A Novel Ratiometric Fluorescent Probe for Mercury (II) ions and Application in Bio-imaging
Abstract
:1. Introduction
2. Results
2.1. Design Strategy of the PMH Probe
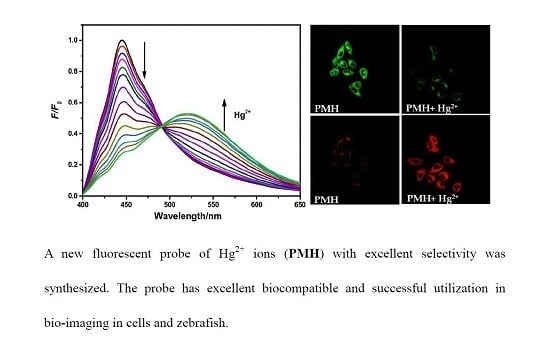
2.2. Spectral Titration of PMH with Hg2+ Ions
2.3. Selectivity and Interference Studies
2.4. Stability and Time Response
2.5. Fluorescence Imaging in Living Cells
2.6. Zebrafish Imaging
3. Experimental
3.1. Materials and General Method
3.2. Synthesis of Compound 1 (4-(1H-phenanthro[9,10-d]imidazol-2-yl)benzaldehyde)
3.3. Synthesis of PMH (((Z)-2-((E)-(4-(1H-phenanthro[9,10-d]imidazol-2-yl) benzylidene) hydrazono)-3-methyl-2,3-dihydrobenzo[d]thiazole))
3.4. Spectrophotometric Experiments
3.5. Cell Culture and Imaging
3.6. Zebrafish Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tan, S.W.; Meiller, J.C.; Mahaffey, K.R. The endocrine effects of mercury in humans and wildlife. Crit. Rev. Toxicol. 2009, 39, 228–269. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; Dong, M.; Wang, Y.-W.; Chen, Y.-T.; Wang, H.-Z.; Su, C.-Y.; Wang, W. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury (II). J. Am. Chem. Soc. 2016, 138, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Lippard, S.J. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef] [PubMed]
- Renzoni, A.; Zino, F.; Franchi, E. Mercury levels along the food chain and risk for exposed populations. Environ. Res. 1998, 77, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Malm, O. Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ. Res. 1998, 77, 73–78. [Google Scholar] [CrossRef]
- Bolger, P.M.; Schwetz, B. Mercury and health. New Engl. J. Med. 2002, 347, 1735–1736. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Akaoka, K.; Iyoshi, S.; Yamamoto, Y. Rosamine-based fluorescent sensor with femtomolar affinity for the reversible detection of a mercury ion. Inorg. Chem. 2012, 51, 13075–13077. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, L.; She, M.; Mo, Y.; Zhang, S.; Liu, P.; Li, J. Synthesis and application of highly sensitive fluorescent probe for Hg2+ regulated by sulfur. Chin. Chem. Lett. 2017, 28, 2014–2018. [Google Scholar] [CrossRef]
- Li, X.-M.; Zhao, R.-R.; Wei, Y.-L.; Yang, D.; Zhou, Z.-J.; Zhang, J.-F.; Zhou, Y. A rhodamine derivative for Hg2+-selective colorimetric and fluorescent sensing and its application to in vivo imaging. Chin. Chem. Lett. 2016, 27, 813–816. [Google Scholar] [CrossRef]
- Park, J.-D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X. Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2001, 222, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Chen, X.; Meng, X.; Wang, S.; Cai, Y.; Wu, Y.; Feng, Y.; Zhu, M.; Guo, Q. A rhodamine-based fluorescent probe for detecting Hg2+ in a fully aqueous environment. Dalton Trans. 2013, 42, 14819–14825. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, L.; Zhou, P. A rhodamine B-based fluorescent sensor toward highly selective mercury (II) ions detection. Talanta 2016, 150, 14–19. [Google Scholar] [CrossRef]
- Bernaus, A.; Gaona, X.; Esbrí, J.M.; Higueras, P.; Falkenberg, G.; Valiente, M. Microprobe techniques for speciation analysis and geochemical characterization of mine environments: The mercury district of Almadén in Spain. Environ. Sci. Technol. 2006, 40, 4090–4095. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, J.-B.; Wang, X.; Wang, Z.-H.; Zhao, R.-S. Simultaneous determination of copper, cobalt, and mercury ions in water samples by solid-phase extraction using carbon nanotube sponges as adsorbent after chelating with sodium diethyldithiocarbamate prior to high performance liquid chromatography. Anal. Bioanal. Chem. 2016, 408, 4445–4453. [Google Scholar] [CrossRef] [PubMed]
- Abollino, O.; Giacomino, A.; Malandrino, M.; Piscionieri, G.; Mentasti, E. Determination of mercury by anodic stripping voltammetry with a gold nanoparticle-modified glassy carbon electrode. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 75–83. [Google Scholar] [CrossRef]
- Chen, J.; Shu, W.; Wang, E. A fluorescent and colorimetric probe based on isatin-appended rhodamine for the detection of Hg2+. Chem. Res. Chin. Univ. 2016, 32, 742–745. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Lan, H.; Gong, G.; Zhu, Y.; Tse, Y.C.; Wong, K.M.C. Rhodol derivatives as selective fluorescent probes for the detection of HgII Ions and the bioimaging of hypochlorous acid. ChemistryOpen 2018, 7, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-L.; Wang, W.-K.; Tan, X.-X.; Luo, X.-F.; Zhang, M.-L.; Zhang, M.; Zang, S.-Q. A new quinoline-based fluorescent probe for Cd2+ and Hg2+ with an opposite response in a 100% aqueous environment and live cell imaging. Dalton Trans. 2016, 45, 8174–8181. [Google Scholar] [CrossRef]
- Dong, J.; Hu, J.; Baigude, H.; Zhang, H. A novel ferrocenyl–naphthalimide as a multichannel probe for the detection of Cu (II) and Hg (II) in aqueous media and living cells. Dalton Trans. 2018, 47, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, H.; Zhu, L.; Wang, Y.; Chen, Z.; Tian, Z. A indole-trizole-rhodamine triad as ratiometric fluorescent probe for nanomolar-concentration level Hg2+ sensing with high selectivity. J. Fluoresc. 2015, 25, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Mallick, D.; Bag, B. Signaling preferences of substituted pyrrole coupled six-membered rhodamine spirocyclic probes for Hg2+ ion detection. Org. Biomol. Chem. 2016, 14, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lü, X.; Chen, Z.; Yu, G.; Yin, J.; Liu, S. A fluorescent probe for Hg2+ based on gold (I) complex with an aggregation-induced emission feature. Chin. J. Chem. 2015, 33, 1064–1068. [Google Scholar] [CrossRef]
- Mahato, P.; Saha, S.; Das, P.; Agarwalla, H.; Das, A. An overview of the recent developments on Hg2+ recognition. RSC Adv. 2014, 4, 36140–36174. [Google Scholar] [CrossRef]
- Gülle, S.; Erbaş, S.Ç.; Uzel, A. Synthesis and spectroscopic studies of phenanthroimidazole-imine derivatives and evaluation of their antioxidant activity. J. Fluoresc. 2018, 28, 217–223. [Google Scholar] [CrossRef]
- Hwang, S.M.; Chae, J.B.; Kim, C. A Phenanthroimidazole-based Fluorescent Turn-Off Chemosensor for the Selective Detection of Cu2+ in Aqueous Media. Bull. Korean Chem. Soc. 2018, 39, 925–930. [Google Scholar] [CrossRef]
- Anbu, S.; Shanmugaraju, S.; Ravishankaran, R.; Karande, A.A.; Mukherjee, P.S. A phenanthrene based highly selective fluorogenic and visual sensor for Cu2+ ion with nanomolar detection limit and its application in live cell imaging. Inorg. Chem. Commun. 2012, 25, 26–29. [Google Scholar] [CrossRef]
- Sinha, S.; Chowdhury, B.; Ghosh, P. A highly sensitive ESIPT-based ratiometric fluorescence sensor for selective detection of Al3+. Inorg. Chem. 2016, 55, 9212–9220. [Google Scholar] [CrossRef]
- Ramu, V.; Roy, S.; Taye, N.; Chattopadhyay, S.; Das, A. A specific probe for Hg2+ to delineate even H+ in pure aqueous buffer/Hct116 colon cancer cells: Hg (ii)–η2-arene π-interaction and a TBET-based fluorescence response. Chem. Commun. 2014, 50, 14421–14424. [Google Scholar]
- Jiao, X.; Liu, C.; He, S.; Zhao, L.; Zeng, X. Highly selective and sensitive ratiometric near-infrared fluorescent probe for rel-time detection of Hg2+ and its bioapplications in live cells. Dyes Pigments 2019, 160, 86–92. [Google Scholar] [CrossRef]
- Fan, J.; Hu, M.; Zhan, P.; Peng, X. Energy transfer cassettes based on organic fluorophores: Construction and applications in ratiometric sensing. Chem. Soc. Rev. 2013, 42, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-based small-molecule fluorescent probes: rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, C.-F.; Shi, W.-J.; Tan, H.-Y.; He, Z.-Z.; Zheng, L.; Liu, F.; Yan, J.-W. A near-infrared BODIPY-based fluorescent probe for ratiometric and discriminative detection of Hg2+ and Cu2+ ions in living cells. Talanta 2019, 198, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.-H.; Zhou, Y.; Fang, Y.; Wang, L.-H.; Li, J.-Y.; Yao, C. Rhodamine–pyrene conjugated chemosensors for ratiometric detection of Hg2+ ions: Different sensing behavior between a spirolactone and a spirothiolactone. Dye Pigment 2013, 98, 339–346. [Google Scholar] [CrossRef]
- Li, J.; Yim, D.; Jang, W.-D.; Yoon, J. Recent progress in the design and applications of fluorescence probes containing crown ethers. Chem. Soc. Rev. 2017, 46, 2437–2458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, X.; Chen, H.; Wang, Y.; Xiao, S.; Zhang, N.; Li, D.; Zheng, K. An ESIPT/ICT modulation based ratiometric fluorescent probe for sensitive and selective sensing Hg2+. Sens. Actuat. B Chem. 2017, 247, 626–631. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Hyun, J.Y.; Wei, T.; Qiang, J.; Ren, X.; Shin, I.; Yoon, J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2016, 45, 2976–3016. [Google Scholar] [CrossRef]
- Hu, B.; Hu, L.-L.; Chen, M.-L.; Wang, J.-H. A FRET ratiometric fluorescence sensing system for mercury detection and intracellular colorimetric imaging in live Hela cells. Biosens. Bioelectron. 2013, 49, 499–505. [Google Scholar] [CrossRef]
- Wu, C.; Wang, J.; Shen, J.; Bi, C.; Zhou, H. Coumarin-based Hg2+ fluorescent probe: Synthesis and turn-on fluorescence detection in neat aqueous solution. Sens. Actuat. B Chem. 2017, 243, 678–683. [Google Scholar] [CrossRef]
- Lin, W.; Long, L.; Yuan, L.; Cao, Z.; Chen, B.; Tan, W. A ratiometric fluorescent probe for cysteine and homocysteine displaying a large emission shift. Org. Lett. 2008, 10, 5577–5580. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Liu, X.; Zhou, L.; He, H.; Zhou, P.; Duan, C. A schiff-base dual emission ratiometric fluorescent chemosensor for Hg2+ ions and its application in cellular imaging. Sens. Actuat. B Chem. 2017, 247, 950–956. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds PMH are available from the authors. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Jiao, Y.; He, C.; Duan, C. A Novel Ratiometric Fluorescent Probe for Mercury (II) ions and Application in Bio-imaging. Molecules 2019, 24, 2268. https://doi.org/10.3390/molecules24122268
Gao Q, Jiao Y, He C, Duan C. A Novel Ratiometric Fluorescent Probe for Mercury (II) ions and Application in Bio-imaging. Molecules. 2019; 24(12):2268. https://doi.org/10.3390/molecules24122268
Chicago/Turabian StyleGao, Qianmiao, Yang Jiao, Cheng He, and Chunying Duan. 2019. "A Novel Ratiometric Fluorescent Probe for Mercury (II) ions and Application in Bio-imaging" Molecules 24, no. 12: 2268. https://doi.org/10.3390/molecules24122268