Trimethylcyanosilane as a Convenient Reagent for the Preparation of Trimethylsilyl Enol Ethers of 1,3-Diketones
Abstract
:Introduction
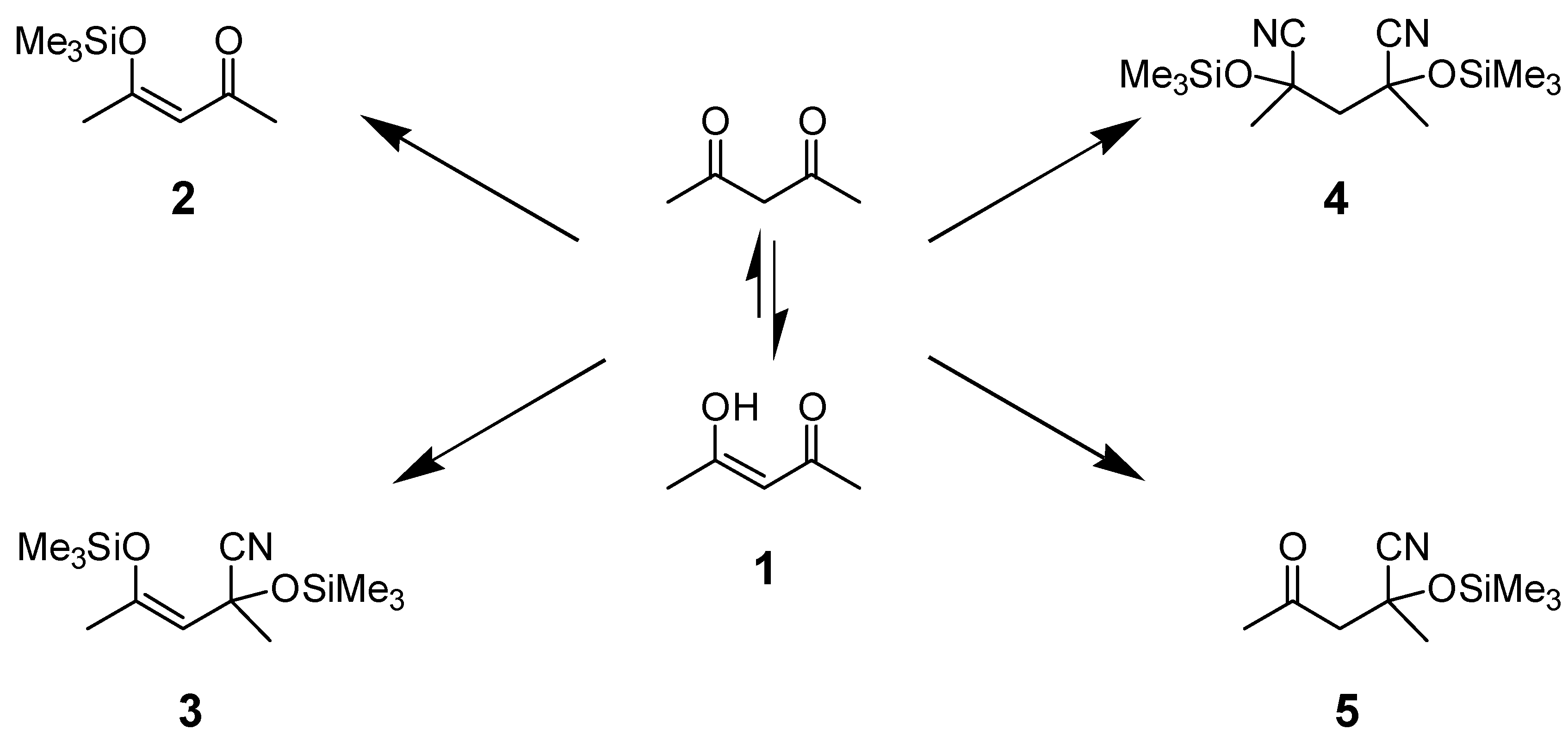
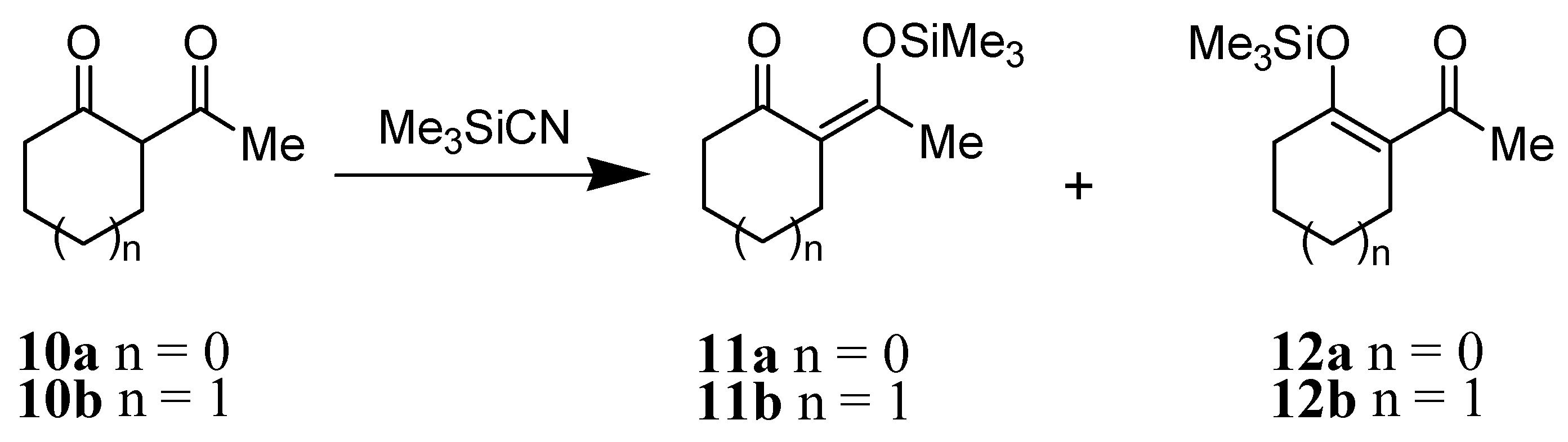
Results and Discussion
Conclusion
Experimental
Acknoledgement
References and Notes
- West, R. J. Am. Chem. Soc. 1958, 80, 3246–3249. House, H. O.; Czuba, L. J.; Gall, M.; Olmstead, H. D. J. Org. Chem. 1969, 34, 2324–2336. Pawlenko, S. Houben-Weyl, Methoden der Organischen Chemie, 4th Edn; Muller, E., Bayer, O., Eds.; Vol. XIII/5, Georg Thieme Verlag: Stuttgart, 1980; pp. 193–201. [Google Scholar]
- Kantlehner, W.; Kugel, W.; Bredereck, H. Chem. Ber. 1972, 105, 2264–2270.
- Dedier, J.; Gerval, P.; Frainnet, E. J. J. Organomet. Chem. 1980, 185, 183–197. Klebe, J. F.; Finkbeiner, H.; White, D. M. J. Am. Chem. Soc. 1966, 88, 3390–3395.
- Ojima, I.; Nagai, Y. J. Organomet. Chem. 1973, 57, C42–C44.
- Emde, H.; Domsch, D.; Feger, H.; Frick, U.; Goetz, A.; Hergott, H. H.; Hofmann, K.; Kober, W.; Kraegeloh, K.; Oesterle, T.; Steppan, W.; West, W.; Schimchen, G. Synthesis 1982, 1–26. Emde, H.; Goetz, A.; Hofmann, K.; Simchen, G. Justus Liebigs Ann. Chem. 1981, 1643–1657; Simchen, G.; Kober, W. Synthesis 1976, 259–261.
- Nakamura, E.; Murotushi, T.; Shimizu, M.; Kuwajima, I. J. Am. Chem. Soc. 1976, 98, 2346–2348.
- Chu, D. T.; W. Huckin, S. N. Can. J. Chem. 1980, 58, 138–142.
- Torkelson, S.; Ainsworth, C. Synthesis 1976, 722–724.
- Aizpurua, J. M.; Palomo, C. Synthesis 1982, 280–281.
- Yamamoto, Y.; Matui, C. Organometallics 1997, 16, 2204–2206.
- Rasmussen, J. K.; Heilmann, S. M.; Krepski, L. R. Advances in Silicon Chemistry; Larson, G.L., Ed.; JAI Press INC.: London, England, 1991; Volume 1, pp. 64–187. [Google Scholar]
- Furin, G. G.; Vyazankina, O. A.; Gostevsky, B. A.; Vyazankin, N. S. Tetrahedron 1988, 44, 2675–2749. Colvin, E. W. Chem. Soc. Rev. 1978, 7, 15. Groutas, W. C.; Felker, D. Synthesis 1980, 861–868.
- Mai, K.; Paril, G. J. Org. Chem. 1986, 51, 3545–3548. Corey, E. J.; Wu, Y.-J. J. Am. Chem. Soc. 1993, 115, 8871–8872. Becu, C.; Reyniers, M.-F.; Anteuuuunis, M. J. O. Bull. Soc. Chim. Belg. 1990, 99, 779–782. Antenuis, M. J. O.; Becu, C.; Becu, F. Bull. Soc. Chim. Belg. 1990, 99, 361–377.
- Zhuo, J.-C.; Wyler, H. Helv. Chim. Acta 1993, 76, 1916–1927.
- Gostevskii, B. A.; Kruglaya, O. A.; Albanov, A. I.; Vyazankin, N. S. Zh. Obshch. Khim. 1981, 51, 817–820. Gostevskii, B. A.; Vyazankina, O. A.; Vyazankin, N. S. Zh. Obshch. Khim. 1983, 53, 1843–1846.
- Foley, L. H. J. Org. Chem. 1985, 50, 5204–5209.
- Neef, H.; Müller, R. J. Prakt. Chem. 1973, 315, 367–374.
- Samples Availability: Not available.

© 1999 by the authors. Reproduction of this article, by any means, is permitted for noncommercial purposes.
Share and Cite
Zhuo, J.-C. Trimethylcyanosilane as a Convenient Reagent for the Preparation of Trimethylsilyl Enol Ethers of 1,3-Diketones. Molecules 1999, 4, 310-315. https://doi.org/10.3390/41000310
Zhuo J-C. Trimethylcyanosilane as a Convenient Reagent for the Preparation of Trimethylsilyl Enol Ethers of 1,3-Diketones. Molecules. 1999; 4(10):310-315. https://doi.org/10.3390/41000310
Chicago/Turabian StyleZhuo, Jin-Cong. 1999. "Trimethylcyanosilane as a Convenient Reagent for the Preparation of Trimethylsilyl Enol Ethers of 1,3-Diketones" Molecules 4, no. 10: 310-315. https://doi.org/10.3390/41000310



