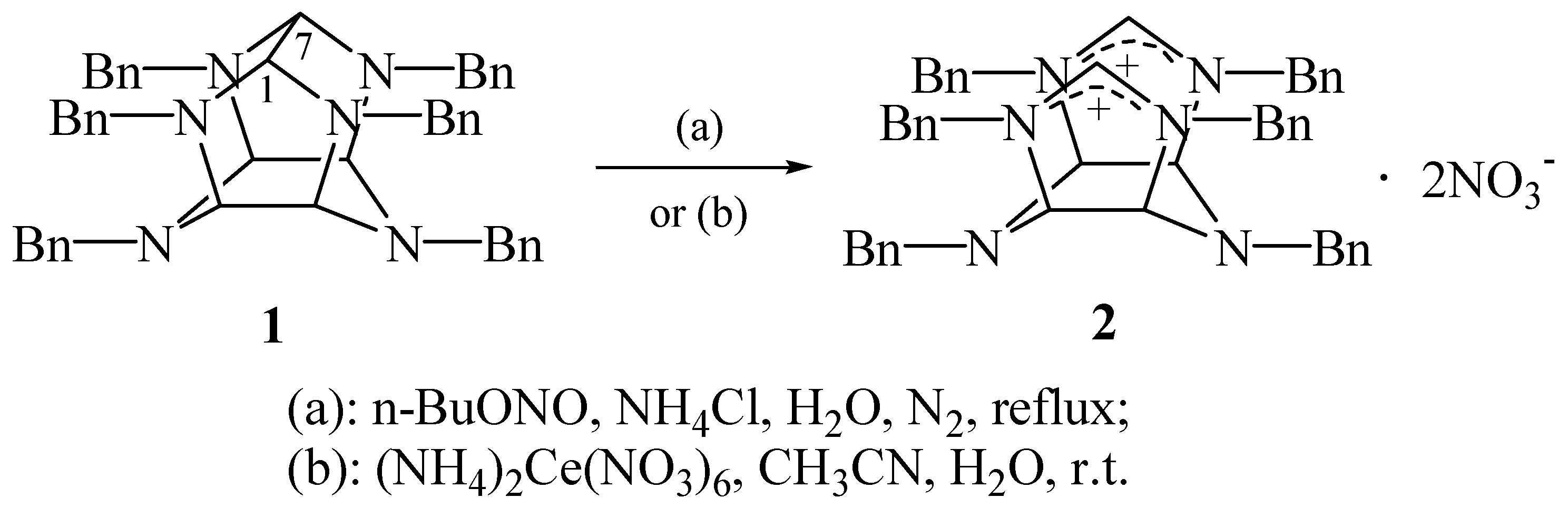
1 caused by
n-BuONO and (NH
4)
2Ce(NO
3)
6, respectively (
Scheme 1).
Results and Discussion
n-BuONO is a convenient reagent for
N-dealkylnitrosation of
N,N-dialkyl aromatic amines with preferential debenzylation [
4]. However, under the same condition, compound
1 did not go through
N-dealkylnitrosation. Instead, diimidazolinium dinitrate
2 was obtained in 42% yield, whose structure was established through the analyses of IR,
1H and
13C NMR, MS and elemental microanalysis. In particular, the IR spectrum shows a strong peak at 1640 cm
−1, indicating the existence of iminium cation, which was further confirmed by
1H NMR spectrum (δ 9.04 ppm, 2H) and
13C NMR spectrum (δ 158.4 ppm, d), and a very intense band of nitrate anion at 1382 cm
−1. The elemental analysis and the field desorption mass spectrum (
m/z 770, M–NO
3−, 100%), is consistent with formula C
48H
48N
8O
6 . In addition, the
1H and
13C NMR spectra also indicated that the molecule was mirror symmetric. These results demonstrate that the product
2 is 1,3,4,5,7,8-hexabenzyl-
cis-cisoid-cis-hexahydro-1
H,5
H-diimidazolinium[4,5-
b:4′,5′-
e]pyrazine.
Under mild conditions, (NH
4)
2Ce(NO
3)
6 can remove the
N-(4-methoxybenzyl) group on 2,5-piperazinediones [
5]. However, when
1 was exposed to (NH
4)
2Ce(NO
3)
6, instead of debenzylation,
2 was isolated in 59% yield.
The easy cleavage of
C(1)-
C(7) bond reflects that the cage skeleton of
1 is somewhat strained. Crystal structure analysis of hexa(4′-chlorobenzyl)hexaazaisowurtzitane shows that the framework
C-C bonds are longer than usual value for unstrained
Csp3-
Csp3 bond and
C(1)-
C(7) bond [1.579(3)Å] is the longest [
1b]. This implys that
C(1)-
C(7) bond is probably the weakest
C-
C bond in this cage skeleton.
A mechanism that accounts for the observed oxidative cleavage of
1 to
2 is depicted in
Scheme 2. The initially formed aminium radical cation
3 rapidly undergoes
C(1)-
C(7) cleavage followed by a second electron loss to give diimidazolinium dinitrate
2. It has been reported that 1,2-diamine radical cations undergo rapid C-C bond fragmentation to form an α-amino radical and an iminium ion [
6], and iminium salts are generally believed to be the key intermediates during the oxidation of tertiary amines [
7]. The readily oxidative cleavage of
1 must arise from the through-bond interaction between
N(2),
N(6),
N(8) and
N(12) atoms, which stabilizes the intermediate-like transition state relative to the ground state. Relief of strain energy contained within the hexaazaisowurtzitane framework and formation of the resonance-stabilized imidazolinium ion provides the driving force for the oxidative cleavage of
C(1)-
C(7) bond. The similar type of Grob fragmentation was also observed in the reaction of triethylenediamine with either ClO
2 or HOCl [
8].
In oxidative dealkylation of tertiary amine, the intermediate iminium cation, which are generally believed to be the key intermediates, is hydrolyzed more or less rapidly, in aqueous solution, with the formation of the corresponding secondary amine [
7]. Usually, imidazolinium salts are also too labile to resist hydrolysis [
9]. The high stability of
2 towards hydrolysis may be partially attributed to being capped of the cage skeleton by the six sterically hindered and hydrophobic benzyl groups.
Experimental
Oxidation of Hexaazahexbenzylisowurtzitane (1) with n-BuONO
A mixture of 1 (501 mg, 0.707 mmol), n-BuONO (5.0 mL, 55 mmol), NH4Cl (50 mg, 0.99 mmol) and H2O (150 μL, 8.3 mmol) was refluxed under N2 for 1h. Volatile material was then evaporated in vacua and the residue was washed with a small amount of water. Recrystallization of the crude solid from CH3CN/CHCl3/hexane afforded pure 2 as colorless crystals (249 mg, 42.3%), mp 224.2~225.0 °C; IR (KBr, cm−1): 1640(C=N+), 1382(NO3−;), 692; 1H NMR (DMSO-d6 ,60MHz): δ 3.93(s, 4H, CH2), 4.59(d, 4H, JAB=15.0Hz, CH2), 4.99(d, 4H, JAB=15.0Hz, CH2), 5.33(s, 4H, CH), 6.9~7.6 (m, 30H, Ph), 9.04(s, 2H, HC=N+) ; 13C NMR (DMSO-d6, 75Hz): δ 48.2(t), 58.4(t), 68.9(d), 127.9(d), 128.0(d), 128.3(d), 128.5(d), 128.7(d), 129.0(d), 133.4(s), 135.9(s), 158.4(d); MS (FD, m/z): 770(M+–NO3, 100%). Anal. Calcd. for C48H48N8O6: C, 69.21; H, 5.81; N, 13.45; O, 11.52; Found: C, 69.13; H, 6.00; N, 13.67; O, 11.37.
Oxidation of Hexaazahexbenzylisowurtzitane (1) with (NH4)2Ce(NO3)6
A solution of 1 (710 mg, 1.00 mmol) and (NH4)2Ce(NO3)6 (12.995 g, 23.70 mmol) in acetonitrile (75 mL) and water (25 mL) was stirred at r.t. for 24h. EtOAc (50 mL) was then added. The organic layer was separated, washed with water, dried over MgSO4 and evaporated in vacua. The residual solid was recrystallized from CH3CN/CHCl3/ hexane to give pure 2 as colorless crystals (490 mg, 58.8%).