Metabolomics Profiling Reveals Rehmanniae Radix Preparata Extract Protects against Glucocorticoid-Induced Osteoporosis Mainly via Intervening Steroid Hormone Biosynthesis
Abstract
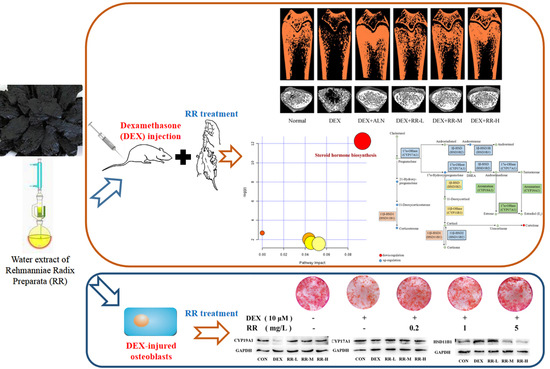
:1. Introduction
2. Results
2.1. UHPLC-MS Analysis of RR Extract
2.2. RR Improves the Micro-Architecture and BMD of the Femur in DEX-Treated Rats
2.3. RR Regulates Bone Metabolism-Related Biochemical Markers in DEX-Treated Rats
2.4. RR Enhances the Proliferation, Differentiation, and Mineralization Levels of Osteoblasts Injured by DEX
2.5. Metabolomics Analysis
2.6. Identification of Potential Biomarkers in DEX-treated Rats
2.7. RR Reverses Metabolic Dysregulation in DEX-Treated Rats
2.8. RR Regulates the Expressions of Key Proteins in Steroid Hormone Biosynthesis
3. Discussion
4. Methods and Materials
4.1. Chemicals and Reagents
4.2. Preparation of RR Extract
4.3. UHPLC-MS Analysis of RR Extract
4.4. Animal Treatment and Samples Collection
4.5. Micro-CT Analysis and Biochemical Marker Measurement
4.6. UHPLC-Q-TOF/MS Metabolomics Analysis
4.7. Data Processing and Multivariate Analysis
4.8. Osteoblasts Culture
4.9. Osteoblastic MTT and ALP Activity Assay
4.10. Osteoblastic Bone Matrix Mineralization Assay
4.11. Western Blotting
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Seibel, M.J.; Cooper, M.S.; Zhou, H. Glucocorticoid-induced osteoporosis: Mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol. 2013, 1, 59–70. [Google Scholar] [CrossRef]
- Yang, Y.; Nian, H.; Tang, X.; Wang, X.; Liu, R. Effects of the combined Herba Epimedii and Fructus Ligustri Lucidi on bone turnover and TGF-β1/Smads pathway in GIOP rats. J. Ethnopharmacol. 2017, 201, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.; Shao, X.; Dunstan, C.R.; Seibel, M.J.; Zhou, H. Biphasic Glucocorticoid-dependent regulation of Wnt expression and its inhibitors in mature osteoblastic cells. Calcif. Tissue Int. 2009, 85, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, B. Adverse effects of bisphosphonates. Calcif. Tissue Int. 2010, 86, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, S.; Marcheselli, R.; Sacchi, S.; Baldini, L.; Angrilli, F.; Pennese, E.; Quarta, G.; Stelitano, C.; Caparotti, G.; Luminari, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: A review of 35 cases and an evaluation of its frequency in multiple myeloma patients. Leuk. Lymphoma 2007, 48, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, R.; Wang, L.; Zhu, R.; Liu, H.; Guo, Y.; Zhao, B.; Zhao, S.; Tang, J.; Li, Y.; et al. Rehmanniae Radix in osteoporosis: A review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J. Ethnopharmacol. 2017, 198, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.O.; Kim, S.W.; Kim, J.Y.; Ko, S.Y.; Kim, H.M.; Baek, J.H.; Ryoo, H.M.; Kim, J.K. Effect of Rehmannia glutinosa Libosch extracts on bone metabolism. Clin. Chim. Acta 2003, 334, 185–195. [Google Scholar] [CrossRef]
- Xia, B.; Xu, B.; Sun, Y.; Xiao, L.; Pan, J.; Jin, H.; Tong, P. The effects of Liuwei Dihuang on canonical Wnt/β-catenin signaling pathway in osteoporosis. J. Ethnopharmacol. 2014, 153, 133–141. [Google Scholar] [CrossRef]
- Ge, J.; Xie, L.; Chen, J.; Li, S.; Xu, H.; Lai, Y.; Qiu, L.; Ni, C. Liuwei Dihuang Pill treats postmenopausal osteoporosis with Shen (Kidney) yin deficiency via Janus kinase/signal transducer and activator of transcription signal pathway by up-regulating cardiotrophin-like cytokine factor 1 expression. Chin. J. Integr. Med. 2016, 24, 1–8. [Google Scholar]
- Gao, Z. ER, PR are up-regulated by rehmannia in aging female mice. J. Shanxi Coll. Tradit. Chin. Med. 2000, 1, 1–3. [Google Scholar]
- Chao, J.; Huo, T.I.; Cheng, H.Y.; Tsai, J.C.; Liao, J.W.; Lee, M.S.; Qin, X.M.; Hsieh, M.T.; Pao, L.H.; Peng, W.H. Gallic acid ameliorated impaired glucose and lipid homeostasis in high fat diet-induced NAFLD mice. PLoS ONE 2014, 9, e96969. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S. Glucocorticoid-Induced Osteoporosis and Osteonecrosis. Endocrinol. Metab. Clin. N. Am. 2012, 41, 595–611. [Google Scholar] [CrossRef] [Green Version]
- Vimalraj, S.; Arumugam, B.; Miranda, P.J.; Selvamurugan, N. Runx2: Structure, function, and phosphorylation in osteoblast differentiation. Int. J. Biol. Macromol. 2015, 78, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Azuma, Y.; Fukuyama, R.; Hattori, Y.; Yoshida, C.; Koida, M.; Ogita, K.; Komori, T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J. Cell Biol. 2004, 166, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yodthong, T.; Kedjarune-Leggat, U.; Smythe, C.; Wititsuwannakul, R.; Pitakpornpreecha, T. l-Quebrachitol Promotes the Proliferation, Differentiation, and Mineralization of MC3T3-E1 Cells: Involvement of the BMP-2/Runx2/MAPK/Wnt/β-Catenin Signaling Pathway. Molecules 2018, 23, 3086. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2017, 8, a031211. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, Y.; Honda, A. Dehydroepiandrosterone and its derivatives: Potentially novel anti-proliferative and chemopreventive agents. Curr. Pharm. Des. 2006, 12, 3411–3421. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Androgen biosynthesis from cholesterol to DHEA. Mol. Cell Endocrinol. 2002, 198, 7–14. [Google Scholar] [CrossRef]
- Marx, C.E.; Bradford, D.W.; Hamer, R.M.; Naylor, J.C.; Allen, T.B.; Lieberman, J.A.; Strauss, J.L.; Kilts, J.D. Pregnenolone as a novel therapeutic candidate in schizophrenia: Emerging preclinical and clinical evidence. Neuroscience 2011, 191, 78–90. [Google Scholar] [CrossRef]
- Maurya, S.W.; Dev, K.; Singh, K.B.; Rai, R.; Siddiqui, I.R.; Singh, D.; Maurya, R. Synthesis and biological evaluation of heterocyclic analogues of pregnenolone as novel anti-osteoporotic agents. Bioorg. Med. Chem. Lett. 2017, 27, 1390–1396. [Google Scholar] [CrossRef]
- Yantsevich, A.V.; Dichenko, Y.V.; Mackenzie, F.; Mukha, D.V.; Baranovsky, A.V.; Gilep, A.A.; Usanov, S.A.; Strushkevich, N.V. Human steroid and oxysterol 7α-hydroxylase CYP7B1: Substrate specificity, azole binding and misfolding of clinically relevant mutants. FEBS J. 2014, 281, 1700–1713. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Asri, S.F.; Mohd Ramli, E.S.; Soelaiman, I.N.; Mat Noh, M.A.; Abdul Rashid, A.H.; Suhaimi, F. Piper sarmentosum Effects on 11β-Hydroxysteroid Dehydrogenase Type 1 Enzyme in Serum and Bone in Rat Model of Glucocorticoid-Induced Osteoporosis. Molecules 2016, 21, 1523. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Odermatt, A. Readjusting the glucocorticoid balance: An opportunity for modulators of 11beta-hydroxysteroid dehydrogenase type 1 activity? Endocr. Metab. Immune Disord. Drug Targets 2007, 7, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Eijken, M.; Hewison, M.; Cooper, M.S.; de Jong, F.H.; Chiba, H.; Stewart, P.M.; Uitterlinden, A.G.; Pols, H.A.P.; van Leeuwen, J.P.T.M. 11β-Hydroxysteroid Dehydrogenase Expression and Glucocorticoid Synthesis Are Directed by a Molecular Switch during Osteoblast Differentiation. Mol. Endocrinol. 2005, 19, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulun, S.E. Aromatase and estrogen receptor α deficiency. Fertil. Steril. 2014, 101, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, J.A.; Manolagas, S.C. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone 2015, 80, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somner, J.; McLellan, S.; Cheung, J.; Mak, Y.T.; Frost, M.L.; Knapp, K.M.; Wierzbicki, A.S.; Wheeler, M.; Fogelman, I.; Ralston, S.H.; et al. Polymorphisms in the P450 c17 (17-Hydroxylase/17,20-Lyase) and P450 c19 (Aromatase) Genes: Association with Serum Sex Steroid Concentrations and Bone Mineral Density in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2004, 89, 344–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, G.; Hentunen, T.A.; Nars, M.; Härkönen, P.L.; Väänänen, H.K. Estrogen protects primary osteocytes against glucocorticoid-induced apoptosis. Apoptosis 2005, 10, 583–595. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds catalpol, echinacoside, and acteoside are available from the authors. |







| NO. | Metabolite Identification | Rt (min) | m/z | Adduct | VIP | Fold Change Value | P-Value | Related Pathway | HMDB/ MELIN ID | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DEX/Control | DEX+RR/DEX | DEX/Control | DEX+RR/DEX | ||||||||
| 1 | Glycine | 3.818 | 76.0395 | [M+H]+ | 1.78 | 0.491 | 1.280 | 0.025 * | 0.845 | Amino acid metabolism | HMDB0000123 |
| 2 | Benzoic acid | 2.449 | 140.0701 | [M+NH4]+ | 1.23 | 2.219 | 0.496 | 0.006 ** | 0.013 * | Amino acid metabolism | HMDB0001870 |
| 3 | N-Acetylproline | 2.450 | 158.0807 | [M+H]+ | 2.29 | 2.155 | 0.492 | 0.008 ** | 0.012 * | Amino acid metabolism | HMDB0094701 |
| 4 | Naphthol | 0.842 | 162.0926 | [M+NH4]+ | 2.20 | 2.192 | 0.574 | 0.047 * | 0.158 | Metabolism of xenobiotics by cytochrome P450 | HMDB0012138/ HMDB0012322 |
| 5 | Indoleacetic acid | 3.823 | 198.0513 | [M+Na]+ | 1.30 | 0.467 | 1.280 | 0.013 * | 0.848 | Amino acid metabolism | HMDB0000197 |
| 6 | 4-Pyridoxic acid | 1.156 | 201.0860 | [M+H]+ | 1.05 | 0.373 | 2.167 | 0.000 ** | 0.014 * | Vitamin B6 metabolism | HMDB0000017 |
| 7 | N-Hydroxy-L-tyrosine | 1.563 | 215.1006 | [M+NH4]+ | 2.08 | 0.307 | 1.869 | 0.000 ** | 0.312 | Amino acid metabolism | HMDB0038750 |
| 8 | N-alpha-Acetylcitrulline | 1.154 | 218.1137 | [M+H]+ | 1.21 | 0.396 | 1.964 | 0.001 ** | 0.057 | Amino acid metabolism | HMDB0000856 |
| 9 | 3-Oxododecanoic acid | 6.690 | 232.1898 | [M+NH4]+ | 1.24 | 2.714 | 0.720 | 0.010 * | 0.442 | Fatty acid biosynthesis | HMDB0010727 |
| 10 | Valerylcarnitine/Isovalerylcarnitine | 5.306 | 246.1698 | [M+H]+ | 1.15 | 2.136 | 0.725 | 0.042 * | 0.479 | Fatty acid biosynthesis | HMDB0013128/HMDB0000688 |
| 11 | N-Phenylacetylaspartic acid | 4.080 | 252.0842 | [M+H]+ | 1.53 | 2.064 | 0.551 | 0.007 ** | 0.022 * | Amino acid metabolism | HMDB0029355 |
| 12 | Palmitic acid/Isopalmitic acid | 9.939 | 274.2743 | [M+NH4]+ | 5.52 | 0.741 | 1.156 | 0.013 * | 0.463 | Fatty acid biosynthesis | HMDB0000220/HMDB0031068 |
| 13 | Androsterone/Epiandrosterone | 8.665 | 313.2156 | [M+Na]+ | 1.61 | 3.719 | 0.592 | 0.001 ** | 0.048 * | Steroid hormone biosynthesis | HMDB0000031/ HMDB0000365 |
| 14 | Hydroxypregnenolone | 10.545 | 333.2407 | [M+H]+ | 1.01 | 0.414 | 1.438 | 0.029 * | 0.795 | Steroid hormone biosynthesis | — |
| 15 | Galactosylhydroxylysine | 6.575 | 342.1904 | [M+NH4]+ | 1.01 | 6.878 | 0.867 | 0.006 ** | 0.942 | Energy metabolism | HMDB0000600 |
| 16 | 11-Dehydrocorticosterone | 9.011 | 345.2061 | [M+H]+ | 1.90 | 0.325 | 3.389 | 0.003 ** | 0.001 ** | Steroid hormone biosynthesis | HMDB0004029 |
| 17 | Corticosterone | 9.537 | 347.2219 | [M+H]+ | 2.53 | 0.289 | 4.163 | 0.128 | 0.035 * | Steroid hormone biosynthesis | HMDB0001547 |
| 18 | Arachidonic acid/Arachidonate | 10.055 | 349.2378 | [M+FA-H]- | 1.87 | 0.306 | 0.853 | 0.014 * | 0.989 | Arachidonic acid metabolism | HMDB0001043/HMDB0060102 |
| 19 | Docosapentaenoic acid | 9.795 | 353.2475 | [M+Na]+ | 3.45 | 3.137 | 0.413 | 0.032 * | 0.077 | Fatty acid biosynthesis | HMDB0006528/ HMDB0001976 |
| 20 | MG(0:0/16:0/0:0) | 13.949 | 353.2667 | [M+Na]+ | 2.57 | 0.670 | 0.964 | 0.010 * | 0.991 | Fatty acid biosynthesis | — |
| 21 | 18-Hydroxycorticosterone | 9.008 | 361.2016 | [M-H]- | 1.59 | 0.215 | 4.840 | 0.001 ** | 0.000 ** | Steroid hormone biosynthesis | HMDB0000319 |
| 22 | HETE | 8.666 | 365.2332 | [M+FA-H]- | 3.04 | 3.978 | 0.503 | 0.000 ** | 0.110 | Arachidonic acid metabolism | — |
| 23 | Cortolone | 8.667 | 367.2478 | [M+H]+ | 1.40 | 4.327 | 0.553 | 0.000 ** | 0.042 * | Steroid hormone biosynthesis | HMDB0003128 |
| 24 | Carbocyclic Thromboxane A2 | 9.793 | 371.2576 | [M+Na]+ | 4.34 | 2.766 | 0.414 | 0.048 * | 0.078 | Arachidonic acid metabolism | METLIN-45632 |
| 25 | α,α-Dimethyl anandamide | 13.286 | 376.3186 | [M+Na]+ | 1.34 | 1.694 | 1.017 | 0.026 * | 0.999 | Retrograde endocannabinoid signaling | METLIN-36748 |
| 26 | LysoPA(i-14:0/0:0) | 8.665 | 405.2038 | [M+Na]+ | 2.41 | 7.529 | 0.452 | 0.000 ** | 0.027 * | Lysophosphatidic metabolism | HMDB0114765 |
| 27 | O-Arachidonoyl Glycidol | 9.789 | 405.2644 | [M+FA-H]- | 3.23 | 3.122 | 0.286 | 0.035 * | 0.120 | Retrograde endocannabinoid signaling | METLIN-44872 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, T.; Dong, X.; Jiang, Y.; Lin, L.; Dong, Z.; Shen, Y.; Xin, H.; Zhang, Q.; Qin, L. Metabolomics Profiling Reveals Rehmanniae Radix Preparata Extract Protects against Glucocorticoid-Induced Osteoporosis Mainly via Intervening Steroid Hormone Biosynthesis. Molecules 2019, 24, 253. https://doi.org/10.3390/molecules24020253
Xia T, Dong X, Jiang Y, Lin L, Dong Z, Shen Y, Xin H, Zhang Q, Qin L. Metabolomics Profiling Reveals Rehmanniae Radix Preparata Extract Protects against Glucocorticoid-Induced Osteoporosis Mainly via Intervening Steroid Hormone Biosynthesis. Molecules. 2019; 24(2):253. https://doi.org/10.3390/molecules24020253
Chicago/Turabian StyleXia, Tianshuang, Xin Dong, Yiping Jiang, Liuyue Lin, Zhimin Dong, Yi Shen, Hailiang Xin, Qiaoyan Zhang, and Luping Qin. 2019. "Metabolomics Profiling Reveals Rehmanniae Radix Preparata Extract Protects against Glucocorticoid-Induced Osteoporosis Mainly via Intervening Steroid Hormone Biosynthesis" Molecules 24, no. 2: 253. https://doi.org/10.3390/molecules24020253