Pressurized Hot Water Extraction and Capillary Electrophoresis for Green and Fast Analysis of Useful Metabolites in Plants
Abstract
:1. Introduction
2. Results and Discussion
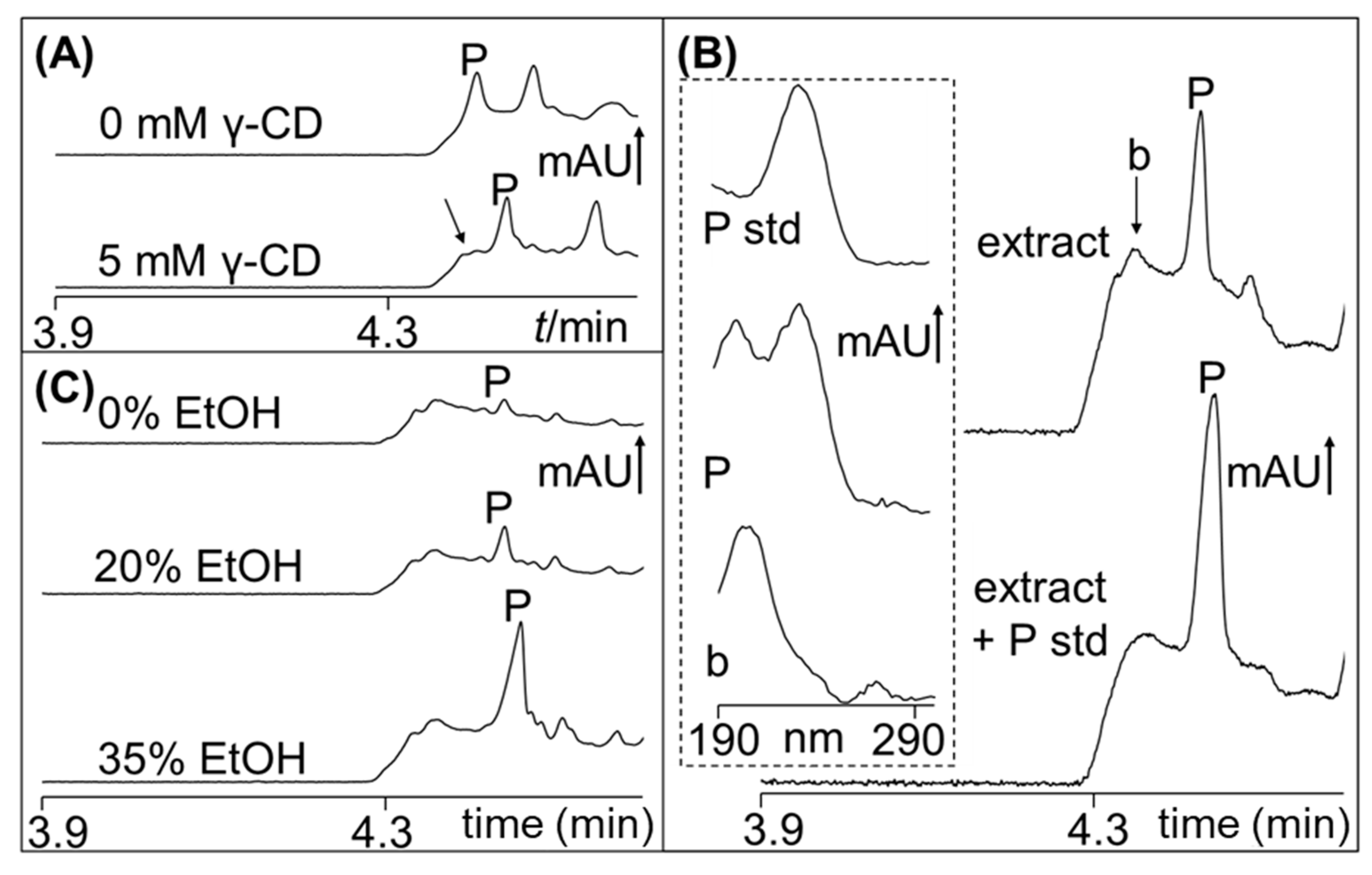
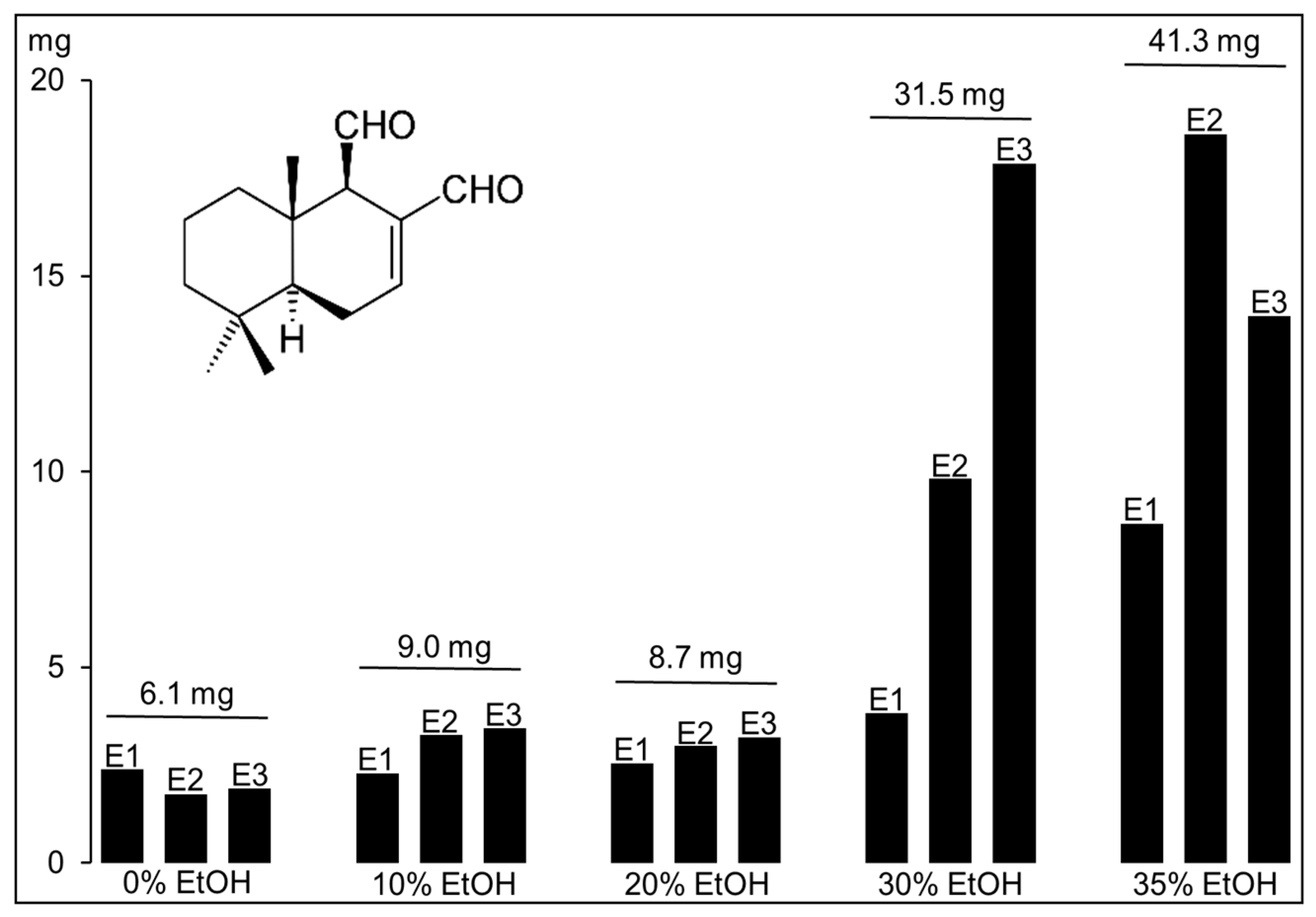
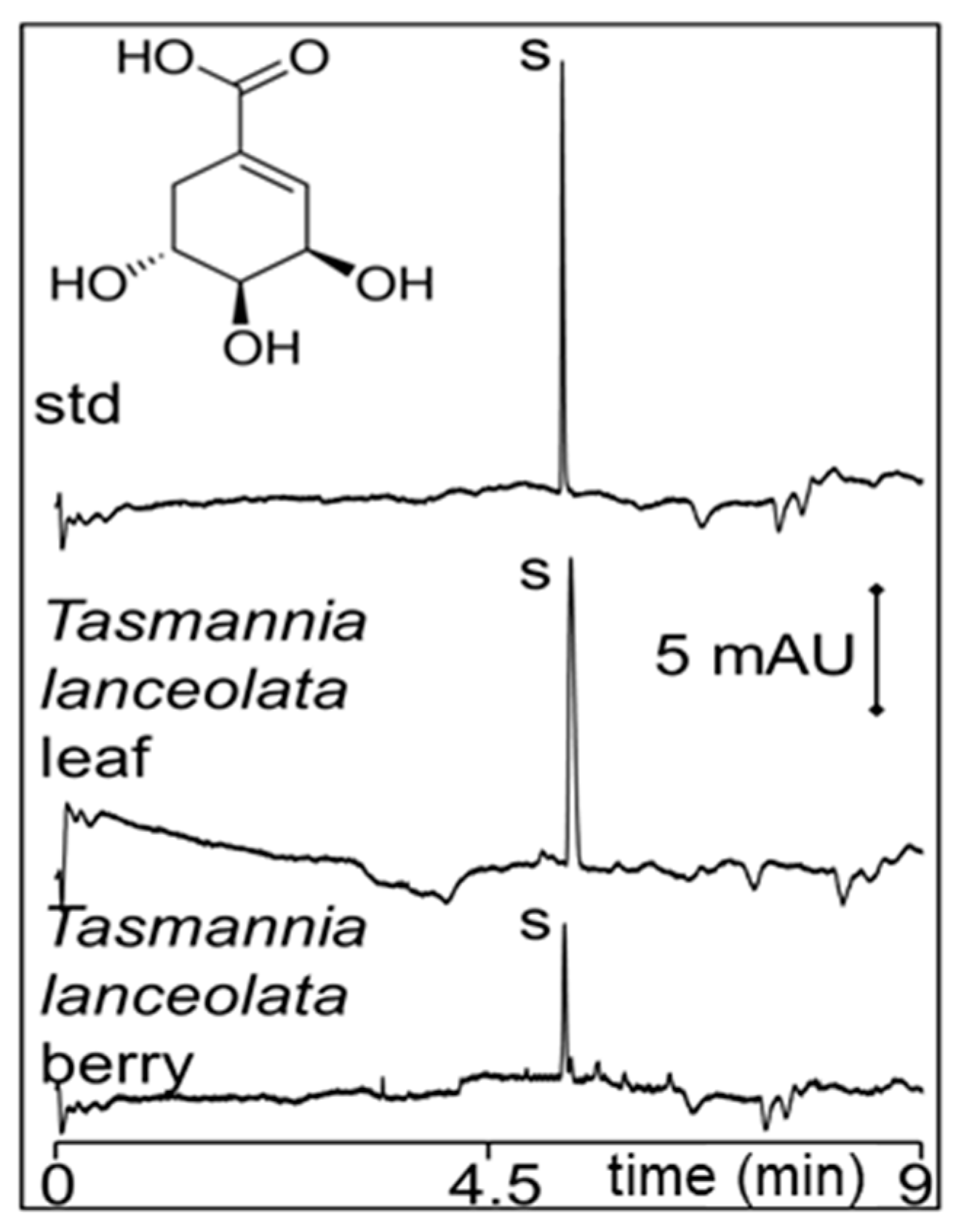
2.1. Polygodial from Tasmannia Lanceolata Leaf
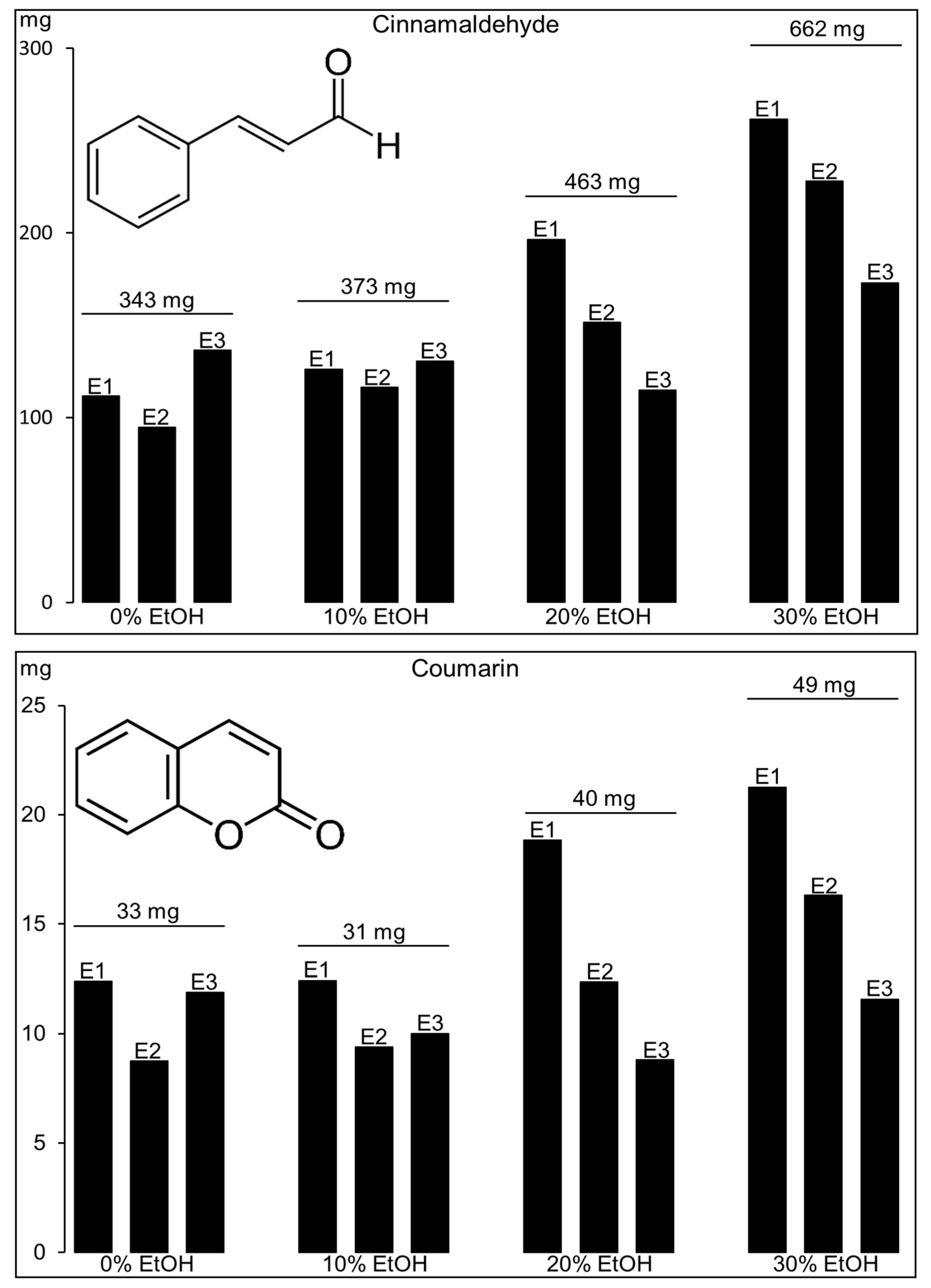
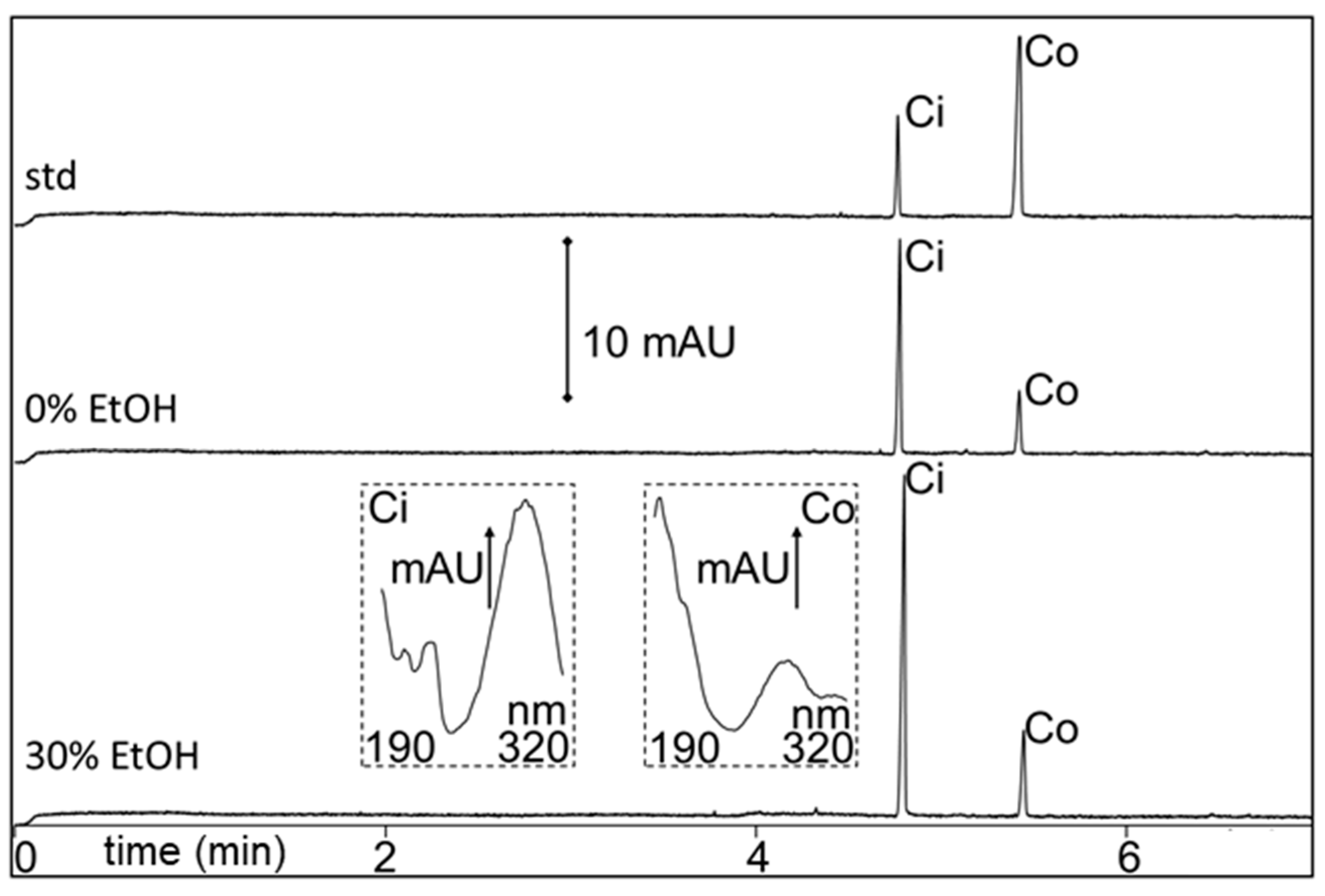
2.2. Cinnamaldehyde and Coumarin from Cinnamomum cassia
2.3. Shikimic Acid from Illicium verum and Other Plants
3. Materials and Methods
3.1. Reagents, Standards, and Solutions
3.2. Plant Materials
3.3. Espresso Machine-Based PHWE
3.4. CE Procedures
3.5. CE Methods Selection/Optimization
3.6. Sample Preparation after PHWE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deans, B.J.; Just, J.; Chhetri, J.; Burt, L.K.; Smith, J.N.; Kilah, N.L.; de Salas, M.; Gueven, N.; Bissember, A.C.; Smith, J.A. Pressurized Hot Water Extraction as a Viable Bioprospecting Tool: Isolation of Coumarin Natural Products from Previously Unexamined Correa (Rutaceae) Species. Chem. Select. 2017, 2, 2439–2443. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Liang, H.; Zhang, Q.; Li, Q. Optimization of ionic liquid based ultrasonic assisted extraction of antioxidant compounds from Curcuma longa L. using response surface methodology. Ind. Crop. Prod. 2015, 76, 487–493. [Google Scholar] [CrossRef]
- Bharate, S.S.; Mignani, S.; Vishwakarma, R.A. Why Are the Majority of Active Compounds in the CNS Domain Natural Products? A Critical Analysis. J. Med. Chem. 2018, 61, 10345–10374. [Google Scholar] [CrossRef] [PubMed]
- Nadia, J.; Shahbaz, K.; Ismail, M.; Farid, M.M. Approach for Polygodial Extraction from Pseudowintera colorata (Horopito) Leaves Using Deep Eutectic Solvents. Acs Sustain. Chem. Eng. 2017. [Google Scholar] [CrossRef]
- Just, J.; Jordan, T.B.; Paull, B.; Bissember, A.C.; Smith, J.A. Practical isolation of polygodial from Tasmannia lanceolata: A viable scaffold for synthesis. Org. Biomol. Chem. 2015, 13, 11200–11207. [Google Scholar] [CrossRef]
- Usuki, T.; Yasuda, N.; Yoshizawa-Fujita, M.; Rikukawa, M. Extraction and isolation of shikimic acid from Ginkgo biloba leaves utilizing an ionic liquid that dissolves cellulose. Chem. Commun. 2011, 47, 10560–10562. [Google Scholar] [CrossRef]
- Davison, E.K.; Sperry, J. Natural Products with Heteroatom-Rich Ring Systems. J. Nat. Prod. 2017, 80, 3060–3079. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Martin-Pastor, M. Experiments for the editing of singlet peaks and simplification of 1H NMR spectra of complex mixtures. J. Agric. Food Chem. 2014, 62, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; D’Abrosca, B.; Scognamiglio, M.; D’Angelo, G.; Gallicchio, M.; Galasso, S.; Monaco, P.; Fiorentino, A. NMR-based metabolic profiling and in vitro antioxidant and hepatotoxic assessment of partially purified fractions from Golden germander (Teucrium polium L.) methanolic extract. Food Chem. 2012, 135, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Vučković, I.; Rapinoja, M.L.; Vaismaa, M.; Vanninen, P.; Koskela, H. Application of comprehensive NMR-based analysis strategy in annotation, isolation and structure elucidation of low molecular weight metabolites of Ricinus communis seeds. Phytochem. Anal. 2016, 27, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qian, Y.; Tian, Y.-J.; Yuan, S.-M.; Wei, W.; Wang, G. Optimization of ionic liquid-assisted extraction of biflavonoids from Selaginella doederleinii and evaluation of its antioxidant and antitumor activity. Molecules 2017, 22, 586. [Google Scholar] [CrossRef] [PubMed]
- Grazhdannikov, A.E.; Kornaukhova, L.M.; Rodionov, V.I.; Pankrushina, N.A.; Shults, E.E.; Fabiano-Tixier, A.S.; Popov, S.A.; Chemat, F. Selecting a Green Strategy on Extraction of Birch Bark and Isolation of Pure Betulin Using Monoterpenes. Acs Sustain. Chem. Eng. 2018, 6, 6281–6288. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiao, J.; Gai, Q.-Y.; Wang, P.; Guo, N.; Niu, L.-L.; Fu, Y.-J. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2017, 145, 339–345. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.-S.; Chemat, F. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Wizi, J.; Wang, L.; Hou, X.; Tao, Y.; Ma, B.; Yang, Y. Ultrasound-microwave assisted extraction of natural colorants from sorghum husk with different solvents. Ind. Crop. Prod. 2018, 120, 203–213. [Google Scholar] [CrossRef]
- Yansheng, C.; Zhida, Z.; Changping, L.; Qingshan, L.; Peifang, Y.; Welz-Biermann, U. Microwave-assisted extraction of lactones from Ligusticum chuanxiong Hort. using protic ionic liquids. Green Chem. 2011, 13, 666–670. [Google Scholar] [CrossRef]
- Goldfarb, J.L.; Buessing, L.; Gunn, E.; Lever, M.; Billias, A.; Casoliba, E.; Schievano, A.; Adani, F. Novel integrated biorefinery for olive mill waste management: Utilization of secondary waste for water treatment. Acs Sustain. Chem. Eng. 2016, 5, 876–884. [Google Scholar] [CrossRef]
- Suomi, J.; Sirén, H.; Hartonen, K.; Riekkola, M.-L. Extraction of iridoid glycosides and their determination by micellar electrokinetic capillary chromatography. J. Chromatogr. A 2000, 868, 73–83. [Google Scholar] [CrossRef]
- Ong, E.S.; Len, S.M. Pressurized hot water extraction of berberine, baicalein and glycyrrhizin in medicinal plants. Anal. Chim. Acta 2003, 482, 81–89. [Google Scholar] [CrossRef]
- Just, J.; Deans, B.J.; Olivier, W.J.; Paull, B.; Bissember, A.C.; Smith, J.A. New method for the rapid extraction of natural products: Efficient isolation of shikimic acid from star anise. Org. Lett. 2015, 17, 2428–2430. [Google Scholar] [CrossRef] [PubMed]
- Corell, L.; Armenta, S.; Esteve-Turrillas, F.A.; de la Guardia, M. Flavonoid determination in onion, chili and leek by hard cap espresso extraction and liquid chromatography with diode array detection. Microchem. J. 2018, 140, 74–79. [Google Scholar] [CrossRef]
- Martinez-Sena, M.T.; de la Guardia, M.; Esteve-Turrillas, F.A.; Armenta, S. Hard cap espresso extraction and liquid chromatography determination of bioactive compounds in vegetables and spices. Food Chem. 2017, 237, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Just, J.; Bunton, G.L.; Deans, B.J.; Murray, N.L.; Bissember, A.C.; Smith, J.A. Extraction of Eugenol from Cloves Using an Unmodified Household Espresso Machine: An Alternative to Traditional Steam-Distillation. J. Chem. Educ. 2016, 93, 213–216. [Google Scholar] [CrossRef]
- Leiman, K.; Colomo, L.; Armenta, S.; de la Guardia, M.; Esteve-Turrillas, F.A. Fast extraction of cannabinoids in marijuana samples by using hard-cap espresso machines. Talanta 2018, 190, 321–326. [Google Scholar] [CrossRef]
- Tubaon, R.M.S.; Rabanes, H.; Haddad, P.R.; Quirino, J.P. Capillary electrophoresis of natural products: 2011–2012. Electrophoresis 2014, 35, 190–204. [Google Scholar] [CrossRef]
- Quirino, J.P.; Terabe, S. Electrokinetic chromatography. J. Chromatogr. A 1999, 856, 465–482. [Google Scholar] [CrossRef]
- Wuethrich, A.; Quirino, J.P. Capillary Electrophoresis as a Green Alternative Separation Technique. In The Application of Green Solvents in Separation Processes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 517–532. [Google Scholar]
- Rabanes, H.R.; Guidote, A.M.; Quirino, J.P. Micellar electrokinetic chromatography of the constituents in Philippine lagundi (Vitex negundo) herbal products. Microchem. J. 2014, 112, 153–158. [Google Scholar] [CrossRef]
- Gomes, A.F.; Ganzera, M.; Schwaiger, S.; Stuppner, H.; Halabalaki, M.; Almeida, M.P.; Leite, M.F.; Amaral, J.G.; David, J.M. Simultaneous determination of iridoids, phenylpropanoids and flavonoids in Lippia alba extracts by micellar electrokinetic capillary chromatography. Microchem. J. 2018, 138, 494–500. [Google Scholar] [CrossRef]
- Ghiasvand, A.; Feng, Z.; Quirino, J.P. Enrichment and Separation of Cationic, Neutral, and Chiral Analytes by Micelle to Cyclodextrin Stacking-Micellar Electrokinetic Chromatography. Anal. Chem. 2019, 91, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Yu, J.W.; Lu, F.L.; Lin, M.C.; Cheng, H.F. Differentiating parts of Cinnamomum cassia using LC-qTOF-MS in conjunction with principal component analysis. Biomed. Chromatogr. 2016, 30, 1449–1457. [Google Scholar] [CrossRef]
- Rawat, G.; Tripathi, P.; Saxena, R. Expanding horizons of shikimic acid. Appl. Microbiol. Biotechnol. 2013, 97, 4277–4287. [Google Scholar] [CrossRef] [PubMed]
- Candeias, N.R.; Assoah, B.; Simeonov, S.P. Production and Synthetic Modifications of Shikimic Acid. Chem. Rev. 2018, 118, 10458–10550. [Google Scholar] [CrossRef]
- Ohira, H.; Torii, N.; Aida, T.M.; Watanabe, M.; Smith, R.L., Jr. Rapid separation of shikimic acid from Chinese star anise (Illicium verum Hook. f.) with hot water extraction. Sep. Purif. Technol. 2009, 69, 102–108. [Google Scholar] [CrossRef]
- Xu, S.; Hossain, M.M.; Lau, B.B.; To, T.Q.; Rawal, A.; Aldous, L. Total quantification and extraction of shikimic acid from star anise (llicium verum) using solid-state NMR and cellulose-dissolving aqueous hydroxide solutions. Sustain. Chem. Pharm. 2017, 5, 115–121. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debruille, K.; Smith, J.A.; Quirino, J.P. Pressurized Hot Water Extraction and Capillary Electrophoresis for Green and Fast Analysis of Useful Metabolites in Plants. Molecules 2019, 24, 2349. https://doi.org/10.3390/molecules24132349
Debruille K, Smith JA, Quirino JP. Pressurized Hot Water Extraction and Capillary Electrophoresis for Green and Fast Analysis of Useful Metabolites in Plants. Molecules. 2019; 24(13):2349. https://doi.org/10.3390/molecules24132349
Chicago/Turabian StyleDebruille, Kurt, Jason A. Smith, and Joselito P. Quirino. 2019. "Pressurized Hot Water Extraction and Capillary Electrophoresis for Green and Fast Analysis of Useful Metabolites in Plants" Molecules 24, no. 13: 2349. https://doi.org/10.3390/molecules24132349