Controllable Preparation of SERS-Active Ag-FeS Substrates by a Cosputtering Technique
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Two-Dimensional (2D) Ordered PSCP Templates
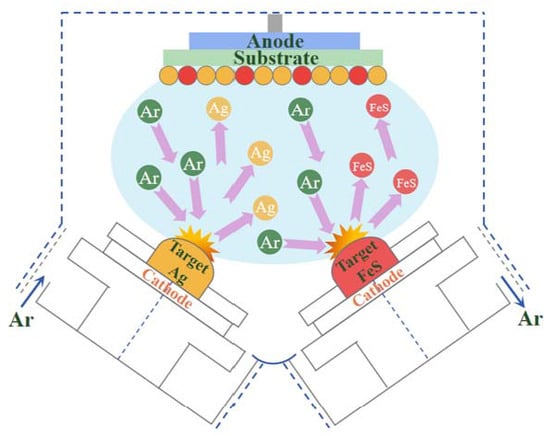
3.3. Fabrication of Ag/FeS Arrays on PSCP Templates
3.4. Characterization and SERS Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleischmann, M.; Hendra, P.J.; Hill, I.R.; Pemble, M.E. Enhanced Raman Spectra from Species Formed by the Coadsorption of Halide Ions and Water Molecules on Silver Electrodes. J. Electroanal. Chem. 1981, 117, 243–255. [Google Scholar] [CrossRef]
- Dai, Z.G.; Xiao, X.H.; Wu, W.; Zhang, Y.P.; Liao, L.; Guo, S.S.; Ying, J.J.; Shan, C.X.; Sun, M.T.; Jiang, C.Z. Plasmon-driven reaction controlled by the number of graphenelayers and localized surface plasmon distribution during optical excitation. Light Sci. Appl. 2015, 4, e342. [Google Scholar] [CrossRef]
- Rusnati, M.; Sala, D.; Orro, A.; Bugatti, A.; Trombetti, G.; Cichero, E.; Urbinati, C.; Di Somma, M.; Millo, E.; Galietta, L.J.V.; et al. Speeding Up the Identification of Cystic Fibrosis Transmembrane Conductance Regulator-Targeted Drugs: An Approach Based on Bioinformatics Strategies and Surface Plasmon Resonance. Molecules 2018, 23, 120. [Google Scholar] [CrossRef] [PubMed]
- Meuser, M.E.; Murphy, M.B.; Rashad, A.A.; Cocklin, S. Kinetic Characterization of Novel HIV-1 Entry Inhibitors: Discovery of a Relationship between Off-Rate and Potency. Molecules 2018, 23, 1940. [Google Scholar] [CrossRef]
- Schatz, G. Theoretical Studies of Surface Enhanced Raman Scattering. Acc. Chem. Res. 1984, 17, 370–376. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar] [CrossRef]
- Adrian, F. Charge transfer effects in surface-enhanced Raman scatteringa. J. Chem. Phys. 1982, 77, 5302–5314. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L.; Lu, T.; Xu, J. Charge-transfer theory of surface enhanced Raman spectroscopy: Herzberg-Teller contributions. J. Chem. Phys. 1986, 84, 4174–4180. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Mock, J.J.; Barbic, M.; Smith, D.A.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Mcfarland, A.D.; Duyne, R.P.V. Single Silver Nanoparticles as Real-Time Optical Sensors with Zeptomole Sensitivity. Nano Lett. 2003, 3, 1057–1062. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.; Li, D.; Shen, Z.; Xiong, Q.; Li, S.; Fan, H.J. Ordered array of gold semishells on TiO2 spheres: An ultrasensitive and recyclable SERS substrate. ACS Appl. Mater. Interfaces 2012, 4, 2180–2185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, L.; Xi, M.; Feng, Q.; Jiang, C.; Fong, H. Electrospun TiO2 nanofelt surface-decorated with Ag nanoparticles as sensitive and UV-cleanable substrate for surface enhanced Raman scattering. ACS Appl. Mater. Interfaces 2014, 6, 5759–5767. [Google Scholar] [CrossRef] [PubMed]
- Harraz, F.A.; Ismail, A.A.; Bouzid, H.; Al-Sayari, S.A.; Al-Hajry, A.; Al-Assiri, M.S. Surface-enhanced Raman scattering (SERS)-active substrates from silver plated-porous silicon for detection of crystal violet. Appl. Surf. Sci. 2015, 331, 241–247. [Google Scholar] [CrossRef]
- Cheng, C.; Yan, B.; Wong, S.M.; Li, X.; Zhou, W.; Yu, T.; Shen, Z.; Yu, H.; Fan, H.J. Fabrication and SERS performance of silver-nanoparticle-decorated Si/ZnO nanotrees in ordered arrays. ACS Appl. Mater. Interfaces 2010, 2, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, X.; Jia, X.; Li, G.; Wang, X.; Wang, G.; Li, Z.; Yang, L.; Zhao, B. Surface-Enhanced Raman Scattering from Synergistic Contribution of Metal and Semiconductor in TiO2/MBA/Ag(Au) and Ag(Au)/MBA/TiO2 Assemblies. J. Phys. Chem. C 2012, 116, 14650–14655. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Sui, H.; Zhang, X.; Su, H.; Cheng, W.; Han, X.X.; Zhao, B. Investigation of Charge Transfer in Ag/N719/TiO2 Interface by Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2016, 120, 13078–13086. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Z.; Ji, W.; Sui, H.; Cong, Q.; Wang, X.; Zhao, B. Charge-Transfer Effect on Surface-Enhanced Raman Scattering (SERS) in an Ordered Ag NPs/4-Mercaptobenzoic Acid/TiO2 System. J. Phys. Chem. C 2015, 119, 22439–22444. [Google Scholar] [CrossRef]
- Otto, A. The ‘chemical’ (electronic) contribution to surface-enhanced Raman scattering. J. Raman Spectrosc. 2005, 36, 497–509. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Cao, E.; Xu, X.; Liang, W.; Zhang, X. Surface Plasmon Resonance Sensors on Raman and Fluorescence Spectroscopy. Sensors 2017, 17, 2719. [Google Scholar] [CrossRef] [PubMed]
- Baffou, G.; Quidant, R. Nanoplasmonics for chemistry. Chem. Soc. Rev. 2014, 43, 3898–3907. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Chen, J.; Mcbride, J.R.; Lian, T. Efficient Hot-Electron Transfer by a Plasmon-Induced Interfacial Charge-Transfer Transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Freitas, I.C.D.; Geonmonond, R.S.; Aquino, C.B.D.; Pieretti, F.C.; Domingues, S.H.; Ando, R.A.; Camargo, P.H.C. Supports Matter: Unraveling the Role of Charge-Transfer over the Plasmonic Catalytic Activity of Silver Nanoparticles. J. Mater. Chem. A 2017, 5, 11720–11729. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, C.; Chen, L.; Zhang, Y.; Yang, J. Controllable Charge Transfer in Ag-TiO2 Composite Structure for SERS Application. Nanomaterials 2017, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, H.; Zhao, Y.; Gao, R.; Wang, Y.; Liu, Y.; Zhang, Y.; Hua, Z.; Yang, J. Iron layer-dependent surface-enhanced raman scattering of hierarchical nanocap arrays. Appl. Surf. Sci. 2017, 423, 1124–1133. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Gao, W.; Chen, L.; Chen, S.; Wei, M.; Gao, M.; Wang, C.; Zhang, Y.; Yang, J. Au/Ag bimetal nanogap arrays with tunable morphologies for surface-enhanced Raman scattering. RSC Adv. 2015, 5, 7454–7460. [Google Scholar] [CrossRef]
- Hou, B.H.; Wang, Y.Y.; Guo, J.Z.; Ning, Q.L.; Xi, X.T.; Pang, W.L.; Cao, A.M.; Wang, X.; Zhang, J.P.; Wu, X.L. Pseudocapacitance-boosted ultrafast Na storage in a pie-like FeS@C nanohybrid as an advanced anode material for sodium-ion full batteries. Nanoscale 2018, 10, 9218–9225. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Meng, G.; Huang, Q.; Xu, W.; Han, F.; Sun, K.; Xu, Q.; Wang, Z. Large-scale homogeneously distributed Ag-NPs with sub-10 nm gaps assembled on a two-layered honeycomb-like TiO2 film as sensitive and reproducible SERS substrates. Nanotechnology 2012, 23, 385705. [Google Scholar] [CrossRef]
- Quyen, T.T.B.; Su, W.N.; Chen, K.J.; Pan, C.J.; Rick, J.; Chang, C.C.; Hwang, B.J. Au@SiO2 core/shell nanoparticle assemblage used for highly sensitive SERS-based determination of glucose and uric acid. J. Raman Spectrosc. 2013, 44, 1671–1677. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Zhao, S.; Zhang, K.; Ma, F.; Liu, X.; Chen, X.; He, D. Microstructural evolution, mechanical properties and strengthening mechanism of TiN/Ni nanocomposite film. J. Alloys Compd. 2017, 691, 159–164. [Google Scholar] [CrossRef]
- Belov, D.S.; Blinkov, I.V.; Volkhonskii, A.O. The effect of Cu and Ni on the nanostructure and properties of arc-PVD coatings based on titanium nitride. Surf. Coat. Tech. 2014, 260, 186–197. [Google Scholar] [CrossRef]
- Regent, F.; Musil, J. Magnetron sputtered Cr-Ni-N and Ti-Mo-N films: Comparison of mechanical properties. Surf. Coat. Tech. 2001, 142–144, 146–151. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, S.; Mitra, R. Effect of Ar:N2 ratio on structure and properties of Ni-TiN nanocomposite thin films processed by reactive RF/DC magnetron sputtering. Surf. Coat. Tech. 2013, 228, 100–114. [Google Scholar] [CrossRef]
- Ganeev, R.; Ryasnyanskiy, A.I.; Stepanov, A.L.; Usmanov, T. Saturated absorption and nonlinear refraction of silicate glasses doped with silver nanoparticles at 532 nm. Opt. Quantum Electron 2004, 36, 949–960. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, Y.; Huang, L.; Li, L.H.; Liu, G.; Zhang, X.; Lyu, X.; Cai, W.; Li, Y. Periodic Porous Alloyed Au-Ag Nanosphere Arrays and Their Highly SensitiveSERS Performance with Good Reproducibility and High Density of Hotspots. ACS Appl. Mater. Interfaces 2018, 10, 9792–9801. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.N.; Devnani, H.; Jha, S.; Ingole, P.P. Fermi Level Equilibration of Ag and Au Plasmonic Metal Nanoparticles supported on Graphene Oxide. Phys. Chem. Chem. Phys. 2018, 20, 26719–26733. [Google Scholar] [CrossRef] [PubMed]
- Ghodselahi, T.; Neishaboorynejad, T.; Arsalani, S. Fabrication LSPR sensor chip of Ag NPs and their biosensor application based on inter-particle coupling. Appl. Surf. Sci. 2015, 343, 194–201. [Google Scholar] [CrossRef]
- Li, D.; Sun, Y.; Chen, S.; Yao, J.; Zhang, Y.; Xia, Y.; Yang, D. Highly Porous FeS/Carbon Fibers Derived from Fe-Carrageenan Biomass: High-capacity and Durable Anodes for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 17175–17182. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Xia, X.; Wang, L.; Dong, W. Fe1-xZnxS ternary solid solution as an efficient Fenton-like catalyst for ultrafast degradation of phenol. J. Hazard. Mater. 2018, 353, 393–400. [Google Scholar] [CrossRef]
- Naujok, R.R.; Duevel, R.V.; Corn, R.M. Fluorescence and Fourier Transform Surface-Enhanced Raman Scattering Measurements of Methylene Blue Adsorbed onto a Sulfur-Modified Gold Electrode. Langmuir 1993, 9, 1771–1774. [Google Scholar] [CrossRef]
- Ruan, C.; Wang, W.; Gu, B. Single-molecule detection of thionine on aggregated gold nanoparticles by surface enhanced Raman scattering. J. Raman Spectrosc. 2007, 38, 568–573. [Google Scholar] [CrossRef]
- Hulchineon, K.; Hester, R.E.; Albery, W.J.; Hillman, A.R. Raman Spectroscopic Studies of a Thionine-modified Electrode. J. Chem. Soc. Faraday Trans. 1984, 80, 2053–2071. [Google Scholar] [CrossRef]
- Niu, Z.; Fang, Y. Surface-enhanced Raman scattering system of sample molecules in silver-modified silver film. Spectrochim. Acta A 2007, 66, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Khorasaninejad, M.; Walia, J.; Saini, S.S. Enhanced first-order Raman scattering from arrays of vertical silicon nanowires. Nanotechnology. 2012, 23, 275706. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.Q.; Ren, B.; Wu, D.Y. Surface-Enhanced Raman Scattering: From Noble to Transition Metals and from Rough Surfaces to Ordered Nanostructures. J. Phys. Chem. B 2002, 106, 9463–9483. [Google Scholar] [CrossRef]
- Green, M.; Liu, F.M. SERS Substrates Fabricated by Island Lithography: The Silver/Pyridine System. J. Phys. Chem. B 2003, 107, 13015–13021. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| MB Raman Spectra | Excitation Wavelength (514 nm) | Band Assignments |
|---|---|---|
| 445 | 449 | δ (C–N–C) |
| 497 | δ (C–N–C) | |
| 677 | 671 | ν (C–H) |
| 768 | 772 | |
| 883 | 862 | |
| 1038 | β (C–H) | |
| 1067 | 1075 | |
| 1181 | ν (C–N) | |
| 1272 | ||
| 1331 | 1365 | |
| 1396 | 1399 | α (C–H) |
| 1441 | 1437 | νasym (C–N) |
| 1544 | νasym (C–C) | |
| 1594 | ν (C–C) ring | |
| 1618 | 1627 | ν (C–C) ring |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, N.; Zhang, X.-Y.; Fan, W.; Han, B.; Jin, S.; Park, Y.; Chen, L.; Zhang, Y.; Liu, Y.; Yang, J.; et al. Controllable Preparation of SERS-Active Ag-FeS Substrates by a Cosputtering Technique. Molecules 2019, 24, 551. https://doi.org/10.3390/molecules24030551
Ma N, Zhang X-Y, Fan W, Han B, Jin S, Park Y, Chen L, Zhang Y, Liu Y, Yang J, et al. Controllable Preparation of SERS-Active Ag-FeS Substrates by a Cosputtering Technique. Molecules. 2019; 24(3):551. https://doi.org/10.3390/molecules24030551
Chicago/Turabian StyleMa, Ning, Xin-Yuan Zhang, Wenyue Fan, Bingbing Han, Sila Jin, Yeonju Park, Lei Chen, Yongjun Zhang, Yang Liu, Jinghai Yang, and et al. 2019. "Controllable Preparation of SERS-Active Ag-FeS Substrates by a Cosputtering Technique" Molecules 24, no. 3: 551. https://doi.org/10.3390/molecules24030551