Multi-Organs-on-Chips: Towards Long-Term Biomedical Investigations
Abstract
:1. Introduction
2. Development of Long-Term Testing in MOC Systems
2.1. Advances in Microfluidic Technology for Long-Term Investigations
2.2. Biomedical Sensors for Long-Term as well as Real-Time Monitoring of MOC Platforms
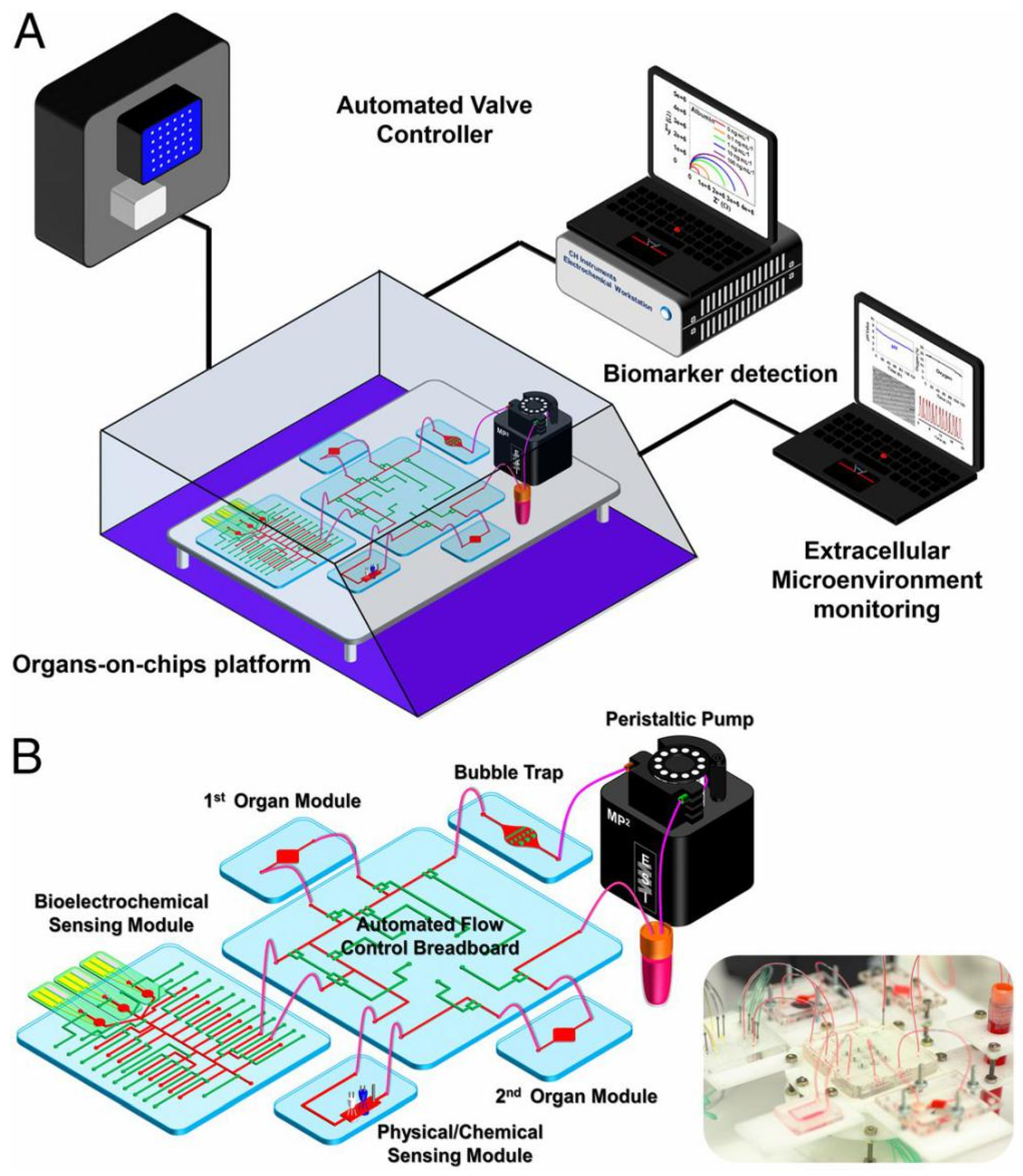
2.3. Multisensor-Integrated MOC Systems
3. Biomedical Applications of Long-Term Testing in MOC Platforms
3.1. Proposed Biomedical Applications of Long-Term Testing in MOC Systems
3.1.1. Drug Testing/Toxicology
3.1.2. Disease Modeling
3.2. Potential Applications of Long-Term Testing using MOCs
3.2.1. Drug Screening
3.2.2. Cancer Metastasis
3.2.3. Biomarker Detection
3.2.4. Personalized Medicine
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Avior, Y.; Sagi, I.; Benvenisty, N. Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Esch, M.B.; Smith, A.S.T.; Prot, J.-M.; Oleaga, C.; Hickman, J.J.; Shuler, M.L. How multi-organ microdevices can help foster drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz-Schughart, L.; Freyer, J.; Hofstaedter, F.; Ebner, R. The use of 3-D cultures for high-throughput screening: The multicellular spheroid model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Pham, E.A.; Kim, J.W.; Ng, C.W.; Kim, J.H.; Kamei, D.T.; Wu, B.M. Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs. Cancer Sci. 2010, 101, 2637–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drewitz, M.; Helbling, M.; Fried, N.; Bieri, M.; Moritz, W.; Lichtenberg, J.; Kelm, J.M. Towards automated production and drug sensitivity testing using scaffold-free spherical tumor microtissues. Biotechnol. J. 2011, 6, 1488–1496. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839. [Google Scholar] [CrossRef]
- Jamieson, L.E.; Harrison, D.J.; Campbell, C.J. Chemical analysis of multicellular tumour spheroids. Analyst 2015, 140, 3910–3920. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.H.; Srinivasan, B.; Esch, M.B.; McLamb, W.T.; Bernabini, C.; Shuler, M.L.; Hickman, J.J. Using physiologically-based pharmacokinetic-guided “body-on-a-chip” systems to predict mammalian response to drug and chemical exposure. Exp. Biol. Med. 2014, 239, 1225–1239. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.H. Chapter 10—Pharmacokinetic-based multi-organ chip for recapitulating organ interactions. In Methods in Cell Biology; Doh, J., Fletcher, D., Piel, M., Eds.; Academic Press: San Diego, CA, USA, 2018; Volume 146, pp. 183–197. [Google Scholar]
- Greek, R.; Menache, A. Systematic reviews of animal models: Methodology versus epistemology. Int. J. Med. Sci. 2013, 10, 206–221. [Google Scholar] [CrossRef]
- Wagner, I.; Materne, E.-M.; Brincker, S.; Süssbier, U.; Frädrich, C.; Busek, M.; Sonntag, F.; Sakharov, D.A.; Trushkin, E.V.; Tonevitsky, A.G.; et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip 2013, 13, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, P.J.K.; Kooijman, M.; Gispen-de Wied, C.C.; Moors, E.H.M.; Schellekens, H. The ability of animal studies to detect serious post marketing adverse events is limited. Regul. Toxicol. Pharmacol. 2012, 64, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Torisawa, Y.-S.; Hamilton, G.A.; Kim, H.J.; Ingber, D.E. Microengineered physiological biomimicry: Organs-on-chips. Lab Chip 2012, 12, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Radisic, M. Organ-on-a-chip devices advance to market. Lab Chip 2017, 17, 2395–2420. [Google Scholar] [CrossRef] [PubMed]
- Caplin, J.D.; Granados, N.G.; James, M.R.; Montazami, R.; Hashemi, N. Microfluidic Organ-on-a-Chip Technology for Advancement of Drug Development and Toxicology. Adv. Healthc. Mater. 2015, 4, 1426–1450. [Google Scholar] [CrossRef] [PubMed]
- Ronaldsonbouchard, K.; Vunjaknovakovic, G. Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development. Cell Stem Cell 2018, 22, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.S.; Jothimuthu, P.; Papautsky, I. Photodefinable polydimethylsiloxane (PDMS) for rapid lab-on-a-chip prototyping. Lab Chip 2007, 7, 1192–1197. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, G.; Siong, C.F. Novel polydimethylsiloxane (PDMS) based microchannel fabrication method for lab-on-a-chip application. Sens. Actuators B Chem. 2009, 137, 754–761. [Google Scholar]
- Knowlton, S.; Yenilmez, B.; Tasoglu, S. Towards single-step biofabrication of organs on a chip via 3D printing. Trends Biotechnol. 2016, 34, 685–688. [Google Scholar] [CrossRef]
- Young, P.J.; Jinah, J.; Hyun-Wook, K. 3D Bioprinting and its application to organ-on-a-chip. Microelectron. Eng. 2018, 200, 1–11. [Google Scholar]
- Amin, R.; Knowlton, S.; Hart, A. 3D-printed microfluidic devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Lu, F.J.; Liu, C.G. Effect of icariin on engineered 3D-printed porous scaffolds for cartilage repair. Materials 2018, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Hee-Gyeong, Y.; Hyungseok, L.; Dong-Woo, C. 3D Printing of Organs-On-Chips. Bioengineering 2017, 4, 10. [Google Scholar] [Green Version]
- Kankala, R.K.; Xu, X.M.; Liu, C.G. 3D-printing of microfibrous porous scaffolds based on hybrid approaches for bone tissue engineering. Polymers 2018, 10, 807. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhu, K.; Li, J. Fabrication of arbitrary 3D components in cardiac surgery: From macro-, micro- to nanoscale. Biofabrication 2017, 9, 032002. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhu, K.; Sun, X. Cardiac Tissue Engineering on the Nanoscale. ACS Biomater. Sci. Eng. 2018, 4, 800–818. [Google Scholar] [CrossRef]
- Kieninger, J.; Weltin, A.; Flamm, H.; Urban, G.A. Microsensor systems for cell metabolism—From 2D culture to organ-on-chip. Lab Chip 2018, 18, 1274–1291. [Google Scholar] [CrossRef]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120. [Google Scholar] [CrossRef]
- Midwoud, P.M.V.; Merema, M.T.; Verpoorte, E.; Groothuis, G.M.M. A microfluidic approach for in vitro assessment of interorgan interactions in drug metabolism using intestinal and liver slices. Lab Chip 2010, 10, 2778–2786. [Google Scholar] [CrossRef]
- Toh, Y.C.; Lim, T.L.; Tai, D.; Xiao, G.; Van Noort, D.; Yu, H. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip 2009, 9, 2026–2035. [Google Scholar] [CrossRef]
- van Midwoud, P.M.; Verpoorte, E.; Groothuis, G.M. Microfluidic devices for in vitro studies on liver drug metabolism and toxicity. Integr. Biol. 2011, 3, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.T.; Boucher, Y.; Kozin, S.V.; Winkler, F.; Hicklin, D.J.; Jain, R.K. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004, 64, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Wlodkowic, D.; Cooper, J.M. Tumors on chips: Oncology meets microfluidics. Curr. Opin. Chem. Biol. 2010, 14, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Romero, I.A. Transporting therapeutics across the blood-brain barrier. Mol. Med. Today 1996, 2, 106–113. [Google Scholar] [CrossRef]
- Prabhakarpandian, B.; Shen, M.C.; Nichols, J.B.; Mills, I.R.; Sidoryk-Wegrzynowicz, M.; Aschner, M.; Pant, K. SyM-BBB: A microfluidic Blood Brain Barrier model. Lab Chip 2013, 13, 1093–1101. [Google Scholar] [CrossRef]
- Patton, J.S. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 1996, 19, 3–36. [Google Scholar] [CrossRef]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248. [Google Scholar] [CrossRef]
- Polini, A.; Prodanov, L.; Bhise, N.S.; Manoharan, V.; Dockmeci, M.R.; Khademhosseini, A. Organs-on-a-chip: A new tool for drug discovery. Expert Opin. Drug Discov. 2014, 9, 335–352. [Google Scholar] [CrossRef]
- Selimović, S.; Dokmeci, M.R.; Khademhosseini, A. Organs-on-a-chip for drug discovery. Curr. Opin. Pharmacol. 2013, 13, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Smith, A.S.; Lone, S.; Kwon, S.; Kim, D.H. Biomimetic 3D Tissue Models for Advanced High-Throughput Drug Screening. J. Lab. Autom. 2015, 20, 201–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Sung, J.H. Organ-on-a-Chip Technology for Reproducing Multiorgan Physiology. Adv. Healthc. Mater. 2017, 7, 1700419. [Google Scholar] [CrossRef] [PubMed]
- Atac, B.; Wagner, I.; Horland, R.; Lauster, R.; Marx, U.; Tonevitsky, A.G.; Azar, R.P.; Lindner, G. Skin and hair on-a-chip: In vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip 2013, 13, 3555. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materne, E.M.; Maschmeyer, I.; Lorenz, A.K.; Horland, R.; Schimek, K.M.S.; Busek, M.; Sonntag, F.; Lauster, R.; Marx, U. The Multi-organ Chip—A Microfluidic Platform for Long-term Multi-tissue Coculture. J. Vis. Exp. 2015, 2015, e52526. [Google Scholar] [CrossRef] [PubMed]
- Bugrim, A.; Nikolskaya, T.; Nikolsky, Y. Early prediction of drug metabolism and toxicity: Systems biology approach and modeling. Drug Discov. Today 2004, 9, 127–135. [Google Scholar] [CrossRef]
- Lahoz, A.; Gombau, L.; Donato, M.T.; Castell, J.V.; Gómezlechón, M.J. In vitro ADME medium/high-throughput screening in drug preclinical development. Mini Rev. Med. Chem. 2006, 6, 1053–1062. [Google Scholar] [CrossRef]
- Sung, J.H.; Shuler, M.L. In vitro microscale systems for systematic drug toxicity study. Bioprocess Biosyst. Eng. 2010, 33, 5–19. [Google Scholar] [CrossRef]
- Dingemanse, D.J.; Appel-Dingemanse, S. Integrated Pharmacokinetics and Pharmacodynamics in Drug Development. Clin. Pharmacokinet. 2007, 46, 713–737. [Google Scholar] [CrossRef]
- Seung, L.; Jong, S. Microtechnology-Based Multi-Organ Models. Bioengineering 2017, 4, 46. [Google Scholar] [Green Version]
- Lee, J.B.; Sung, J.H. Organ-on-a-chip technology and microfluidic whole-body models for pharmacokinetic drug toxicity screening. Biotechnol. J. 2014, 8, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–715. [Google Scholar] [CrossRef]
- Dickson, M.; Gagnon, J.P. Key factors in the rising cost of new drug discovery and development. Nat. Rev. Drug Discov. 2004, 3, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Agoram, B.M.; Martin, S.W.; Graaf, P.H.V.D. The role of mechanism-based pharmacokinetic–pharmacodynamic (PK–PD) modelling in translational research of biologics. Drug Discov. Today 2007, 12, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.I.; Oleaga, C.; Long, C.J. Self-contained, low-cost Body-on-a-Chip systems for drug development. Exp. Biol. Med. 2017, 242, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.; Mehta, G.; Lesher-Perez, S.C.; Takayama, S. Organs-on-a-chip: A focus on compartmentalized microdevices. Ann. Biomed. Eng. 2012, 40, 1211–1227. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Wikswo, J.P.; Curtis, E.L.; Eagleton, Z.E.; Evans, B.C.; Kole, A.; Hofmeister, L.H.; Matloff, W.J. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab Chip 2013, 13, 3496–3511. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Mousavi Shaegh, S.A.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293. [Google Scholar] [CrossRef]
- Horland, R.; Materne, E.M.; Wagner, I.; Schimek, K.; Hasenberg, T.; Lorenz, A.; Jaenicke, A.; Ramme, A.; Sonntag, F.; Lauster, R. The Multi-Organ-Chip (MOC)—A universal microfluidic platform for long-term tissue maintenance and substance testing. Toxicol. Lett. 2014, 229, S139. [Google Scholar] [CrossRef]
- Kim, L.; Toh, Y.C.; Voldman, J.; Yu, H. A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip 2007, 7, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Huang, S.B.; Lee, G.B. Microfluidic cell culture systems for drug research. Lab Chip 2010, 10, 939–956. [Google Scholar] [CrossRef] [PubMed]
- Young, E.W.; Beebe, D.J. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem. Soc. Rev. 2010, 39, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.U.R.; Geng, C.; Fu, M.; Yu, X.; Qin, K.; Liu, B. The Role of Microfluidics for Organ on Chip Simulations. Bioengineering 2017, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Tourovskaia, A.; Figueroa-Masot, X.; Folch, A. Differentiation-on-a-chip: A microfluidic platform for long-term cell culture studies. Lab Chip 2004, 5, 14–19. [Google Scholar] [CrossRef]
- Korin, N.; Bransky, A.; Dinnar, U.; Levenberg, S. A parametric study of human fibroblasts culture in a microchannel bioreactor. Lab Chip 2007, 7, 611–617. [Google Scholar] [CrossRef]
- Zhu, X.; Yi, C.L.; Chueh, B.H.; Shen, M.; Hazarika, B.; Phadke, N.; Takayama, S. Arrays of horizontally-oriented mini-reservoirs generate steady microfluidic flows for continuous perfusion cell culture and gradient generation. Analyst 2004, 129, 1026–1031. [Google Scholar] [CrossRef]
- Liu, M.C.; Ho, D.; Tai, Y.C. Monolithic fabrication of three-dimensional microfluidic networks for constructing cell culture array with an integrated combinatorial mixer. Sens. Actuators B Chem. 2008, 129, 826–833. [Google Scholar] [CrossRef]
- Lii, J.; Hsu, W.J.; Parsa, H.; Das, A.; Rouse, R.; Sia, S.K. Real-Time Microfluidic System for Studying Mammalian Cells in 3D Microenvironments. Anal. Chem. 2008, 80, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhu, X.; Futai, N.; Cho, B.S.; Takayama, S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc. Natl. Acad. Sci. USA 2004, 101, 15861–15866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, H.; Yamamoto, T.; Sakai, H.; Sakai, Y.; Fujii, T. An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab Chip 2008, 8, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Huang, S.B.; Cui, Z.; Zheng, C.; Lee, G.B. A high throughput perfusion-based microbioreactor platform integrated with pneumatic micropumps for three-dimensional cell culture. Biomed. Microdevices 2008, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, M.; Kim, S. Pumpless steady-flow microfluidic chip for cell culture. Anal. Biochem. 2013, 437, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.G.; Shuler, M.L. Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol. Bioeng. 2016, 113, 2213–2227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.I.; Shuler, M.L. UniChip enables long-term recirculating unidirectional perfusion with gravity-driven flow for microphysiological systems. Lab Chip 2018, 18, 2563–2574. [Google Scholar] [CrossRef]
- Weltin, A.; Slotwinski, K.; Kieninger, J.; Moser, I.; Jobst, G.; Wego, M.; Ehret, R.; Urban, G.A. Cell culture monitoring for drug screening and cancer research: A transparent, microfluidic, multi-sensor microsystem. Lab Chip 2014, 14, 138–146. [Google Scholar] [CrossRef]
- Li, Y.; Tao, X.; Zou, H.; Chen, X.; Dong, S.; Yang, M. Cell migration microfluidics for electrotaxis-based heterogeneity study of lung cancer cells. Biosens. Bioelectron. 2016, 89, 837. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Abdolahad, M.; Zanganeh, S.; Dahmardeh, M.; Gharooni, M.; Abiri, H.; Alikhani, A.; Mohajerzadeh, S.; Mashinchian, O. Nanoelectromechanical Chip (NELMEC) Combination of Nanoelectronics and Microfluidics to Diagnose Epithelial and Mesenchymal Circulating Tumor Cells from Leukocytes. Small 2016, 12, 883–891. [Google Scholar] [CrossRef]
- Riahi, R.; Shaegh, S.A.M.; Ghaderi, M.; Yu, S.Z.; Su, R.S.; Aleman, J.; Massa, S.; Kim, D.; Dokmeci, M.R.; Khademhosseini, A. Automated microfluidic platform of bead-based electrochemical immunosensor integrated with bioreactor for continual monitoring of cell secreted biomarkers. Sci. Rep. 2016, 6, 24598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero, D.; Kaushik, S.; Correlo, V.M.; Oliveira, J.M.; Reis, R.L.; Kundu, S.C. Organ-on-chip models of cancer metastasis for future personalized medicine: From chip to the patient. Biomaterials 2017, 149, 98. [Google Scholar] [CrossRef]
- Javanmard, M.; Davis, R.W. A microfluidic platform for electrical detection of DNA hybridization. Sens. Actuators B Chem. 2011, 154, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De-Carvalho, J.; Rodrigues, R.M.; Tomé, B.; Henriques, S.F.; Mira, N.P.; Sá-Correia, I.; Ferreira, G.N. Conformational and mechanical changes of DNA upon transcription factor binding detected by a QCM and transmission line model. Analyst 2014, 139, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Chen, H.Y. Amperometric Glucose Sensor Based on Coimmobilization of Glucose Oxidase and Poly( p -phenylenediamine) at a Platinum Microdisk Electrode. Anal. Biochem. 2000, 280, 221–226. [Google Scholar]
- Mishra, G.K.; Sharma, A.; Deshpande, K.; Bhand, S. Flow injection analysis biosensor for urea analysis in urine using enzyme thermistor. Appl. Biochem. Biotechnol. 2014, 174, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yin, B.C.; Wang, X.F.; Ye, B.C. Interaction of peptides with graphene oxide and its application for real-time monitoring of protease activity. Chem. Commun. 2011, 47, 2399–2401. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Chirumamilla, M.; Toma, A.; Gopalakrishnan, A.; Zaccaria, R.P.; Alabastri, A.; Leoncini, M.; Di, F.E. Plasmon based biosensor for distinguishing different peptides mutation states. Sci. Rep. 2013, 3, 1792. [Google Scholar] [CrossRef] [Green Version]
- Vistas, C.R.; Soares, S.S.; Rodrigues, R.M.; Chu, V.; Conde, J.P.; Ferreira, G.N. An amorphous silicon photodiode microfluidic chip to detect nanomolar quantities of HIV-1 virion infectivity factor. Analyst 2014, 139, 3709–3713. [Google Scholar] [CrossRef]
- Estevesvillanueva, J.O.; Trzeciakiewicz, H.; Martic, S. A protein-based electrochemical biosensor for detection of tau protein, a neurodegenerative disease biomarker. Analyst 2014, 139, 2823–2831. [Google Scholar] [CrossRef]
- Moral-Vico, J.; Barallat, J.; Abad, L.; Olivé-Monllau, R.; Muñoz-Pascual, F.X.; Ortega, A.G.; Campo, F.J.D.; Baldrich, E. Dual chronoamperometric detection of enzymatic biomarkers using magnetic beads and a low-cost flow cell. Biosens. Bioelectron. 2015, 69, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Loke, W.K.; Nguyen, N.T.; Tan, S.N.; Tay, N.B.; Wang, W.; Ng, S.H. Lab-on-a-chip for rapid electrochemical detection of nerve agent Sarin. Biomed. Microdevices 2014, 16, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Klauke, N.; Sedgwick, H.; Smith, G.L.; Cooper, J.M. Metabolic monitoring of the electrically stimulated single heart cell within a microfluidic platform. Lab Chip 2006, 6, 1424–1431. [Google Scholar] [CrossRef]
- Sun, Y.S.; Peng, S.W.; Cheng, J.Y. In vitro electrical-stimulated wound-healing chip for studying electric field-assisted wound-healing process. Biomicrofluidics 2012, 6, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Goss, J.A.; Cho, A.; Mccain, M.L.; Parker, K.K. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip 2013, 13, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R. When Microfluidic Devices Go Bad. Anal. Chem. 2005, 77, 429A–432A. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xiong, P.; Yun, C.S.; Strouse, G.F.; Zheng, J.P.; Yang, R.S.; Wang, Z.L. Mechanism and optimization of pH sensing using SnO2 nanobelt field effect transistors. Nano Lett. 2008, 8, 4179. [Google Scholar] [CrossRef]
- Wu, M.H.; Urban, J.P.; Cui, Z.F.; Cui, Z.; Xu, X. Effect of extracellular ph on matrix synthesis by chondrocytes in 3D agarose gel. Biotechnol. Prog. 2010, 23, 430–434. [Google Scholar] [CrossRef]
- Paradise, R.K.; Lauffenburger, D.A.; Vliet, K.J.V. Acidic Extracellular pH Promotes Activation of Integrin αvβ3. PLoS ONE 2011, 6, e15746. [Google Scholar] [CrossRef]
- Radisic, M.; Malda, J.; Epping, E.; Geng, W.; Langer, R.; Vunjaknovakovic, G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol. Bioeng. 2010, 93, 332–343. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V.; Husak, V.V.; Luzhna, L.I.; Lushchak, O.V.; Storey, K.B. Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in goldfish tissues. Int. J. Biochem. Cell Biol. 2005, 37, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Sud, D.; Mehta, G.; Mehta, K.; Linderman, J.; Takayama, S.; Mycek, M.A. Optical imaging in microfluidic bioreactors enables oxygen monitoring for continuous cell culture. J. Biomed. Opt. 2006, 11, 050504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi Shaegh, S.A.; De, F.F.; Zhang, Y.S.; Nabavinia, M.; Binth, M.N.; Ryan, J.; Pourmand, A.; Laukaitis, E.; Banan, S.R.; Nadhman, A. A microfluidic optical platform for real-time monitoring of pH and oxygen in microfluidic bioreactors and organ-on-chip devices. Biomicrofluidics 2016, 10, 760–793. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, D.J.; Iii, F.E.B.; Cliffel, D.E.; Daniels, J.S.; Ellacott, K.L.; Goodwin, C.R.; Hofmeister, L.H.; Li, D.; Markov, D.A.; May, J.C. Neurovascular unit on a chip: Implications for translational applications. Stem Cell Res. Ther. 2013, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Fabio, B.; João, R.; Julio, A.; Nascimento, R.T.; Mousavi, S.S.A.; Solange, M.; Baj, R.C.; Irene, T.; Su-Ryon, S. Google Glass-Directed Monitoring and Control of Microfluidic Biosensors and Actuators. Sci. Rep. 2016, 6, 22237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungerböck, B.; Mistlberger, G.; Charwat, V.; Ertl, P.; Mayr, T. Oxygen imaging in microfluidic devices with optical sensors applying color cameras. Procedia Eng. 2010, 5, 456–459. [Google Scholar] [CrossRef] [Green Version]
- Mckenzie, J.R.; Cliffel, D.E.; Wikswo, J.P. Electrochemical Monitoring of Cellular Metabolism. Encycl. Appl. Electrochem. 2014, 522–528. [Google Scholar]
- Wu, C.C.; Lin, W.C.; Fu, S.Y. The open container-used microfluidic chip using IrO(x) ultramicroelectrodes for the in situ measurement of extracellular acidification. Biosens. Bioelectron. 2011, 26, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Andreas, R.; Georgi, P.; Stephan, K.; Jens, L.; Karl-Friedrich, A.; Adler, H.-J.P. Review on Hydrogel-based pH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [Green Version]
- Koman, V.B.; Santschi, C.; Martin, O.J.F. Multiscattering-enhanced optical biosensor: Multiplexed, non-invasive and continuous measurements of cellular processes. Biomed. Opt. Express 2015, 6, 2353–2365. [Google Scholar] [CrossRef] [PubMed]
- Hondebrink, L.; Verboven, A.H.; Drega, W.S.; Schmeink, S.; de Groot, M.W.; van Kleef, R.G.; Wijnolts, F.M.; De, G.A.; Meulenbelt, J.; Westerink, R.H. Neurotoxicity screening of drugs of abuse using novel methods for analysis of microelectrode array (MEA) recordings. Neurotoxicology 2016, 55, 1–9. [Google Scholar] [CrossRef]
- Wainger, B.J.; Kiskinis, E.; Mellin, C.; Wiskow, O.; Han, S.S.; Sandoe, J.; Perez, N.P.; Williams, L.A.; Lee, S.; Boulting, G. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muratore, C.R.; Rice, H.C.; Srikanth, P.; Callahan, D.G.; Shin, T.; Benjamin, L.N.; Walsh, D.M.; Selkoe, D.J.; Youngpearse, T.L. The familial Alzheimer’s disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum. Mol. Genet. 2014, 23, 3523–3536. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, K.H.; Lewis, G.F.; Gay, E.A.; Sellgren, K.L.; Grego, S. High-throughput cardiac safety evaluation and multi-parameter arrhythmia profiling of cardiomyocytes using microelectrode arrays. Toxicol. Appl. Pharmacol. 2015, 288, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Mcconnell, E.R.; Mcclain, M.A.; Ross, J.; Lefew, W.R.; Shafer, T.J. Evaluation of multi-well microelectrode arrays for neurotoxicity screening using a chemical training set. Neurotoxicology 2012, 33, 1048–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groot, M.W.G.D.M.d.; Dingemans, M.M.L.; Rus, K.H.; Groot, A.D.; Westerink, R.H.S. Characterization of Calcium Responses and Electrical Activity in Differentiating Mouse Neural Progenitor Cells In Vitro. Toxicol. Sci. 2014, 137, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.; Shieh, J. Chapter 8—Manipulating Neural Activity. In Guide to Research Techniques in Neuroscience, 2nd ed.; Carter, M., Shieh, J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 185–201. [Google Scholar]
- Kim, R.; Joo, S.; Jung, H.; Hong, N.; Nam, Y. Recent trends in microelectrode array technology for in vitro neural interface platform. Biomed. Eng. Lett. 2014, 4, 129–141. [Google Scholar] [CrossRef]
- Oleaga, C.; Riu, A.; Rothemund, S.; Lavado, A.; McAleer, C.W.; Long, C.J.; Persaud, K.; Narasimhan, N.S.; Tran, M.; Roles, J.; et al. Investigation of the effect of hepatic metabolism on off-target cardiotoxicity in a multi-organ human-on-a-chip system. Biomaterials 2018, 182, 176–190. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Seeking the right context for evaluating nanomedicine: From tissue models in petri dishes to microfluidic organs-on-a-chip. Nanomedicine 2015, 10, 685–688. [Google Scholar] [CrossRef]
- Wikswo, J.P.; Rd, B.F.; Cliffel, D.E.; Goodwin, C.R.; Marasco, C.C.; Markov, D.A.; Mclean, D.L.; Mclean, J.A.; Mckenzie, J.R.; Reiserer, R.S. Engineering challenges for instrumenting and controlling integrated organ-on-chip systems. IEEE Trans. Biomed. Eng. 2013, 60, 682–690. [Google Scholar] [CrossRef]
- Zhang, Y.S. Modular multi-organ-on-chips platform with physicochemical sensor integration. In Proceedings of the IEEE International Midwest Symposium on Circuits and Systems, Boston, MA, USA, 6–9 August 2017; pp. 80–83. [Google Scholar]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.D.; Douville, N.J.; Takayama, S.; Elsayed, M. Quantitative analysis of molecular absorption into PDMS microfluidic channels. Ann. Biomed. Eng. 2012, 40, 1862–1873. [Google Scholar] [CrossRef] [PubMed]
- Luni, C.; Serena, E.; Elvassore, N. Human-on-chip for therapy development and fundamental science. Curr. Opin. Biotechnol. 2014, 25, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Qin, J.; Shi, W.; Liu, X.; Lin, B. Cell-based high content screening using an integrated microfluidic device. Lab Chip 2007, 7, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Kam, C.; Shuler, M.L. A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab Chip 2010, 10, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 4250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Fluri, D.A.; Kelm, J.M.; Hierlemann, A.; Frey, O. 96-well format-based microfluidic platform for parallel interconnection of multiple multicellular spheroids. J. Lab. Autom. 2015, 20, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Fluri, D.A.; Marchan, R.; Boonen, K.; Mohanty, S.; Singh, P.; Hammad, S.; Landuyt, B.; Hengstler, J.G.; Kelm, J.M. 3D spherical microtissues and microfluidic technology for multi-tissue experiments and analysis. J. Biotechnol. 2015, 205, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materne, E.-M.; Ramme, A.P.; Terrasso, A.P.; Serra, M.; Alves, P.M.; Brito, C.; Sakharov, D.A.; Tonevitsky, A.G.; Lauster, R.; Marx, U. A multi-organ chip co-culture of neurospheres and liver equivalents for long-term substance testing. J. Biotechnol. 2015, 205, 36–46. [Google Scholar] [CrossRef] [PubMed]
- HE, A.; ML, S. Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr. Biol. Quant. Biosci. Nano Macro 2015, 7, 383. [Google Scholar]
- Vunjak-Novakovic, G.; Bhatia, S.; Chen, C.; Hirschi, K. HeLiVa platform: Integrated heart-liver-vascular systems for drug testing in human health and disease. Stem Cell Res. Ther. 2013, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tatosian, D.; Shuler, M. A Novel System for Evaluation of Drug Mixtures for Potential Efficacy in Treating Multidrug Resistant Cancers. Biotechnol. Bioeng. 2010, 103, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Yuki, I.; Kiichi, S.; Etsuro, Y. Micro total bioassay system for ingested substances: Assessment of intestinal absorption, hepatic metabolism, and bioactivity. Anal. Chem. 2010, 82, 9983–9988. [Google Scholar]
- Bersini, S.; Jeon, J.S.; Dubini, G.; Arrigoni, C.; Chung, S.; Charest, J.L.; Moretti, M.; Kamm, R.D. A Microfluidic 3D In Vitro Model for Specificity of Breast Cancer Metastasis to Bone. Biomaterials 2014, 35, 2454–2461. [Google Scholar] [CrossRef]
- Xu, Z.; Li, E.; Guo, Z.; Yu, R.; Hao, H.; Xu, Y.; Sun, Z.; Li, X.; Lyu, J.; Wang, Q. Design and Construction of a Multi-Organ Microfluidic Chip Mimicking the in vivo Microenvironment of Lung Cancer Metastasis. ACS Appl. Mater. Interfaces 2016, 8, 25840–25847. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Devarasetty, M.; Forsythe, S.; Atala, A.; Soker, S. A reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol. Bioeng. 2016, 113, 2020–2032. [Google Scholar] [CrossRef]
- Skardal, A.; Murphy, S.V.; Devarasetty, M. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep. 2017, 7, 8837. [Google Scholar] [CrossRef] [Green Version]
- Satoh, T.; Sugiura, S.; Shinji, K. A multi-throughput multi-organ-on-a-chip system on a plate formatted pneumatic pressure-driven medium circulation platform. Lab Chip 2018, 18, 115. [Google Scholar] [CrossRef]
- Hassell, B.A.; Goyal, G.; Lee, E. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Rep. 2017, 21, 508. [Google Scholar] [CrossRef]
- ML, S. Organ-, body- and disease-on-a-chip systems. Lab Chip 2017, 17, 2345–2346. [Google Scholar]
- Hajba, L.; Guttman, A. Continuous-flow-based microfluidic systems for therapeutic monoclonal antibody production and organ-on-a-chip drug testing. J. Flow Chem. 2017, 7, 118–123. [Google Scholar] [CrossRef]
- van de Stolpe, A.; Den, T.J. Workshop meeting report Organs-on-Chips: Human disease models. Lab Chip 2013, 13, 3449. [Google Scholar] [CrossRef] [PubMed]
- Casavant, B.P.; Strotman, L.N.; Tokar, J.J.; Thiede, S.M.; Traynor, A.M.; Ferguson, J.S.; Lang, J.M.; Beebe, D.J. Paired diagnostic and pharmacodynamic analysis of rare non-small cell lung cancer cells enabled by the VerIFAST platform. Lab Chip 2013, 14, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Allen, S.G.; Ingram, P.N.; Buckanovich, R.; Merajver, S.D.; Yoon, E. Single-cell Migration Chip for Chemotaxis-based Microfluidic Selection of Heterogeneous Cell Populations. Sci. Rep. 2015, 5, 9980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyunjae, L.; Woohyun, P.; Hyunryul, R.; Noo Li, J. A microfluidic platform for quantitative analysis of cancer angiogenesis and intravasation. Biomicrofluidics 2014, 8, 401–410. [Google Scholar]
- Fabrizio, M.; Giovanna, S.; Adele, D.N.; Valeria, L.; Paola, S.; Antonella, S.; Alessandra, F.; Massimo, S.; Massimo, S.; Annamaria, G. A multidisciplinary study using in vivo tumor models and microfluidic cell-on-chip approach to explore the cross-talk between cancer and immune cells. J. Immunotoxicol. 2014, 11, 337–346. [Google Scholar] [Green Version]
- Zhang, Y.; Zhou, L.; Qin, L. High-Throughput 3D Cell Invasion Chip Enables Accurate Cancer Metastatic Assays. J. Am. Chem. Soc. 2014, 136, 15257–15262. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Yue, W.; Yu, W.K.; Liu, D.; Fong, C.C.; Zhao, J.; Yang, M. Microfluidic Platform for Studying Chemotaxis of Adhesive Cells Revealed a Gradient-Dependent Migration and Acceleration of Cancer Stem Cells. Anal. Chem. 2015, 87, 7098–7108. [Google Scholar] [CrossRef] [PubMed]
- Riahi, R.; Yang, Y.L.; Kim, H.; Jiang, L.; Wong, P.K.; Zohar, Y. A microfluidic model for organ-specific extravasation of circulating tumor cells. Biomicrofluidics 2014, 8, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Pei, Y.; Xie, M.; Jin, Z.H.; Xiao, Y.S.; Wang, Y.; Zhang, L.N.; Li, Y.; Huang, W.H. An artificial blood vessel implanted three-dimensional microsystem for modeling transvascular migration of tumor cells. Lab Chip 2015, 15, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, A.R.; Águas, A.C.; Rainer, A.; Forte, G. Microfluidic Organ/Body-on-a-Chip Devices at the Convergence of Biology and Microengineering. Sensors 2015, 15, 31142–31170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skardal, A.; Shupe, T.; Atala, A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today 2016, 21, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.I.; Park, M.H.; Heo, S.C. Quantification and application of a liquid chromatography-tandem mass spectrometric method for the determination of WKYMVm peptide in rat using solid phase extraction. Biomed. Chromatogr. 2018, 32, e4107. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Tian, T. Recent advances in organ-on-a-chip: Biomarker analysis and applications. Anal. Methods 2018, 10, 1039. [Google Scholar]
- Arrigoni, C.; Gilardi, M.; Bersini, S.; Candrian, C.; Moretti, M. Bioprinting and Organ-on-Chip Applications Towards Personalized Medicine for Bone Diseases. Stem Cell Rev. Rep. 2017, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, M.A.; Kuo, C.J. Organoid modeling for cancer precision medicine. Genome Med. 2015, 7, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Ian, V.; Andrea, S.; Iaquinta, P.J.; Karthaus, W.R.; Anuradha, G.; Catherine, D.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187. [Google Scholar]

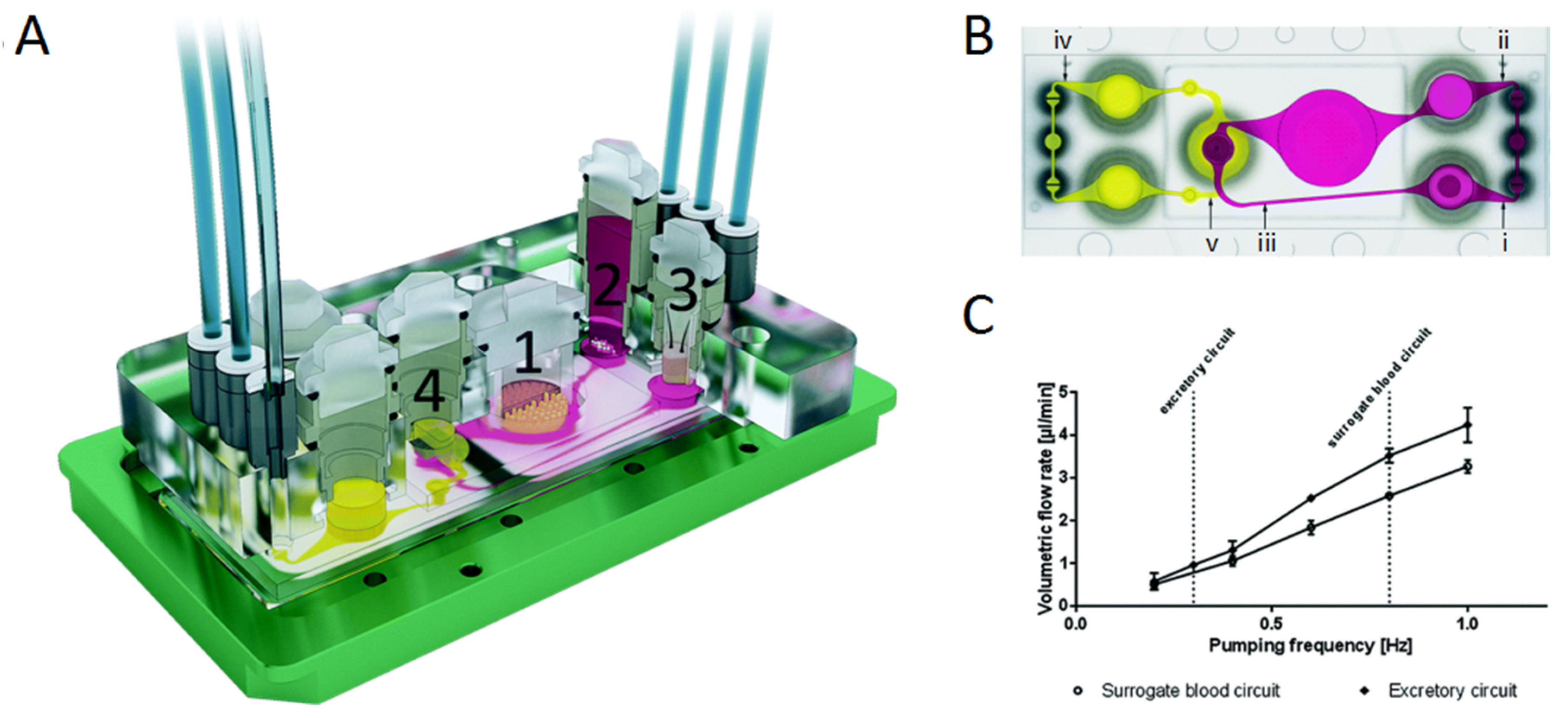
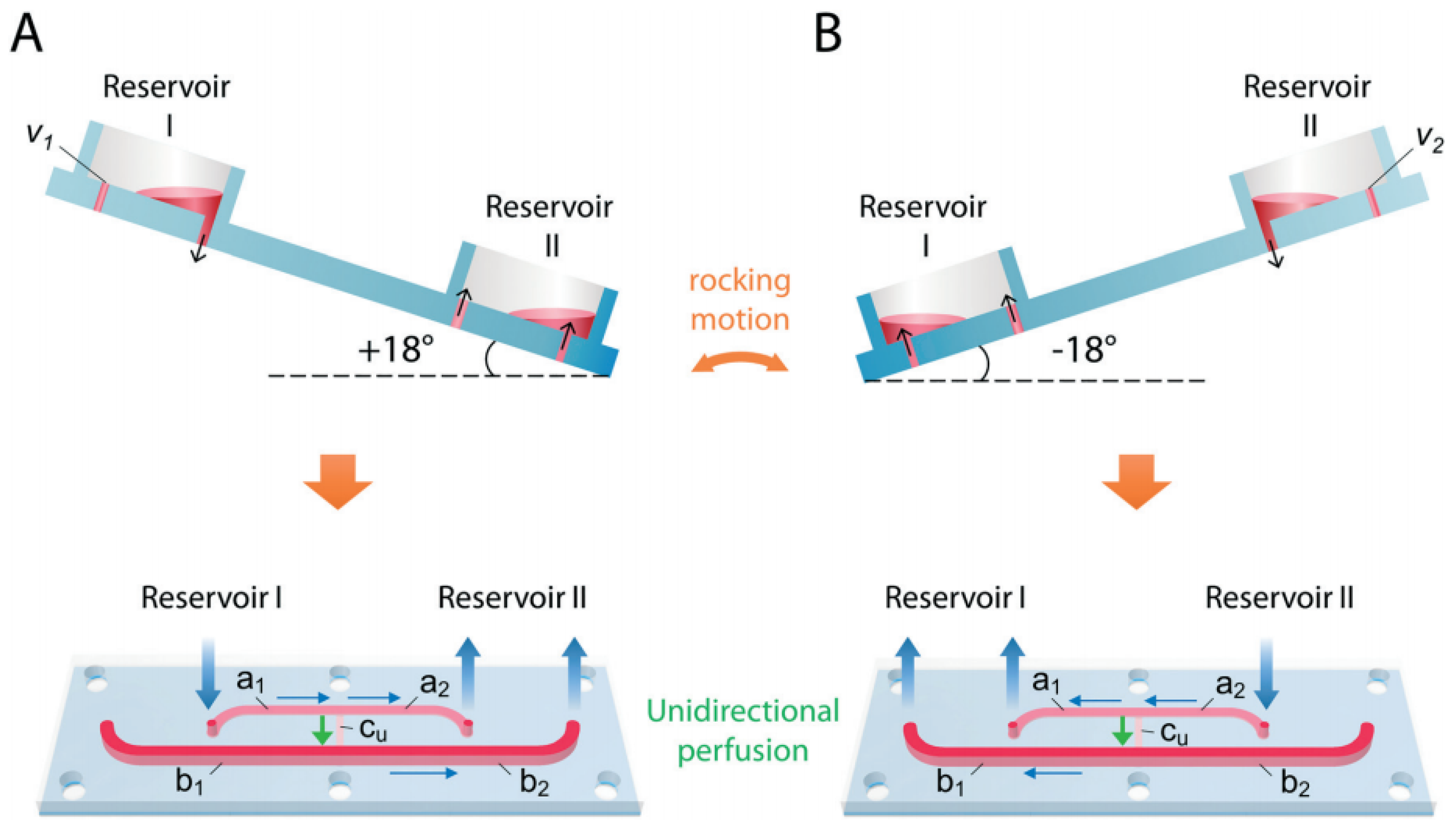
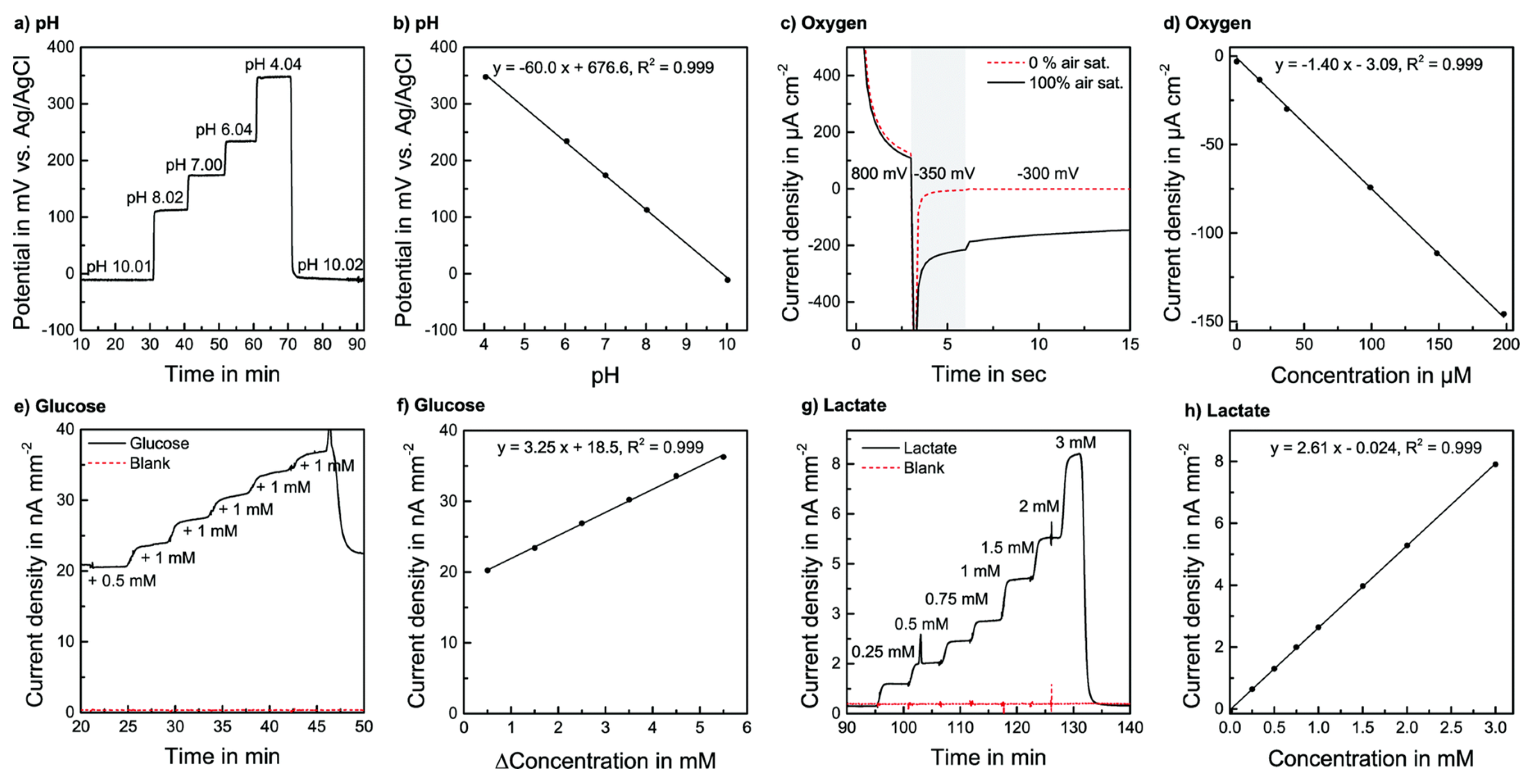
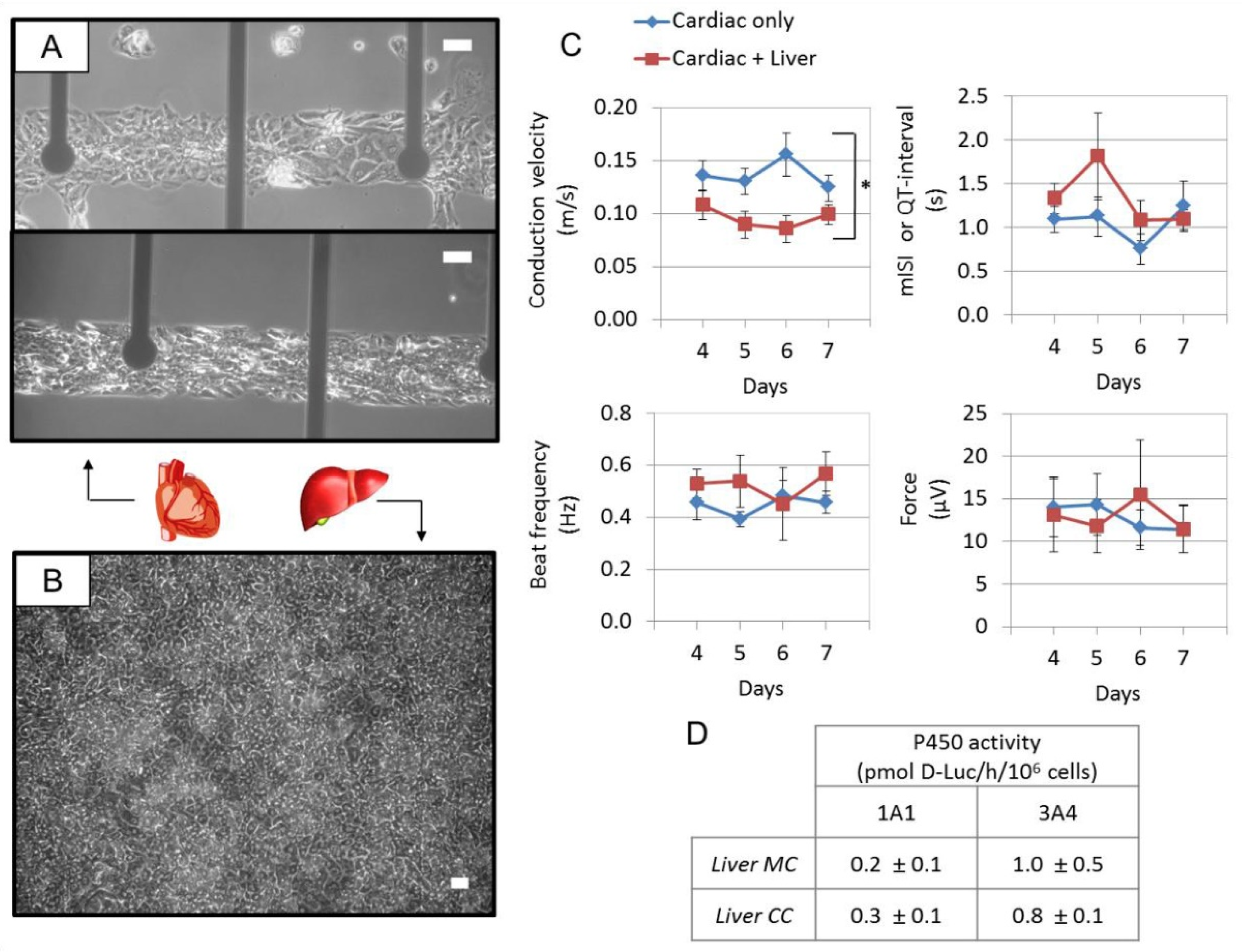
| Application | Multi-Organ/Tissue System | Fabrication Approach | Outcome | References |
|---|---|---|---|---|
| Drug testing/toxicology | Liver, tumor, and marrow | This model combined a three-compartment microscale cell culture analog (µCCA) device exposed to a pumpless gravity-induced flow with a mathematical pharmacokinetic and pharmacodynamic (PK-PD) model. | This model promoted the analysis and prediction of the effects of 5-fluorouracil (5-FU). | [128] |
| Liver, intestine, skin, and kidney | This model integrated two peristaltic on-chip micropumps and microfluidic channels connecting four tissue culture chambers for two microfluidic circuits into the four-organ-chip. | This model was helpful for repeated dose toxicity testing of drug candidates and further in vitro absorption, distribution, metabolism and elimination (ADME) observation. | [46] | |
| Liver, colorectal tissues | These models cultured spherical microtissues in parallel, connected by a microfluidic-channel network, with liquid flow controlled through a hanging-drop device. | These models were helpful for testing drug effects at different concentrations. | [129,130,131] | |
| Liver, nerve tissues | This model connecting two tissue compartments exposed by microfluidic channels was maintained in a combined media circuit. | This model showed the dose-dependent cytotoxicity result of the neurotoxic compound 2,5-hexanedione. | [132] | |
| Liver, heart | This model contained human-induced pluripotent stem cells (iPSCs)-derived liver and heart tissues, which were exposed to serum-free medium flow using a pumpless system. | This model was helpful for the prediction of the cardiotoxicity transformation of drugs through hepatic metabolism. | [120] | |
| Liver, skin tissues | This model used a single polydimethylsiloxane (PDMS) layer integrating the respectively arranged channels interconnecting the tissue counterparts, peristaltic on-chip micropumps, media reservoirs, and openings for culture compartments. | This model tested the liver toxicity of troglitazone at different molecular levels. | [12] | |
| Lung, gut, skin, vascular, liver, and kidney | This model, using physiologically-based pharmacokinetics with pharmacodynamic (PBPK/PD) models for estimating ADME parameters, was made of PDMS and microfluidic channels for connecting different organ compartments. | This model was helpful for PBPK/PD modeling and drug development in different stages. | [133] | |
| Disease modeling | Liver, heart, and vascular system | This model interconnected iPSCs-derived cardiomyocytes and hepatocytes by 3D-printed rigid filament networks of a carbohydrate glass with endothelial cells, and perfused the networks with high-pressure pulsatile blood flow. | This model was helpful for predictions of physiological responses in the diseased microenvironment. | [134] |
| Drug screening | Liver, heart, lung, and kidney | This model adopted allometric scaling for coupled non-linear organ-on-a-chip (OOC)/ multi-organ-on-chip (MOC) systems to create micro-organs maintained by a universal media. | This model was helpful for the screening of new drugs for efficacy and potential side-effects | [60] |
| Liver, marrow, megakaryoblast, and cancerous tissues | This model integrated a µCCA device into a silicon chip, on which four functional tissues were cultured in corresponding chambers connected by Pharmed tubing, with recirculating flow being provided by a peristaltic pump. | This model was helpful to predict the selectivity of chemotherapeutic/modulator mixtures for killing or reducing the growth of multidrug resistance (MDR) tumor cells in vivo. | [135] | |
| Liver, intestine, and breast carcinoma cells | This model containing microtissues of liver, intestine and the breast carcinoma cells cultured in the target components consisting of a slide and PDMS layers, having microchannels made by photolithography. | This model was helpful for the evaluation overall properties of orally ingested drugs, foods, and chemicals. | [136] | |
| Cancer metastasis | Marrow, mesenchymal stem cells, and breast cancer cells | This model bonded a bored PDMS layer to a cover glass to create microfluidic channels with oxygen plasma treatment, and provided eight cell-culture gel regions connected to the central media channel. | This model was helpful to mimic the dissemination of breast cancer cells into bone. | [137] |
| Brain, bone, liver, and lung carcinoma cells | This model combined three PDMS sheets and two thin PDMS microporous membranes to create three parallel microchannels connecting an upstream micro-lung and three downstream micro-organs. | This model was helpful for observing lung cancer cell behaviors in a physiologically relevant context. | [138] | |
| Intestine, liver, and colon carcinoma tissues | This model, comprising two independent cell-culture chambers connected by a circulating fluid flow, was fabricated with a hyaluronic acid-based hydrogel system in which the metastatic colon carcinoma tumor foci were created. | This model was helpful for studying the process of the migration of colon carcinoma cells. | [139] | |
| Biomarker detection | Heart, liver, and lung | This model comprised lung tissues based on the PDMS model and bioprinted spherical liver and heart organoids, which are connected via a central fluid channel with fluid flow driven by a peristaltic micropump. | This model was helpful to utilize enzyme-linked immunosorbent assays (ELISAs) to determine the effect of bleomycin to quantify the levels of interleukin-8 (IL-8) and interleukin-1β (IL-1β). | [140] |
| Liver, intestine, cancer, and connective cells | This model contained two culture chambers interconnected in each culture unit via microchannels with a medium driven by a sequential pneumatic pressure-control system. | This model was helpful for liquid chromatography coupled with a mass spectrometry (LC-MS) system, to measure the concentrations of capecitabine and 5-FU in the medium of the model. | [141] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Multi-Organs-on-Chips: Towards Long-Term Biomedical Investigations. Molecules 2019, 24, 675. https://doi.org/10.3390/molecules24040675
Zhao Y, Kankala RK, Wang S-B, Chen A-Z. Multi-Organs-on-Chips: Towards Long-Term Biomedical Investigations. Molecules. 2019; 24(4):675. https://doi.org/10.3390/molecules24040675
Chicago/Turabian StyleZhao, Yi, Ranjith Kumar Kankala, Shi-Bin Wang, and Ai-Zheng Chen. 2019. "Multi-Organs-on-Chips: Towards Long-Term Biomedical Investigations" Molecules 24, no. 4: 675. https://doi.org/10.3390/molecules24040675






