Heterologous Expression of Ilicicolin H Biosynthetic Gene Cluster and Production of a New Potent Antifungal Reagent, Ilicicolin J
Abstract
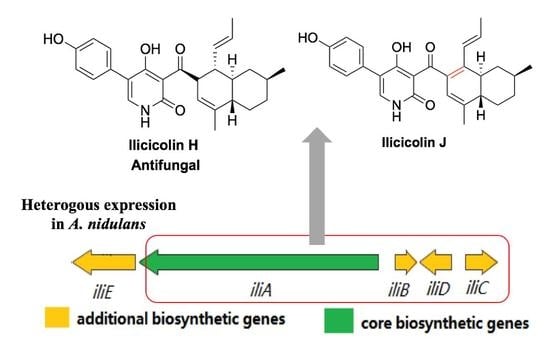
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Strains, Plasmids, and Culture Conditions
3.3. Isolation and Manipulation of DNA
3.4. Construction of Recombinant Plasmids
3.5. Transformation of A. nidulans
3.6. Isolation and Purification
3.7. Overproduction of iliD in E. coli
3.8. Antifungal Test of Compounds towards Candida Albicans ATCC10231
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Alberti, F.; Foster, G.D.; Bailey, A.M. Natural products from filamentous fungi and production by heterologous expression. Appl. Microbiol. Biotechnol. 2017, 101, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Minato, H.; Katagiri, K. The ilicicolins, antibiotics from Cylindrocladium Ilicicola. J. Antibiot. (Tokyo). 1971, 24, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Cirlos, E.B.; Merbitz-Zahradnik, T.; Trumpower, B.L. Inhibition of the Yeast Cytochrome bc1 Complex by Ilicicolin H, a Novel Inhibitor that Acts at the Qn Site of the bc1 Complex. J. Biol. Chem. 2004, 279, 8708–8714. [Google Scholar] [CrossRef]
- Singh, S.B.; Li, X.; Chen, T. Biotransformation of antifungal ilicicolin H. Tetrahedron Lett. 2011, 52, 6190–6191. [Google Scholar] [CrossRef]
- Singh, S.B.; Liu, W.; Li, X.; Chen, T.; Shafiee, A.; Card, D.; Abruzzo, G.; Flattery, A.; Gill, C.; Thompson, J.R.; et al. Antifungal spectrum, in vivo efficacy, and structure-activity relationship of ilicicolin H. ACS Med. Chem. Lett. 2012, 3, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Liu, W.; Li, X.; Chen, T.; Shafiee, A.; Dreikorn, S.; Hornak, V.; Meinz, M.; Onishi, J.C. Structure-activity relationship of cytochrome bc1 reductase inhibitor broad spectrum antifungal ilicicolin H. Bioorganic Med. Chem. Lett. 2013, 23, 3018–3022. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Guan, Z.; Singh, S.B. A new method for the synthesis of 1,4,5-oxadiazocines and its application in the structure modification of natural products. Tetrahedron Lett. 2005, 46, 8009–8012. [Google Scholar] [CrossRef]
- Eley, K.L.; Halo, L.M.; Song, Z.; Powles, H.; Cox, R.J.; Bailey, A.M.; Lazarus, C.M.; Simpson, T.J. Biosynthesis of the 2-pyridone tenellin in the insect pathogenic fungus Beauveria bassiana. ChemBioChem 2007, 8, 289–297. [Google Scholar] [CrossRef]
- Halo, L.M.; Heneghan, M.N.; Yakasai, A.A.; Song, Z.; Williams, K.; Bailey, A.M.; Cox, R.J.; Lazarus, C.M.; Simpson, T.J. Late stage oxidations during the biosynthesis of the 2-pyridone tenellin in the entomopathogenic fungus beauveria bassiana. J. Am. Chem. Soc. 2008, 130, 17988–17996. [Google Scholar] [CrossRef] [PubMed]
- Yakasai, A.A.; Davison, J.; Wasil, Z.; Halo, L.M.; Butts, C.P.; Lazarus, C.M.; Bailey, A.M.; Simpson, T.J.; Cox, R.J. Nongenetic reprogramming of a fungal highly reducing polyketide synthase. J. Am. Chem. Soc. 2011, 133, 10990–10998. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C.; Fisch, K.M.; Bakeer, W.; Yakasai, A.A.; Song, Z.; Pedrick, J.; Wasil, Z.; Bailey, A.M.; Lazarus, C.M.; Simpson, T.J.; et al. Rational domain swaps decipher programming in fungal highly reducing polyketide synthases and resurrect an extinct metabolite. J. Am. Chem. Soc. 2011, 133, 16635–16641. [Google Scholar]
- Bergmann, S.; Schümann, J.; Scherlach, K.; Lange, C.; Brakhage, A.A.; Hertweck, C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 2007, 3, 213–217. [Google Scholar] [CrossRef]
- Xu, W.; Cai, X.; Jung, M.E.; Tang, Y. Analysis of intact and dissected fungal polyketide synthase-nonribosomal peptide synthetase in vitro and in saccharomyces cerevisiae. J. Am. Chem. Soc. 2010, 132, 13604–13607. [Google Scholar] [CrossRef] [PubMed]
- Cary, J.W.; Uka, V.; Han, Z.; Buyst, D.; Harris-Coward, P.Y.; Ehrlich, K.C.; Wei, Q.; Bhatnagar, D.; Dowd, P.F.; Martens, S.L.; et al. An Aspergillus flavus secondary metabolic gene cluster containing a hybrid PKS-NRPS is necessary for synthesis of the 2-pyridones, leporins. Fungal Genet. Biol. 2015, 81, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, M.; Liu, F.; Hai, Y.; Chen, M.; Tang, M.C.; Yang, Z.; Sato, M.; Watanabe, K.; Houk, K.N.; Tang, Y. SAM-dependent enzyme-catalysed pericyclic reactions in natural product biosynthesis. Nature 2017, 549, 502–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.M.; Li, J.W.H.; Choi, J.W.; Zhou, H.; Lee, K.K.M.; Moorthie, V.A.; Xie, X.; Kealey, J.T.; Da Silva, N.A.; Vederas, J.C.; et al. Complete reconstitution of a highly reducing iterative polyketide synthase. Science 2009, 326, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.; Auclair, K.; Kendrew, S.G.; Park, C.; Vederas, J.C.; Hutchinson, C.R. Modulation of polyketide synthase activity by accessory proteins during lovastatin biosynthesis. Science 1999, 284, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Ruszczycky, M.W.; Choi, S.H.; Liu, Y.N.; Liu, H.W. Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A. Nature 2011, 473, 109–112. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, P.; Tang, M.C.; Zou, Y.; Gao, S.S.; Hung, Y.S.; Zhao, M.; Watanabe, K.; Houk, K.N.; Tang, Y. Biochemical Characterization of a Eukaryotic Decalin-Forming Diels-Alderase. J. Am. Chem. Soc. 2016, 138, 15837–15840. [Google Scholar] [CrossRef]
- Tan, D.; Jamieson, C.S.; Ohashi, M.; Tang, M.C.; Houk, K.N.; Tang, Y. Genome-Mined Diels-Alderase Catalyzes Formation of the cis-Octahydrodecalins of Varicidin A and B. J. Am. Chem. Soc. 2019, 141, 769–773. [Google Scholar] [CrossRef]
- Tanabe, M.; Urano, S. Biosynthetic studies with 13C. The antifungal antibiotic ilicicolin H. Tetrahedron 1983, 39, 3569–3574. [Google Scholar] [CrossRef]
- Yaegashi, J.; Oakley, B.R.; Wang, C.C.C. Recent advances in genome mining of secondary metabolite biosynthetic gene clusters and the development of heterologous expression systems in Aspergillus nidulans. J. Ind. Microbiol. Biotechnol. 2014, 41, 433–442. [Google Scholar] [CrossRef]
- Zhang, Z.; Jamieson, C.S.; Zhao, Y.-L.; Li, D.; Ohashi, M.; Houk, K.N.; Tang, Y. Enzyme-Catalyzed Inverse-Electron Demand Diels–Alder Reaction in the Biosynthesis of Antifungal Ilicicolin H. J. Am. Chem. Soc. 2019, 141, 5659–5663. [Google Scholar] [CrossRef]
- Uk, H.; Yup, S.; Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Uk Kim, H.; Bruccoleri, R.; Yup Lee, S.; Fischbach, M.A.; et al. antiSMASH 3.0—A comprehensive resource for the genome mining of biosynthetic gene clusters antiSMASH 3.0—A comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar]
- Halo, L.M.; Marshall, J.W.; Yakasai, A.A.; Song, Z.; Butts, C.P.; Crump, M.P.; Heneghan, M.; Bailey, A.M.; Simpson, T.J.; Lazarus, C.M.; et al. Authentic heterologous expression of the tenellin iterative polyketide synthase nonribosomal peptide synthetase requires cooexpression with an enoyl reductase. ChemBioChem 2008, 9, 585–594. [Google Scholar] [CrossRef]
- Fage, C.D.; Isiorho, E.A.; Liu, Y.; Wagner, D.T.; Liu, H.W.; Keatinge-Clay, A.T. The structure of SpnF, a standalone enzyme that catalyzes [4 + 2] cycloaddition. Nat. Chem. Biol. 2015, 11, 256–258. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-C.; Major, D.T.; Guo, R.-T.; Zhang, G.; Ko, T.-P.; Zhang, L.; Dai, L.; Liu, W.; Ansbacher, T.; Chang, Z.; et al. Crystal structure of LepI, a multifunctional SAM-dependent enzyme which catalyzes pericyclic reactions in leporin biosynthesis. Org. Biomol. Chem. 2019, 17, 2070. [Google Scholar]
- Clevenger, K.D.; Bok, J.W.; Ye, R.; Miley, G.P.; Verdan, M.H.; Velk, T.; Chen, C.; Yang, K.H.; Robey, M.T.; Gao, P.; et al. A scalable platform to identify fungal secondary metabolites and their gene clusters. Nat. Chem. Biol. 2017, 13, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Houk, K.N.; Ohashi, M.; Tang, Y.; Jamieson, C.S.; Liu, F. The expanding world of biosynthetic pericyclases: Cooperation of experiment and theory for discovery. Nat. Prod. Rep. 2019, 36, 698–713. [Google Scholar]
- Kato, N.; Nogawa, T.; Hirota, H.; Jang, J.H.; Takahashi, S.; Ahn, J.S.; Osada, H. A new enzyme involved in the control of the stereochemistry in the decalin formation during equisetin biosynthesis. Biochem. Biophys. Res. Commun. 2015, 460, 210–215. [Google Scholar] [CrossRef]
- Rosenberg, A.; Ene, I.V.; Bibi, M.; Zakin, S.; Segal, E.S.; Ziv, N.; Dahan, A.M.; Colombo, A.L.; Bennett, R.J.; Berman, J. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat. Commun. 2018, 9, 2470. [Google Scholar] [CrossRef]
- Tu, Z.; He, G.; Li, K.X.; Chen, M.J.; Chang, J.; Chen, L.; Yao, Q.; Liu, D.P.; Ye, H.; Shi, J.; et al. An improved system for competent cell preparation and high efficiency plasmid transformation using different Escherichia coli strains. Electron. J. Biotechnol. 2005, 8, 113–120. [Google Scholar]
- Kildgaard, S.; Subko, K.; Phillips, E.; Goidts, V.; De la Cruz, M.; Díaz, C.; Gotfredsen, C.H.; Andersen, B.; Frisvad, J.C.; Nielsen, K.F.; et al. A Dereplication and Bioguided Discovery Approach to Reveal New Compounds from a Marine-Derived Fungus Stilbella fimetaria. Mar. Drugs 2017, 15, 253. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 and 2 are available from the authors. |


| Protein | Size (aa) | Proposed Function | Tenellin BGC Homolog | Similarity/Identity (%) | DMBA BGC Homolog | Similarity/IDentity (%) |
|---|---|---|---|---|---|---|
| IliA | 2000 | PKS-NRPS | tenS | 78/66 | dmbS | 80/68 |
| IliB | 381 | ER | tenC | 67/52 | dmbC | 67/53 |
| IliC | 505 | cytochrome P450 | tenA | 78/63 | dmbA | 78/63 |
| IliD | 244 | methyltransferase | - | - | - | - |
| IliE | 766 | NADH:Flavin Oxidoreductase | - | - | - | - |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Yuan, S.; Chen, S.; Chen, B.; Xu, H.; Liu, L.; Li, H.; Gao, Z. Heterologous Expression of Ilicicolin H Biosynthetic Gene Cluster and Production of a New Potent Antifungal Reagent, Ilicicolin J. Molecules 2019, 24, 2267. https://doi.org/10.3390/molecules24122267
Lin X, Yuan S, Chen S, Chen B, Xu H, Liu L, Li H, Gao Z. Heterologous Expression of Ilicicolin H Biosynthetic Gene Cluster and Production of a New Potent Antifungal Reagent, Ilicicolin J. Molecules. 2019; 24(12):2267. https://doi.org/10.3390/molecules24122267
Chicago/Turabian StyleLin, Xiaojing, Siwen Yuan, Senhua Chen, Bin Chen, Hui Xu, Lan Liu, Huixian Li, and Zhizeng Gao. 2019. "Heterologous Expression of Ilicicolin H Biosynthetic Gene Cluster and Production of a New Potent Antifungal Reagent, Ilicicolin J" Molecules 24, no. 12: 2267. https://doi.org/10.3390/molecules24122267