Functional Fragments of AIMP1-Derived Peptide (AdP) and Optimized Hydrosol for Their Topical Deposition by Box-Behnken Design
Abstract
:1. Introduction
2. Results
2.1. Functional Fragments of AIMP1-Derived Peptide (AdP)
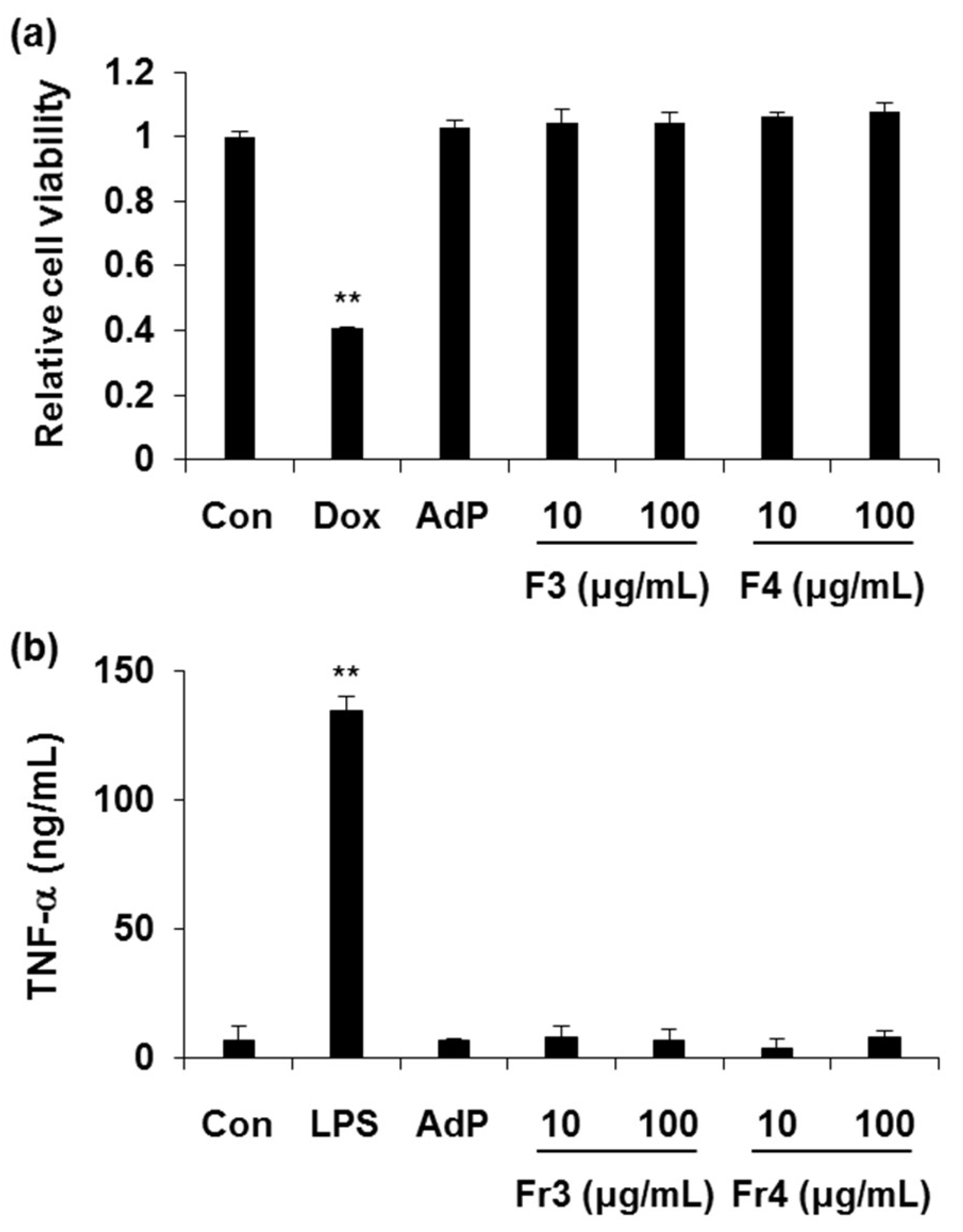
2.2. Cytotoxicity and Immunogenicity Tests of FA- and MI-AdP
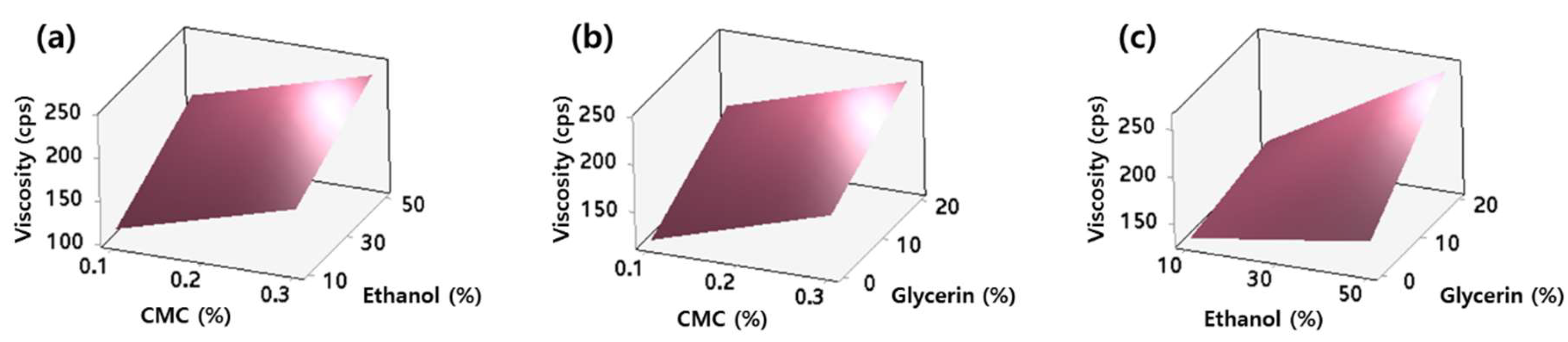
2.3. Viscosity of AdP Hydrosol
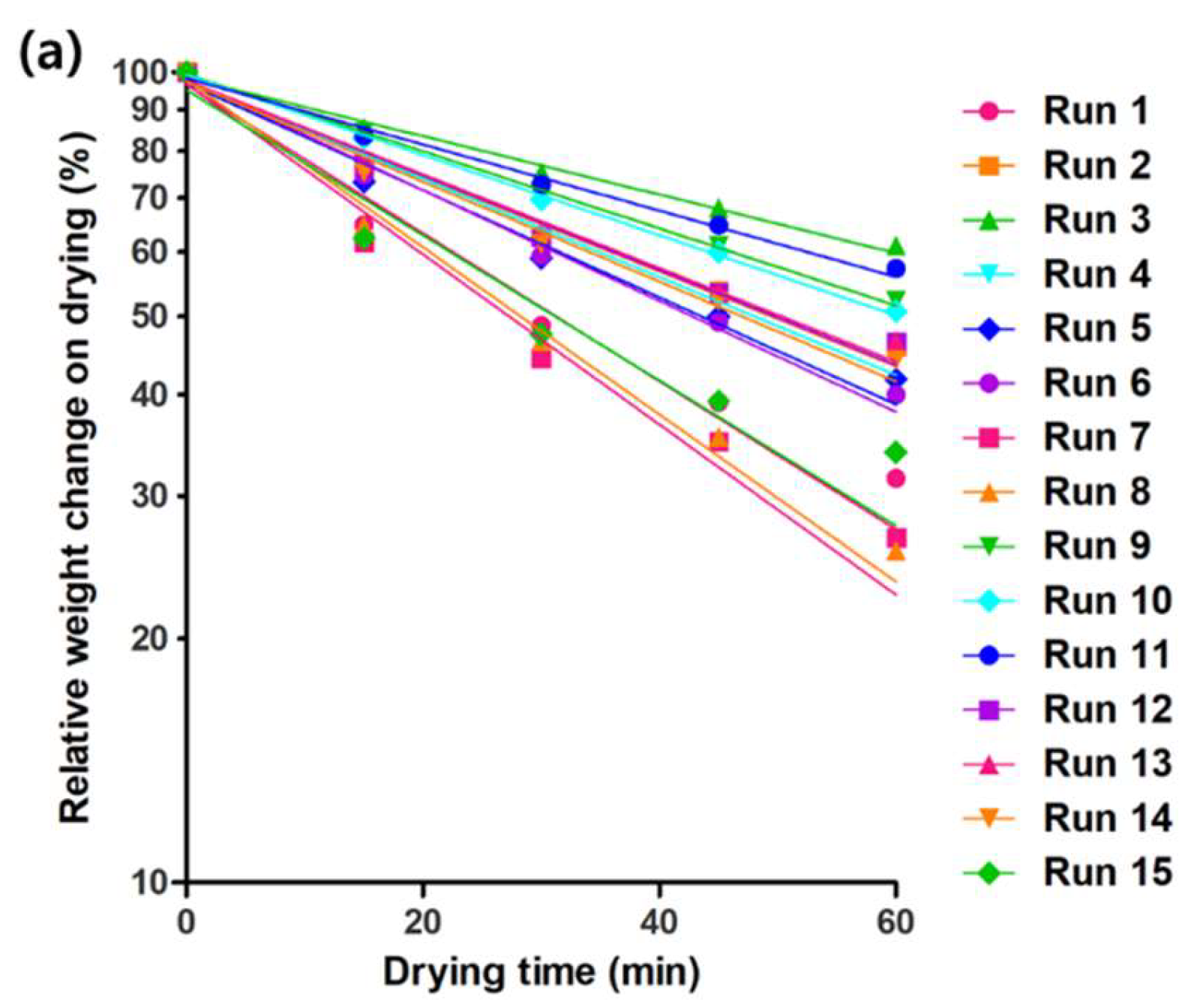
2.4. Drying Rate of AdP Hydrosol
2.5. Optimization of AdP Hydrosol Based on Composite Desirability
2.6. Artificial Skin Deposition Study
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Collagen ELISA
4.3. Proliferation Assay
4.4. Measurement of Melanin
4.5. Cytotoxicity and Immunogenicity Tests
4.6. Preparation of FA-AdP-Loaded Hydrosol
4.7. Experimental Design for Hydrosol Optimization
4.8. Measurement of Viscosity and Drying Rate
4.9. Evaluation of Artificial Skin Deposition by Near-Infrared Fluorescence Imaging
4.10. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermato-endocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Pena Ferreira, M.R.; Costa, P.C.; Bahia, F.M. Efficacy of anti-wrinkle products in skin surface appearance: a comparative study using non-invasive methods. Skin. Res. Technol. 2010, 16, 444–449. [Google Scholar] [CrossRef]
- Hynes, R.O. The extracellular matrix: not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Clause, K.C.; Barker, T.H. Extracellular matrix signaling in morphogenesis and repair. Curr. Opin. Biotechnol. 2013, 24, 830–833. [Google Scholar] [CrossRef] [Green Version]
- Malerich, S.; Berson, D. Next generation cosmeceuticals: the latest in peptides, growth factors, cytokines, and stem cells. Dermatol. clin. 2014, 32, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J. Drugs Dermato. 2008, 7, s12–s16. [Google Scholar]
- Fligiel, S.E.; Varani, J.; Datta, S.C.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J. Investig. Dermatol. 2003, 120, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Perone, P.; Fligiel, S.E.; Fisher, G.J.; Voorhees, J.J. Inhibition of type I procollagen production in photodamage: correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J. Invest. Dermatol. 2002, 119, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Carsberg, C.J.; Warenius, H.M.; Friedmann, P.S. Ultraviolet radiation-induced melanogenesis in human melanocytes. Effects of modulating protein kinase C. J. Cell Sci. 1994, 107, 2591–2597. [Google Scholar]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Lintner, K.; Mas-Chamberlin, C.; Mondon, P.; Peschard, O.; Lamy, L. Cosmeceuticals and active ingredients. Clin. Dermatol. 2009, 27, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, S.; Kwon, H.; Moon, H.; Park, M.C. Dual functional bioactive-peptide, AIMP1-derived peptide (AdP), for anti-aging. J. Cosmet. Dermatol. 2019, 18, 251–257. [Google Scholar] [CrossRef]
- Kim, S.; You, S.; Hwang, D. Aminoacyl-tRNA synthetases and tumorigenesis: more than housekeeping. Nat. Rev. Cancer 2011, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, G.; Kim, S. Aminoacyl-tRNA synthetase-interacting multi-functional protein 1/p43: an emerging therapeutic protein working at systems level. Expert Opin. Drug Discov. 2008, 3, 945–957. [Google Scholar] [CrossRef]
- Helfrich, Y.R.; Sachs, D.L.; Voorhees, J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 2008, 20, 177–183. [Google Scholar] [PubMed]
- Park, S.G.; Shin, H.; Shin, Y.K.; Lee, Y.; Choi, E.C.; Park, B.J.; Kim, S. The novel cytokine p43 stimulates dermal fibroblast proliferation and wound repair. Am. J. Pathol. 2005, 166, 387–398. [Google Scholar] [CrossRef]
- Han, J.M.; Park, S.G.; Lee, Y.; Kim, S. Structural separation of different extracellular activities in aminoacyl-tRNA synthetase-interacting multi-functional protein, p43/AIMP1. Biochem. Biophys. Res. Commun. 2006, 342, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.; Obst, K.; Friess, W.; Hedtrich, S. Recent advances in topical delivery of proteins and peptides mediated by soft matter nanocarriers. Biotechnol. Adv. 2015, 33, 1355–1369. [Google Scholar] [CrossRef]
- Kang, N.W.; Kim, M.H.; Sohn, S.Y.; Kim, K.T.; Park, J.H.; Lee, S.Y.; Lee, J.Y.; Kim, D.D. Curcumin-loaded lipid-hybridized cellulose nanofiber film ameliorates imiquimod-induced psoriasis-like dermatitis in mice. Biomaterials 2018, 182, 245–258. [Google Scholar] [CrossRef]
- Aufenvenne, K.; Larcher, F.; Hausser, I.; Duarte, B.; Oji, V.; Nikolenko, H.; Del Rio, M.; Dathe, M.; Traupe, H. Topical enzyme-replacement therapy restores transglutaminase 1 activity and corrects architecture of transglutaminase-1-deficient skin grafts. Am. J. Hum. Genet. 2013, 93, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Goebel, A.S.; Schmaus, G.; Neubert, R.H.; Wohlrab, J. Dermal peptide delivery using enhancer molecules and colloidal carrier systems--part I: carnosine. Skin Pharmacol. Physiol. 2012, 25, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Denet, A.R.; Vanbever, R.; Preat, V. Skin electroporation for transdermal and topical delivery. Adv. Drug Deliv. Rev. 2004, 56, 659–674. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Magnusson, B.M.; Runn, P. Effect of penetration enhancers on the permeation of the thyrotropin releasing hormone analogue pGlu-3-methyl-His-Pro amide through human epidermis. Int. J. Pharm. 1999, 178, 149–159. [Google Scholar] [CrossRef]
- Butun, S.; Ince, F.G.; Erdugan, H.; Sahiner, N. One-step fabrication of biocompatible carboxymethyl cellulose polymeric particles for drug delivery systems. Carbohydr. Polym. 2011, 86, 636–643. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, K.-T.; Park, J.-H.; Lee, J.-Y.; Kim, M.; Min, H.G.; Moon, I.-H.; Choi, C.-Y.; Kim, B.H.; Kim, D.-D. Coacervate microcapsules of vitamin U optimized by central composite design (CCD). J. Pharm. Investig. 2018. [Google Scholar] [CrossRef]
- Ahmed, Z.Z.; Khan, F.N.; Shaikh, D.A. Reverse engineering and formulation by QBD of olopatadine hydrochloride ophthalmic solution. J. Pharm. Investig. 2018, 48, 279–293. [Google Scholar] [CrossRef]
- Navamanisubramanian, R.; Nerella, R.; Duraipandian, C.; Seetharaman, S. Quality by design approach for optimization of repaglinide buccal tablets using Box-Behnken Design. Future J. Pharm. Sci. 2018, 4, 265–272. [Google Scholar] [CrossRef]
- Prabhakaran, D.; Basha, C.A.; Kannadasan, T.; Aravinthan, P. Removal of hydroquinone from water by electrocoagulation using flow cell and optimization by response surface methodology. J. Environ. Sci. Health A 2010, 45, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Natrella, M. NIST/SEMATECH e-Handbook of Statistical Methods; NIST: Gaithersburg, MA, USA, 2010.
- Amir, A.I.; van Rosmalen, M.; Mayer, G.; Lebendiker, M.; Danieli, T.; Friedler, A. Highly homologous proteins exert opposite biological activities by using different interaction interfaces. Sci. Rep. 2015, 5, 11629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Megrab, N.A.; Williams, A.C.; Barry, B.W. Oestradiol permeation across human skin, silastic and snake skin membranes: the effects of ethanol/water co-solvent systems. Int. J. Pharm. 1995, 116, 101–112. [Google Scholar] [CrossRef]
- Morimoto, H.; Wada, Y.; Seki, T.; Sugibayashi, K. In vitro skin permeation of morphine hydrochloride during the finite application of penetration-enhancing system containing water, ethanol and l-menthol. Biol. Pharm. Bull. 2002, 25, 134–136. [Google Scholar] [CrossRef]
- Löffler, H.; Kampf, G.; Schmermund, D.; Maibach, H. How irritant is alcohol? Br. J. Dermatol. 2007, 157, 74–81. [Google Scholar] [CrossRef]
- Maibach, H.I.; Lodén, M. Dry Skin and Moisturizers: Chemistry and Function; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Chang, R.-K.; Raw, A.; Lionberger, R.; Yu, L. Generic development of topical dermatologic products: formulation development, process development, and testing of topical dermatologic products. AAPS J. 2012, 15, 41–52. [Google Scholar] [CrossRef]
- Lu, W.; Luo, H.; Zhu, Z.; Wu, Y.; Luo, J.; Wang, H. Preparation and the biopharmaceutical evaluation for the metered dose transdermal spray of dexketoprofen. J. Drug Deliv. 2014, 2014. [Google Scholar] [CrossRef]
- Adler, S.; Basketter, D.; Creton, S.; Pelkonen, O.; van Benthem, J.; Zuang, V.; Andersen, K.E.; Angers-Loustau, A.; Aptula, A.; Bal-Price, A.; et al. Alternative (non-animal) methods for cosmetics testing: current status and future prospects—2010. Arch. Toxicol. 2011, 85, 367–485. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds FA-AdP and MI-AdP are available from the authors. |




| Run Order | Independent Variables | Dependent Variables | |||
|---|---|---|---|---|---|
| x1 | x2 | x3 | y1 | y2 | |
| CMC (%, w/v) | Ethanol (%, v/v) | Glycerin (%, v/v) | Viscosity (cps) | Drying Rate Constant (1/min) | |
| 1 | 0.3 | 50 | 10 | 261.1 | 0.0210 |
| 2 | 0.1 | 30 | 20 | 173.7 | 0.0135 |
| 3 | 0.2 | 10 | 20 | 158.7 | 0.0083 |
| 4 | 0.3 | 30 | 0 | 171.0 | 0.0139 |
| 5 | 0.2 | 30 | 10 | 165.0 | 0.0152 |
| 6 | 0.1 | 30 | 0 | 137.1 | 0.0157 |
| 7 | 0.1 | 50 | 10 | 173.7 | 0.0242 |
| 8 | 0.2 | 50 | 0 | 146.4 | 0.0238 |
| 9 | 0.3 | 10 | 10 | 188.1 | 0.0110 |
| 10 | 0.2 | 10 | 0 | 116.1 | 0.0114 |
| 11 | 0.1 | 10 | 10 | 122.1 | 0.0094 |
| 12 | 0.2 | 30 | 10 | 173.7 | 0.0135 |
| 13 | 0.3 | 30 | 20 | 212.4 | 0.0133 |
| 14 | 0.2 | 30 | 10 | 178.5 | 0.0142 |
| 15 | 0.2 | 50 | 20 | 267.1 | 0.0206 |
| Responses | Sources | DF 1 | SS 1 | MS 1 | F-Value | p-Value |
|---|---|---|---|---|---|---|
| y1 | Model | 4 | 23853.5 | 5963.4 | 23.78 | <0.001 |
| x1 | 1 | 6384.5 | 6384.5 | 25.46 | 0.001 | |
| x2 | 1 | 8665.9 | 8665.9 | 34.56 | <0.001 | |
| x3 | 1 | 7278.2 | 7278.2 | 29.02 | <0.001 | |
| x2·x3 | 1 | 1524.9 | 1524.9 | 6.08 | 0.033 | |
| Error | 10 | 2507.6 | 250.8 | |||
| Total | 14 | 26361.1 | ||||
| y2 | Model | 4 | 0.000338 | 0.000085 | 152.99 | <0.001 |
| x2 | 1 | 0.000307 | 0.000307 | 555.63 | <0.001 | |
| x3 | 1 | 0.00001 | 0.00001 | 18.86 | 0.001 | |
| x2·x2 | 1 | 0.000015 | 0.000015 | 27.44 | <0.001 | |
| x1·x2 | 1 | 0.000006 | 0.000006 | 10.04 | 0.01 | |
| Error | 10 | 0.000006 | 0.000001 | |||
| Total | 14 | 0.000344 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-J.; Han, Y.-M.; Kwon, T.-W.; Kim, D.H.; Lee, H.S.; Jung, W.J.; Kim, J.; Kang, S.; Kim, S.K.; Cho, C.-W.; et al. Functional Fragments of AIMP1-Derived Peptide (AdP) and Optimized Hydrosol for Their Topical Deposition by Box-Behnken Design. Molecules 2019, 24, 1967. https://doi.org/10.3390/molecules24101967
Lee J-J, Han Y-M, Kwon T-W, Kim DH, Lee HS, Jung WJ, Kim J, Kang S, Kim SK, Cho C-W, et al. Functional Fragments of AIMP1-Derived Peptide (AdP) and Optimized Hydrosol for Their Topical Deposition by Box-Behnken Design. Molecules. 2019; 24(10):1967. https://doi.org/10.3390/molecules24101967
Chicago/Turabian StyleLee, Jeong-Jun, Young-Min Han, Tae-Wan Kwon, Dong Hyun Kim, Han Sol Lee, Woo Jin Jung, Jina Kim, Sujin Kang, Sang Kyum Kim, Cheong-Weon Cho, and et al. 2019. "Functional Fragments of AIMP1-Derived Peptide (AdP) and Optimized Hydrosol for Their Topical Deposition by Box-Behnken Design" Molecules 24, no. 10: 1967. https://doi.org/10.3390/molecules24101967







