Copper-Catalyzed Regioselective Synthesis of (E)-β-Fluorovinyl Sulfones
Abstract
:1. Introduction
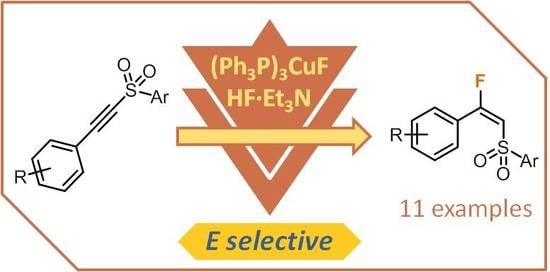
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Method for the Synthesis of (E)-Fluorovinyl Sulfones
3.3. Characterization of (E)-Fluorovinyl Sulfones 2a–2n
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simpkins, N.S. The chemistry of vinyl sulphones. Tetrahedron 1990, 46, 6951–6984. [Google Scholar] [CrossRef]
- Meadows, D.C.; Gervay-Hague, J. Vinyl sulfones: Synthetic preparations and medicinal chemistry applications. Med. Res. Rev. 2006, 26, 793–814. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.-N.R.; Companyó, X.; Rios, R. Sulphones: new reagents in organocatalysis. Chem. Soc. Rev. 2010, 39, 2018–2033. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Inbasekaran, M.; Peet, N.P.; McCarthy, J.R.; LeTourneau, M.E. A novel and efficient synthesis of fluoromethyl phenyl sulphone and its use as a fluoromethyl Wittig equivalent. J. Chem. Soc. Chem. Commun. 1985, 678–679. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Matthews, D.P.; Edwards, M.L.; Stemerick, D.M.; Jarvi, E.T. A new route to vinyl fluorides. Tetrahedron Lett. 1990, 31, 5449–5452. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Matthews, D.P.; Stemerick, D.M.; Huber, E.W.; Bey, P.; Lippert, B.J.; Snyder, R.D.; Sunkara, P.S. Stereospecific method to (E) and (Z) terminal fluoroolefins and its application to the synthesis of 2′-deoxy-2′-fluoromethylenenucleosides as potential inhibitors of ribonucleoside diphosphate reductase. J. Am. Chem. Soc. 1991, 113, 7439–7440. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Huber, E.W.; Le, T.-B.; Laskovics, F.M.; Matthews, D.P. Stereospecific synthesis of 1-fluoro olefins via (fluorovinyl)stannanes and an unequivocal NMR method for the assignment of fluoro olefin geometry. Tetrahedron 1996, 52, 45–58. [Google Scholar] [CrossRef]
- Asakura, N.; Usuki, Y.; Iio, H. A new synthesis of α-fluorovinylsulfones utilizing the Peterson olefination methodology. J. Fluorine Chem. 2003, 124, 81–88. [Google Scholar] [CrossRef]
- Berkowitz, D.B.; de la Salud-Bea, R.; Jiang, W.-J. Synthesis of Quaternary Amino Acids Bearing a (2′Z)-Fluorovinyl α-Branch: Potential PLP Enzyme Inactivators. Org. Lett. 2004, 6, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, S.F.; Garcia, P.I., Jr.; Wang, Z. Radical-Mediated Silyl- and Germyldesulfonylation of Vinyl and (α-Fluoro)vinyl Sulfones: Application of Tris(trimethylsilyl)silanes and Tris(trimethylsilyl)germanes in Pd-Catalyzed Couplings. Org. Lett. 2004, 6, 2047–2049. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.K.S.; Chacko, S.; Vaghoo, H.; Shao, N.; Gurung, L.; Mathew, T.; Olah, G.A. Efficient Nucleophilic Fluoromethylation and Subsequent Transformation of Alkyl and Benzyl Halides Using Fluorobis(phenylsulfonyl)methane. Org. Lett. 2009, 11, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Ghosh, A.K.; Kumar, R.; Zajc, B. Expedient synthesis of α-substituted fluoroethenes. Org. Biomol. Chem. 2012, 10, 3164–3167. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, S.; Hammond, G.B.; Xu, B. Divergent Regio- and Stereoselective Gold-catalyzed Synthesis of α-Fluorosulfones and β-Fluorovinylsulfones from Alkynylsulfones. Chem. Eur. J. 2017, 23, 11977–11981. [Google Scholar] [CrossRef]
- McCune, C.D.; Beio, M.L.; Sturdivant, J.M.; de la Salud-Bea, R.; Darnell, B.M.; Berkowitz, D.B. Synthesis and Deployment of an Elusive Fluorovinyl Cation Equivalent: Access to Quaternary α-(1′-Fluoro)vinyl Amino Acids as Potential PLP Enzyme Inactivators. J. Am. Chem. Soc. 2017, 139, 14077–14089. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, D.M.; Román, R.; Barrio, P.; Morales, C.; Fustero, S. A metal-free and regioselective approach to (Z)-β-fluorovinyl sulfones and their chemoselective hydrogenation to β-fluoroalkyl sulfones. J. Fluorine Chem. 2018, 206, 108–116. [Google Scholar] [CrossRef]
- He, G.; Qiu, S.; Huang, H.; Zhu, G.; Zhang, D.; Zhang, R.; Zhu, H. Cu(I)- or Ag(I)-Catalyzed Regio- and Stereocontrolled trans-Hydrofluorination of Ynamides. Org. Lett. 2016, 18, 1856–1859. [Google Scholar] [CrossRef]
- Chaudhuri, M.K.; Dhar, S.S.; Vijayashree, N. Molecular complexes of copper(I): Easy access to CuF(PPh3)3 · 2ROH (R = Me or Et). Trans. Met. Chem. 2000, 25, 559–561. [Google Scholar] [CrossRef]
- Larsson, J.M.; Pathipati, S.R.; Szabó, K.J. Regio- and Stereoselective Allylic Trifluoromethylation and Fluorination using CuCF3 and CuF Reagents. J. Org. Chem. 2013, 78, 7330–7336. [Google Scholar] [CrossRef]
- Jiménez-Tenorio, M.; Puerta, M.C.; Valerga, P.; Ortuño, M.A.; Ujaque, G.; Lledós, A. Counteranion and Solvent Assistance in Ruthenium-Mediated Alkyne to Vinylidene Isomerizations. Inorg. Chem. 2013, 52, 8919–8932. [Google Scholar] [CrossRef]
- Begley, L.C.; Kakanskas, K.J.; Monaghan, A.; Jackson, S.D. Effect of molecular structure on the hydrogenation and isomerisation of propenylbenzene isomers. Catal. Sci. Technol. 2012, 2, 1287–1291. [Google Scholar] [CrossRef]





| Entry | [cat.] | [F] | Solvent | T °C | Conv.% 2 | E:Z3 |
|---|---|---|---|---|---|---|
| 1 | Ph3PAuMe | HF·DMPU | DCE | 70 | 44 | <1:20 |
| 2 | JohnPhosAuCl | HF·DMPU | DCE | 55 | 58 | 1:9 |
| 3 | AgNTf2 | 3HF·Et3N | DMF | 70 | 55 | 1:5 |
| 4 4 | (Ph3P)3CuF·2MeOH | HF·DMPU | THF | 70 | 73 | 1:1 |
| 5 4 | (Ph3P)3CuF·2MeOH | 3HF·Et3N | THF | 70 | 79 | 2:1 |
| 6 4 | (Ph3P)3CuF·2MeOH | 3HF·Et3N | Dioxane | 70 | 90 | 3:1 |
| 7 4 | (Ph3P)3CuF·2MeOH | 3HF·Et3N | Toluene | 70 | >95 | 4:1 |
| 8 4,5 | (Ph3P)3CuF·2MeOH | - | Toluene | 70 | >95 | 3:1 |
| 9 6 | (Ph3P)3CuF | 3HF·Et3N | Toluene | 70 | <10 | - 7 |
| 10 5,6 | (Ph3P)3CuF | - | Toluene | 70 | >95 | 1:1 |
| 11 8 | (Ph3P)3CuF | 3HF·Et3N | Toluene | 70 | >95 | 3:1 |

| Entry | Substrate | Time, h | Conv.% 1 | 3:4 1 |
|---|---|---|---|---|
| 1 | (Z)-2a 2 | 2 | >98 | 12:1 |
| 2 | (E)-2a | 2 | - | - |
| 3 | (E)-2a | 4 | 10 | 1:1 |
| 4 | (E)-2a | 20 | 95 | 2:3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román, R.; Barrio, P.; Mateu, N.; Sedgwick, D.M.; Fustero, S. Copper-Catalyzed Regioselective Synthesis of (E)-β-Fluorovinyl Sulfones. Molecules 2019, 24, 1569. https://doi.org/10.3390/molecules24081569
Román R, Barrio P, Mateu N, Sedgwick DM, Fustero S. Copper-Catalyzed Regioselective Synthesis of (E)-β-Fluorovinyl Sulfones. Molecules. 2019; 24(8):1569. https://doi.org/10.3390/molecules24081569
Chicago/Turabian StyleRomán, Raquel, Pablo Barrio, Natalia Mateu, Daniel M. Sedgwick, and Santos Fustero. 2019. "Copper-Catalyzed Regioselective Synthesis of (E)-β-Fluorovinyl Sulfones" Molecules 24, no. 8: 1569. https://doi.org/10.3390/molecules24081569