Synthesis and In Vitro Antibacterial Activity of Quaternized 10-Methoxycanthin-6-one Derivatives
Abstract
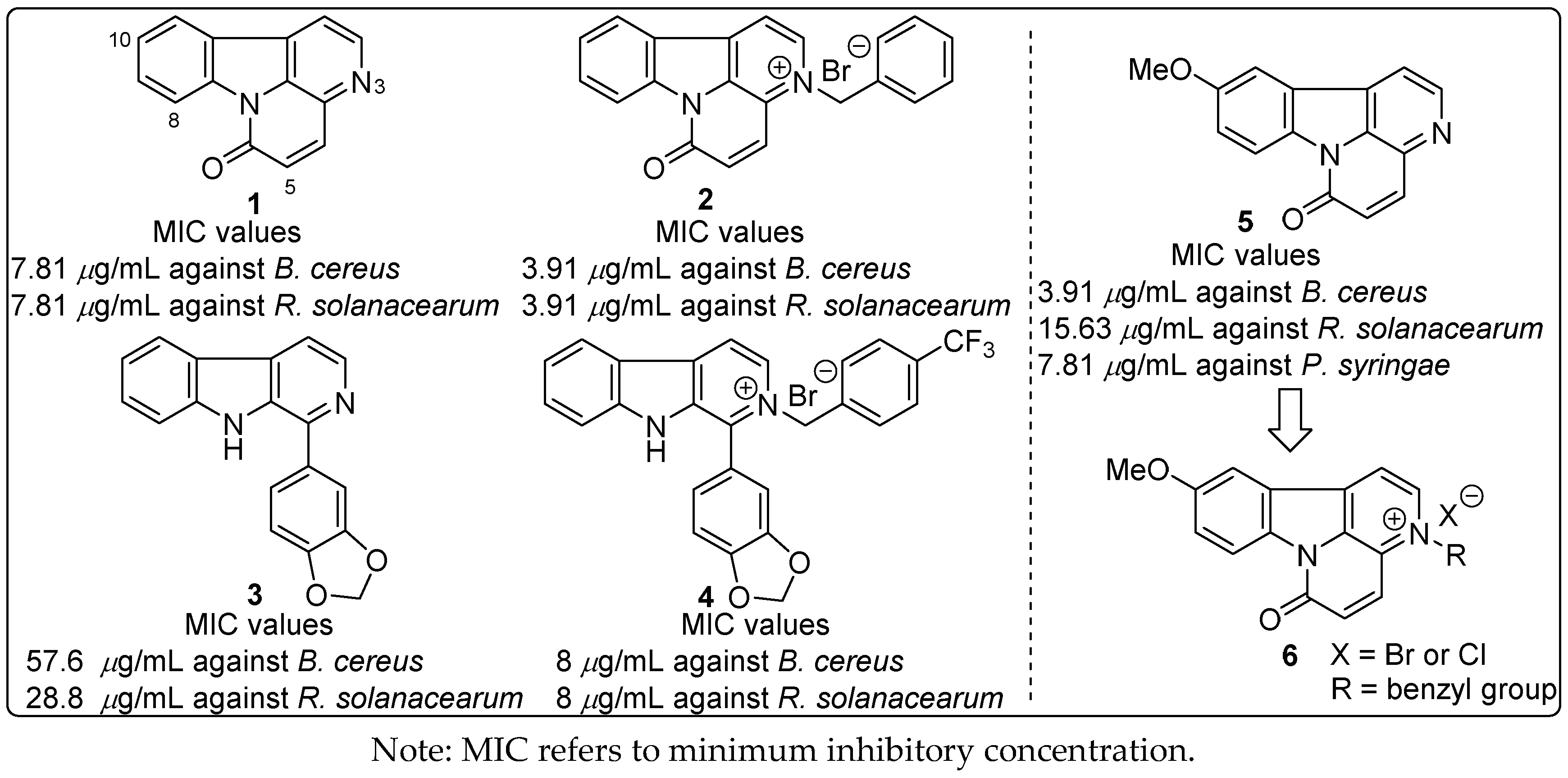
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antibacterial Activity
2.3. Structure–Activity Relationships
2.4. Molecular Physicochemical Properties
2.5. In Vitro Toxicity of the Highly Active Derivatives 6p and 6t
2.6. Germination Rate Evaluation of the Highly Active Derivatives 6p and 6t
3. Materials and Methods
3.1. General Details
3.2. Synthesis of Target Compounds 6a−6v
3.3. Antibacterial Assay
3.4. Calculation of Molecular Physicochemical Properties
3.5. Cell Toxicity Evaluation
3.6. Germination Rate Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. The structure and diversity of human, animal and environmental resistomes. Microbiome 2016, 4, 54. [Google Scholar] [CrossRef]
- Diao, M.; Qi, D.; Xu, M.; Lu, Z.; Lv, F.; Bie, X.; Zhang, C.; Zhao, H. Antibacterial activity and mechanism of monolauroylgalactosylglycerol against Bacillus cereus. Food Control 2018, 85, 339–344. [Google Scholar] [CrossRef]
- Miao, J.X.; Guo, H.X.; Chen, F.L.; Zhao, L.C.; He, L.P.; Ou, Y.W.; Huang, M.M.; Zhang, Y.; Guo, B.Y.; Cao, Y.; et al. Antibacterial effects of a cell-penetrating peptide isolated from Kefir. J. Agric. Food Chem. 2016, 64, 3234–3242. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcus, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Salanoubat, M.; Genin, S.; Artiguenave, F.; Gouzy, J.; Mangenot, S.; Arlat, M.; Billault, A.; Brottier, P.; Camus, J.; Cattolico, L. Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 2002, 415, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Xi, X.; Hu, Z.; Wu, W.J.; Zhang, J.W. Exploration of novel botanical insecticide leads: Synthesis and insecticidal activity of beta-dihydroagarofuran derivatives. J. Agric. Food Chem. 2016, 64, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.C.; Sun, Z.Q.; Lv, M.; Xu, H. Semisynthesis of matrinic acid/alcohol/ester derivatives, their pesticidal activities, and investigation of mechanisms of action against Tetranychus cinnabarinus. J. Agric. Food Chem. 2018, 66, 12898–12910. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.K.; Li, N.; Wang, J.R.; Schneider, U. Fruitful decades for canthin-6-ones from 1952 to 2015: Biosynthesis, chemistry, and biological activities. Molecules 2016, 21, 493. [Google Scholar] [CrossRef]
- Dai, J.K.; Dan, W.J.; Schneider, U.; Wang, J.R. β-Carboline alkaloid monomers and dimers: Occurrence, structural diversity, and biological activities. Eur. J. Med. Chem. 2018, 157, 622–656. [Google Scholar] [CrossRef]
- Dai, J.K.; Dan, W.J.; Li, N.; Du, H.T.; Zhang, J.W.; Wang, J.R. Synthesis, in vitro antibacterial activities of a series of 3-N-substituted canthin-6-ones. Bioorg. Med. Chem. Lett. 2016, 26, 580–583. [Google Scholar] [CrossRef]
- Dai, J.K.; Dan, W.J.; Ren, S.Y.; Shang, C.G.; Wang, J.R. Design, synthesis and biological evaluations of quaternization harman analogues as potential antibacterial agents. Eur. J. Med. Chem. 2018, 160, 23–36. [Google Scholar] [CrossRef]
- Dai, J.K.; Dan, W.J.; Zhang, Y.Y.; He, M.F.; Wang, J.R. Design and synthesis of C10 modified and ring-truncated canthin-6-one analogues as effective membrane-active antibacterial agents. Bioorg. Med. Chem. Lett. 2018, 28, 3123–3128. [Google Scholar] [CrossRef]
- Zhao, F.; Dai, J.K.; Li, D.; Wang, S.J.; Wang, J.R. Synthesis and evaluation of ester derivatives of 10-hydroxycanthin-6-one as potential antimicrobial agents. Molecules 2016, 21, 390. [Google Scholar] [CrossRef]
- Dai, J.K.; Dan, W.J.; Li, N.; Wang, J.R. Computer-aided drug discovery: Novel 3,9-disubstituted eudistomin U derivatives as potent antibacterial agents. Eur. J. Med. Chem. 2018, 157, 333–338. [Google Scholar] [CrossRef]
- Dai, J.K.; Dan, W.J.; Li, N.; Wang, R.X.; Zhang, Y.; Li, N.N.; Wang, R.Z.; Wang, J.R. Synthesis and antibacterial activity of C2 or C5 modified and D ring rejiggered canthin-6-one analogues. Food Chem. 2018, 253, 211–220. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Song, Z.; Li, W.; Zhu, F.; Zhang, J.; Li, S. Synthesis and bio-inspired optimization of drimenal: Discovery of chiral drimane fused oxazinones as promising antifungal and antibacterial candidates. Eur. J. Med. Chem. 2018, 143, 558–567. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Clarke, E.D.; Delaney, J.S. Physical and molecular properties of agrochemicals: An analysis of screen inputs, hits, leads, and products. Chimia 2003, 57, 731–734. [Google Scholar] [CrossRef]
- Hou, W.; Zhang, G.J.; Luo, Z.; Li, D.; Quan, H.Q.; Ruan, B.H.; Su, L.; Xu, H.T. Identification of a diverse synthetic abietane diterpenoid library and insight into the structure–activity relationships for antibacterial activity. Bioorg. Med. Chem. Lett. 2017, 27, 5382–5386. [Google Scholar] [CrossRef]
- Xu, Q.B.; Chen, X.F.; Feng, J.; Miao, J.F.; Liu, J.; Liu, F.T.; Niu, B.X.; Cai, J.Y.; Huang, Y.; Zhang, N.; et al. Design, synthesis and biological evaluation of hybrids of β-carboline and salicylic acid as potential anticancer and apoptosis inducing agents. Sci. Rep. 2016, 6, 36238. [Google Scholar] [CrossRef]
- Elazouni, I.; Abdel-Aziz, S.; Rabea, A. Microbial efficacy as biological agents for potato enrichment as well as bio-controls against wilt disease caused by Ralstonia solanacearum. World J. Microbiol. Biotechnol. 2019, 35, 30. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing; 11th Informational Supplement; M100-S11; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2001; Volume 21. [Google Scholar]
- Feng, L.; Maddox, M.M.; Alam, M.Z.; Tsutsumi, L.S.; Narula, G.; Bruhn, D.F.; Wu, X.; Sandhaus, S.; Lee, R.B.; Simmons, C.J.; et al. Synthesis, structure−activity relationship studies, and antibacterial evaluation of 4-chromanones and chalcones, as well as olympicin A and derivatives. J. Med. Chem. 2014, 57, 8398–8420. [Google Scholar] [CrossRef]
- Su, M.; Xia, D.L.; Teng, P.; Nimmagadda, A.; Zhang, C.; Odom, T.; Cao, A.N.; Hu, Y.; Cai, J.F. Membrane-active hydantoin derivatives as antibiotic agents. J. Med. Chem. 2017, 60, 8456–8465. [Google Scholar] [CrossRef]
- Dan, W.J.; Geng, H.L.; Qiao, J.W.; Guo, R.; Wei, S.P.; Li, L.B.; Wu, W.J.; Zhang, J.W. Efficient synthesis and sntibacterial evaluation of (±)-Yanglingmycin and its analogues. Molecules 2016, 21, 96. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds in this paper are available from the authors. |

| No. | R | X | B. cereus | R. solanacearum | P. syringae |
|---|---|---|---|---|---|
| 6a | -CH2Ph(o-F) | Br | 15.63 | 15.63 | 15.63 |
| 6b | -CH2Ph(p-F) | Br | 15.63 | 15.63 | 15.63 |
| 6c | -CH2Ph(m-F) | Br | 31.25 | 7.81 | 15.63 |
| 6d | -CH2Ph(m-CN) | Cl | 62.50 | 15.63 | 15.63 |
| 6e | -CH2Ph(o-Cl) | Cl | 15.63 | 3.91 | 7.81 |
| 6f | -CH2Ph(m-Cl) | Cl | 15.63 | 3.91 | 3.91 |
| 6g | -CH2Ph(o-Me) | Br | 15.63 | 7.81 | 7.81 |
| 6h | -CH2Ph(p-Me) | Br | 15.63 | 7.81 | 3.91 |
| 6i | -CH2Ph(m-Me) | Br | 15.63 | 3.91 | 3.91 |
| 6j | -CH2Ph(m-OMe) | Cl | 15.63 | 15.63 | 7.81 |
| 6k | -CH2Ph(o-CF3) | Br | 15.63 | 3.91 | 7.81 |
| 6l | -CH2Ph(p-CF3) | Br | 15.63 | 3.91 | 7.81 |
| 6m | -CH2Ph(m-CF3) | Br | 15.63 | 3.91 | 15.63 |
| 6n | -CH2Ph(2,5-difluoro) | Br | 31.25 | 7.81 | 15.63 |
| 6o | -CH2Ph(2,4-difluoro) | Br | 15.63 | 15.63 | 7.81 |
| 6p | -CH2Ph(3,4-dichloro) | Br | 7.81 | 3.91 | 3.91 |
| 6q | -CH2Ph(o-Br) | Br | 7.81 | 7.81 | 15.63 |
| 6r | -CH2Ph(p-Br) | Br | 7.81 | 7.81 | 7.81 |
| 6s | -CH2Ph(m-Br) | Br | 15.63 | 7.81 | 7.81 |
| 6t | -CH2Ph(m-I) | Br | 7.81 | 3.91 | 3.91 |
| 6u | -CH2Ph | Br | 15.63 | 15.63 | 15.63 |
| 6v | -CH2Ph | Cl | 15.63 | 15.63 | 15.63 |
| 5 | - | - | 3.91 | 15.63 | 7.81 |
| F.S. a | - | - | 3.91 | 1.95 | 15.63 |
| A.S. a | - | - | 3.91 | 7.81 | 0.98 |
| S.S. a | - | - | 15.63 | 31.25 | 31.25 |

| Location | Bacteria Strains | SARs |
|---|---|---|
| Ortho-position | R. solanacearum | F < Br = Me < Cl = CF3 |
| P. syringae | F = Br < Cl = Me = CF3 | |
| Meta-position | R. solanacearum | CN = OMe < F = Br < Cl = I = Me = CF3 |
| P. syringae | F = CN = CF3 < OMe = Br < Cl = I = Me | |
| Para-position | R. solanacearum | F < Br = Me < CF3 |
| P. syringae | F < Br = CF3 < Me |
| No. | LogP a | TPSA b | nON c | nONNH d | Nrotb e | MW f |
|---|---|---|---|---|---|---|
| 5 | 2.72 | 43.61 | 4 | 0 | 1 | 250.2 |
| 6p | 1.18 | 34.6 | 4 | 0 | 3 | 410.3 |
| 6t | 0.98 | 34.6 | 4 | 0 | 3 | 467.3 |
| No. | Cell Viability (%) | ||
|---|---|---|---|
| 3.91 μg/mL | 7.81 μg/mL | 15.62 μg/mL | |
| 6p | 99.7 ± 2.9 | 98.2 ± 2.7 | 96.3 ± 4.4 |
| 6t | 98.7 ± 2.2 | 98.2 ± 4.1 | 96.1 ± 3.8 |
| No. | Turnip (%) | Wheat (%) |
|---|---|---|
| 6p | 94 ± 0.33 | 93 ± 0.89 |
| 6t | 91 ± 0.87 | 96 ± 0.55 |
| GP | 76 ± 0.33 | 72 ± 0.83 |
| CK | 93 ± 0.53 | 95 ± 0.57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Liu, D.; Dai, J.-K.; Wang, J.-Y.; Wang, J.-R. Synthesis and In Vitro Antibacterial Activity of Quaternized 10-Methoxycanthin-6-one Derivatives. Molecules 2019, 24, 1553. https://doi.org/10.3390/molecules24081553
Li N, Liu D, Dai J-K, Wang J-Y, Wang J-R. Synthesis and In Vitro Antibacterial Activity of Quaternized 10-Methoxycanthin-6-one Derivatives. Molecules. 2019; 24(8):1553. https://doi.org/10.3390/molecules24081553
Chicago/Turabian StyleLi, Na, Dan Liu, Jiang-Kun Dai, Jin-Yi Wang, and Jun-Ru Wang. 2019. "Synthesis and In Vitro Antibacterial Activity of Quaternized 10-Methoxycanthin-6-one Derivatives" Molecules 24, no. 8: 1553. https://doi.org/10.3390/molecules24081553






