3.1. Raman Spectra on Aqueous Ln(ClO4)3 Solutions (Ln = Lu3+, Yb3+, Tm3+, Er3+ and Ho3+)
The perchlorate spectrum, ClO
4−(aq): The Raman spectrum of the perchlorate ion in aqueous solution (NaClO
4(aq)) has been well characterized and only a brief description shall be given [
14,
15,
16]. The ClO
4− ion possesses T
d symmetry and has nine modes of internal vibrations spanning the representation Γ
vib(T
d) = a
1(Ra) + e(Ra) + 2f
2(Ra, i.r.). All normal modes are Raman active, but in i.r. only the f
2 modes are allowed. The ν
1(a
1) ClO
4− band, centred at 931.5 cm
−1, is totally polarized (ρ = 0.005) whereas ν
3(f
2) ClO
4−, centred at 1108 cm
−1, is depolarized. The deformation modes ν
4(f
2) ClO
4− at 629 cm
−1 and ν
2(e) ClO
4− at 461 cm
−1 [
15] are also depolarized. In dilute aqueous NaClO
4 solutions, the spectrum of ClO
4− (aq) shows no sign of contact ion pairs or outer-sphere ion pairs; the ν
1(a
1) ClO
4− band at 931.5 cm
−1 is quite narrow with a full width at half height (fwhh) = 7.2 cm
−1. However, the ν
1(a
1) ClO
4− band shows an intrinsic low frequency shoulder at 923 cm
−1 which is caused by Fermi resonance of the overtone of ν
2(e) ClO
4−, 2x ν
2(e) = 923 cm
−1 with ν
1(a
1) ClO
4− band [
15]. The antisymmetric stretching mode, ν
3(f
2) ClO
4−, is much weaker in intensity than ν
1(a
1) and it appears at 1106 cm
−1 with a fwhh = 65 cm
−1. In
Figure 1A the scattering profiles in R-format (R
VV, R
VH, and R
iso) of a 3.801 mol·L
−1 NaClO
4(aq) are presented from 50 to 800 cm
−1 and in addition the overview Raman scattering profiles (I
VV, I
VH and I
iso) from 50 to 1800 cm
−1 are given in
Figure S1 together with the band positions and assignments.
The Raman spectra of Lu(ClO
4)
3(aq), Yb(ClO
4)
3(aq), Tm(ClO
4)
3(aq), Er(ClO
4)
3(aq) and Ho(ClO
4)
3(aq): First, we present and discuss the spectroscopic results on Yb(ClO
4)
3 followed below by the spectra of aqueous Tm(ClO
4)
3, Er(ClO
4)
3 and Ho(ClO
4)
3 solutions. The Raman spectra of aqueous Lu(ClO
4)
3 solutions have been reported recently [
17] with the ν
1 LuO
8 breathing mode at 396 cm
−1.
Figure 1B,C show the scattering profiles of aqueous Yb(ClO
4)
3 solutions at 0.240 mol·L
−1 (Rw = 226.6) and, in comparison, a solution at 2.423 mol·L
−1 (R
W = 16.86) respectively. The Raman scattering profiles of the 0.240 mol·L
−1 aqueous Yb(ClO
4)
3 solution (
Figure 1B) from 40–750 cm
−1 shows two ClO
4−(aq) bands at 461 cm
−1 and 629 cm
−1 and a broad, weak polarized mode (isotropic scattering) at 394 cm
−1 which does not occur in NaClO
4(aq). Therefore, the band at 394 cm
−1 has to be assigned to the ν
1 YbO
8 breathing mode of the [Yb(H
2O)
8]
3+ species. In the at 2.423 mol·L
−1 (R
W = 16.86) Yb(ClO
4)
3 solution (Figure C), however, the ν
1 YbO
8 breathing mode is shifted by 4 cm
−1 to lower wavenumbers compared to the dilute solution (
Figure 1B). An overview Raman spectrum of the 2.423 mol·L
−1 Yb(ClO
4)
3 solution is given in
Figure S2 (top panel) from 80–1400 cm
−1 which displays all four perchlorate bands and the weak isotropic mode at 390 cm
−1 assigned to the ν
1 YbO
8 breathing mode. The vibrational bands in the anisotropic scattering could only be detected in the concentrated Yb(ClO
4)
3 solution at 2.423 mol·L
−1 because of their very weak and broad nature. These anisotropic bands are even weaker than the already weak ν
1 YbO
8 breathing mode with an integrated band intensity at 3161. The band fit results for the anisotropic scattering are given in
Table S1 and presented in
Figure 2 Five bands appear at 88.5 cm
−1 (fwhh = 119), 158.7 cm
−1 (fwhh = 97.6.9 cm
−1), 229.4 cm
−1 (fwhh = 75.7 cm
−1), 260.0 cm
−1 (fwhh = 68cm
−1) and 333.2 cm
−1 (fwhh = 85.3) in the anisotropic scattering in the 2.423 mol·L
−1 Yb(ClO
4)
3(aq) solution. These weak, broad bands stem from the YbO
8 skeleton fundamentals of the [Yb(OH
2)
8]
3+ species and break the symmetry of the YbO
8 skeleton. Therefore, they appear only in the anisotropic scattering, but not in the isotropic profile. From group theoretical considerations we expect 7 Raman active modes for the YbO
8 skeleton (ligated water molecules seen as point masses) and a brief group theoretical discussion shall be given. The YbO
8 skeleton (D
4d symmetry) with its 9 atoms leads to 21 normal modes and the irreducible representation follows as: Γ
v(D
4d) = 2a
1(Ra) + b
1(i.a.) + 2b
2(i.r.) + 3e
1(i.r.) + 3e
2(Ra) + 2e
3(Ra). (The YbO
8 skeleton possesses no symmetry centre but the mutual exclusion rule nevertheless applies.) Seven modes with the character a
1, e
2 and e
3 are Raman allowed while six modes with the character b
1, b
2 and e
1 are i.r. active. The totally symmetric Yb–O stretch, the breathing mode, is only Raman active and appears strongly polarized in the Raman spectrum as the strongest band of the YbO
8 skeleton. Two additional depolarized Raman stretching modes are expected (character e
2 and e
3) as well as four other Raman deformation modes (character a
1, e
2 and e
3). In infrared, two stretching modes (character b
2 and e
1) are expected and the remaining are deformations. In reality, however, we observe only six skeleton modes with one unaccounted mode (see also [
17]). From our Raman spectroscopic results, it follows directly that the Yb
3+–OH
2 hydration shell cannot constitute a hexa-hydrate (T
h symmetry) which has been, for instance, characterized for [Al(OH
2)
6]
3+(aq) [
22,
23]. Group theoretical considerations expect only three skeleton modes in Raman for [Al(OH
2)
6]
3+; one of which should be totally polarized (breathing mode for the AlO
6 skeleton) and the remaining two depolarized. All of these bands were detected in the Raman spectrum of an Al(ClO
4)
3(aq) solution with the symmetric stretching mode of [Al(OH
2)
6]
3+ at 525 cm
−1 strongly polarized and two bands at 438 cm
−1 and 332 cm
−1 which are depolarized [
22,
23].
The concentration dependence of the band parameters (peak positions, full width at half height (fwhh) and integrated band areas) of the ν
1 YbO
8 breathing mode for Yb(ClO
4)
3 solutions allows the determination of the change of these band parameters as a function of concentration. The band profiles of the ν
1 YbO
8 breathing mode are given in
Figure 3 at concentrations 0.240 mol·L
−1 (R
W = 268.74), 0.603 mol·L
−1 (R
W = 85.17), 0.808 mol·L
−1 (R
W = 62.13), 1.217 mol·L
−1 (R
W = 16.86) and finally at 2.423 mol·L
−1 (R
W = 16.86). The ν
1Yb–O breathing mode appears at 394 cm
−1 at the lowest concentrations and shifts ~4 cm
−1 to lower wavenumbers at the highest concentration. Furthermore, the bandwidths also increase with increasing solute concentration from 52 cm
−1 for the 0.240 mol·L
−1 solution to 59 cm
−1 for the 2.423 mol·L
−1 solution (
Figure 3). This slight change in band parameters of ν
1 YbO
8 breathing mode with increasing solute concentration may be due to ion pair formation in concentrated solutions (The ion pairing effect in perchlorate solutions is discussed in detail in [
15,
17]). The integrated band intensity of the ν
1YbO
8 breathing mode, A
394, rises linearly with the solution concentration. The dependence of the integrated band intensity of the ν
1 YbO
8 breathing mode of [Yb(OH
2)
8]
3+ as a function of the Yb(ClO
4)
3 concentration is given in
Figure S3 and for the linear relationship follows: A
394 = 1303.7·C
Yb(ClO4)3 (R
2 = 99.9).
In addition to the ν
1YbO
8 of [Yb(OH
2)
8]
3+ an extremely weak and broad band centered at 170 ± 10 cm
−1 appears isotropic Raman scattering in of aqueous Yb(ClO
4)
3 solution (see
Figure S2, top panel). This isotropic band is assigned to a restricted translational mode of the weakly H- bonded water molecules (O-H∙∙∙∙OClO
3−). The mode is strongly anion and concentration-dependent [
14,
15,
16]. The influence of the ClO
4− on the water spectrum has been discussed in recent studies on aqueous Ln(ClO
4)
3 solutions [
14,
15,
16,
17]. The Raman scattering profiles, I
VV, I
VH and I
iso of the O-H stretching region of H
2O and its bending mode of a Yb(ClO
4)
3 solution at 2.423 mol·L
−1 and their peak positions are given in
Figure S2, bottom panel, and for details see [
14,
15,
16].
The triflate ion (trifluoromethanesulfonate) in aqueous solution acts as an even weaker complex forming anion and is suited, therefore, for studying metal ion hydration. In aqueous solution, however, the weak ν
1 band of [Yb(OH
2)
8]
3+ at ~394 cm
−1 is overlapped by a strongly polarized triflate band at 319 cm
−1 and so band fit analysis was applied. The isotropic Raman spectrum of Yb(CF
3SO
3)
3(aq) at 1.25 mol·L
−1 is shown in
Figure S4 and the band fit analysis gave two bands with the first band component at 319 cm
−1 and the second band at 394 cm
−1 (fwhh = 50 cm
−1). The first band, a polarized band, stems from CF
3SO
3− (aq) but the second band is the ν
1 YbO
8 breathing mode of [Yb(H
2O)
8]
3+. Band parameters and assignments of CF
3SO
3−(aq) modes are given in [
16].
The effect of deuteration on the YbO
8 skeleton modes of [Yb(OD
2)
8]
3+ was studied in Yb(ClO
4)
3− D
2O solution and resulted in a shift of the Yb–OD
2 mode down to 374 cm
−1. The shift of ν
1 on deuteration is given as ν
1’ = ν
1[m(H
2O)/m(D
2O)]
1/2 =
= 0.9485 × 394 cm
−1 = 373.7 cm
−1. (The water and heavy water molecules are taken as point masses.) A Raman spectra of Yb(ClO
4)
3 solutions in D
2O at 0.779 mol·L
−1 is presented in
Figure 4 and for a concentration at 1.276 mol·L
−1 in
Figure S5. This isotope shift of the symmetrical stretch of YbO
8 in changing from [Yb(OH
2)
8]
3+(H
2O) to [Yb(OD
2)
8]
3+(D
2O) and the totally symmetric character of the mode, that is, showing a polarization degree ~0, are indeed proof for the character of this mode.
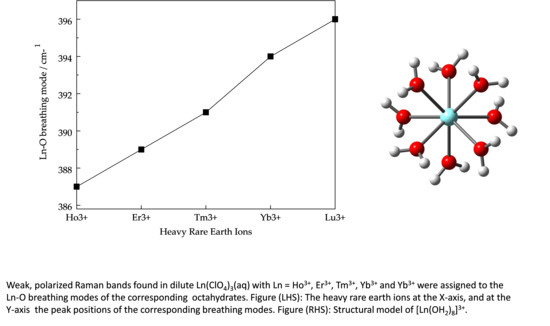
In the Raman spectra of the Tm(ClO4)3, Er(ClO4)3 and Ho(ClO4)3 solutions appear also as strongly polarized bands and were observed at 391, 389 cm−1, and 387 cm−1 respectively. These isotropic bands are unique in these HRE ion solutions and cannot be found in the hydrated ClO4− (aq) spectrum.
The representative Raman spectra of Tm(ClO
4)
3, Er(ClO
4)
3 and Ho(ClO
4)
3 solutions are given in
Figures S6–S8, respectively. Several concentrations of the Tm(ClO
4)
3(aq) were measured but from the coloured Er(ClO
4)
3 and Ho(ClO
4)
3 solutions only the dilute solutions could be reliably measured. (Concentrated Er(ClO
4)
3 and Ho(ClO
4)
3 solutions absorb the laser light markedly.) The peak positions for the ν
1 LnO
8 breathing modes for [Lu(OH
2)
8]
3+ (taken from [
17]), [Yb(OH
2)
8]
3+, [Tm(OH
2)
8]
3+, [Er(OH
2)
8]
3+ and [Ho(OH
2)
8]
3+ are given in
Table 1. Force constant calculations for the ν
1 LnO
8 breathing modes of this species, applying a simple model, have been carried out according to equation (4):
with c, the velocity of light,
the wavenumber of the mode
i, N the Avogadro constant and A
L the molecular weight of the ligand, in our case water. The force constants,
kLn–O, calculated for the measured ν
1 breathing modes are given in
Table 1 together with the corresponding Ln
3+- O bond distances [
7]
. The force constants increase from Lu
3+, Yb
3+, Tm
3+, Er
3+ and Ho
3+ in the same order as the corresponding Ln–O bond distances decrease, namely Lu–O < Yb–O < Tm–O < Er–O < Ho–O (
Figure S9).
Relative scattering intensities, S
h, for the ν
1 Ln–O breathing modes are also given in
Table 1 and for the definitions of the S
h see ref. [
20]. The small scattering intensity values at 0.0156 to 0.0165 for the ν
1 Ln–O modes of the HRE octahydrates reflect the fact that the Ln–OH
2 bonds possess low polarizability and are hard cations [
25]. The accuracy of the scattering coefficient is not better than ± 0.0004 due to the low scattering intensity, the broadness of the modes and the uncertainties in subtracting the baseline. (Note that the S
h value for the totally symmetric stretching mode, ν
1Lu–O is 0.0156 ± 0.0004 and the value reported in [
17] is too small.)
From ab initio quantum mechanical charge field molecular dynamics studies, the mean Ln–O bond distances (Ln = Ho
3+, Er
3+, Tm
3+, Yb
3+ and Lu
3+) of the octahydrates, [Ln(OH
2)
8]
3+, average coordination numbers, vibrational frequencies and the corresponding force constants were presented [
13]. The authors claimed an “excellent agreement with experimental results” [
13] of the computed frequencies with the measured ones in the glassy state [
26]. The theoretical force constants for the ν
1 breathing modes in [
13] deviate considerably from our data in
Table 1and do not follow the expected trend given in
Figure S9 in going from holmium to lutetium. This trend reflects the steady increase of the force constants of the Ln–O breathing modes with decreasing Ln–O bond distances in going from holmium to lutetium (
Table 1;
Figure S9). The force constant for ν
1 Er–OH
2 breathing mode in [
13] was given 360 cm
−1 equal to the one for the ν
1 La–OH
2 breathing mode of [LaOH
2)
9]
3+. However, our recently published datum for the ν
1 La–OH
2 breathing mode [
14,
15] is with 343 cm
−1 much smaller. The calculations in [
13] are based on the simplified model of a heteronuclear diatomic species but such an assumption may not be correct. The character of the symmetrical normal mode ν
1 of the LnO
8 skeleton of the corresponding [Ln(OH
2)
8]
3+ species reveals that the central cations remain stationary and only the water molecules are involved in the breathing motion without disturbing the symmetry and therefore these normal modes are totally polarized.
It is known from kinetic studies [
24,
27,
28] that the water exchange reactions of the [Ln(OH
2)
8]
3+ species for the octahydrates are very fast and these ions are known to be labile. From the rate constants,
kex at 25 °C, given in
Table 1, follow the water residence times, the time the water molecules reside at these cations. The water residence times are in the range of several nanoseconds (see
Table 1) which shows that these ions are indeed quite labile. From the vibration periods of the ν
1 Lu–O modes which are at 0.086 to 0.084 ps in going from holmium(III) to lutetium(III) it follows that these species vibrate several hundred thousand times [
17] before one water exchange occurs. Although the HRE ions are labile structures, Raman spectroscopy probes the actual structure of these octahydrate species. (It is worth mentioning that the intramolecular bond exchange rate is only a few picoseconds, much faster than the water exchange reaction, therefore for such labile structures for instance [Cu(OH
2)
5]
2+ [
27], Raman observes an average structure and, so, a single broad mode appears as a result and at higher peak positions than for comparable divalent metal ions.)
3.2. YbCl3 Solutions
From thermodynamic measurements, it is known that Lu
3+ and Yb
3+ form weak chloro-complexes/ion-pairs [
29,
30,
31,
32]. As a model system for the HRE ion hydrates, aqueous YbCl
3 solutions were investigated. The polarized, depolarized and isotropic Raman scattering profiles of a 3.224 mol·L
−1 YbCl
3 solution compared to a solution at 0.802 mol·L
−1 are presented in
Figure 5. Furthermore, the isotropic scattering profiles of three YbCl
3(aq) solutions at 3.224 mol·L
−1 (R
w = 15.64), 1.600 mol·L
−1 (R
w = 33.06) and 0.802 mol·L
−1 (R
w = 67.55) from 55–700 cm
−1 are given in
Figure 6. Two YbCl
3 solutions in heavy water were also investigated at 0.422 mol·L
−1 and at 0.844 mol·L
−1 and the overview Raman scattering spectra of a 0.422 mol·L
−1 YbCl
3 solution in D
2O is given in
Figure S10. In dilute YbCl
3(aq), the Yb
3+ ion is fully hydrated indicated by the ν
1Yb–OH
2 mode at 394 cm
−1 for the [Yb(OH
2)
8]
3+ species while in dilute YbCl
3 solution in heavy water at 0.422 mol·L
−1 (see
Figure S10), the ν
1Yb–OD
2 mode of [Yb(OD
2)
8]
3+ appears at 376 cm
−1 due to the vibrational isotope effect (see discussion further above; H
2O/D
2O are considered point masses).
The ν
1 Yb–OH
2 stretching mode in the 3.224 mol·L
−1 YbCl
3(aq) solution, with a mole ratio of solute to water at 1 to 15.64 appears at 389 cm
−1 and shifts with dilution to higher frequencies (see
Figure 5). In a 0.400 mol·L
−1 (R
w = 136.98) YbCl
3(aq) solution the ν
1 Yb–OH
2 breathing mode appears at 394 cm
−1 with a fwhh at 52 cm
−1 and these band parameters are comparable to the ones in a dilute Yb(ClO
4)
3(aq) solution in which the fully hydrated [Yb(OH
2)
8]
3+ exists.
A broad isotropic component at 206 cm
−1 and a broad feature at 256 cm
−1 are also observed. The band at 256 cm
−1 is due to the partially hydrated water molecules of the [Yb(OH
2)
7Cl]
2+ species. A Yb-Cl stretching mode should appear at much higher frequencies, namely at ~500 cm
−1, but may be very broad and weak and could not be observed (see spectroscopic and DFT results on ZnCl
2(aq) [
33]). The isotropic component at 206 cm
−1 is assigned to the restricted translation band of water of its O-H∙∙∙O/Cl
− units. These findings are evidence that Cl
− substitutes water from the first hydration shell of Yb
3+ and a partially hydrated Yb
3+- chloro-complex formulated as [Yb(OH
2)
7Cl]
2+ is formed.
The integrated band intensities of ν
1YbO
8 band of the fully hydrated species, [Yb(OH
2)
8]
3+, as a function of concentration was determined from quantitative Raman analysis and it turned out that the integrated band intensity, A
394, does not increase linearly with the total YbCl
3 concentration (C
T). However, a linear increase in band intensity would be expected if the Yb
3+ - octahydrate is the only stable species in YbCl
3 solution and such a linear relationship was observed in Yb(ClO
4)
3(aq) solutions (see
Figure S3). The measured integrated band intensity of the ν
1 YbO
8 band in YbCl
3 (aq), A
394, follows a linear relationship between A
394 and C
T up to ~0.4 mol·L
−1 but then levels off noticeably at higher YbCl
3 concentrations (
Figure S11). Obviously, above ~0.4 mol·L
−1 YbCl
3 fractions of the fully hydrated Yb
3+ (aq) are converted to a 1:1 Yb
3+ chloro-complex species. The existence of higher chloro complexes than 1:1 can be convincingly ruled out taking into account the results of earlier anion exchange studies on aqueous rare earth chloride systems [
32]. The mole fractions of both species are plotted in
Figure 7. The fraction of the chloro-complex at 29%, in the most concentrated solution, is rather small and the fully hydrated species at 71% is still dominant. With dilution, the fraction of the chloro-complex species diminishes quickly and at ~0.4 mol·L
−1 it is zero.
Shown (
Figure 5) is the ν
1YbO
8 symmetric stretching mode at 394 cm
−1 in a 0.802 mol·L
−1 solution compared to the one at 389 cm
−1 in a 3.224 mol·L
−1 solution. An additional isotropic band appears at 256 cm
−1 in the 3.224 mol·L
−1 solution which is due to the stretching mode of the chloro-complex species, [Yb(OH
2)
7Cl]
2+ (details in R
iso scattering in the terahertz region see
Figure 6). The remaining bands in both panels are due to the water being strongly influenced by the solute at the most concentrated solution. First, in the terahertz region (R
VV scattering), weak, broad bands appear at 186 cm
−1 in the 0.802 mol·L
−1 solution and at 202 cm
−1 in the 3.224 mol·L
−1 solution assigned to the restricted translational band of water of the O-H∙∙∙O/Cl
− units. Second, very broad bands (R
VV scattering) with peak maxima which appear at 712 cm
−1 (0.802 mol·L
−1) and 684 cm
−1 (3.224 mol·L
−1) are due to the librational bands of water. Third, the band at 1272 (0.802 mol·L
−1) and 1204 cm
−1 (3.224 mol·L
−1) are due to overtones of water librations. Finally, the bands at 1645 and 1647 cm
−1 respectively are due to the deformation mode of water, ν
2 H
2O.
The formation of a 1:1 complex with Cl
− at higher YbCl
3 concentrations may be written as:
The formation constant for the 1:1 Yb
3+-chloro-complex, K
1, may be formulated according to Equation (6):
with K
1′ the “concentration quotient” we get:
The concentration quotient can be measured by Raman spectroscopy according to equation (7):
where C
T is the total YbCl
3 concentration and the concentrations in brackets denote the equilibrium concentrations of the fully hydrated Yb
3+ and Cl
−. The equilibrium concentration of Yb
3+ determined by Raman spectroscopy allows us to calculate
.
The estimated K
1 value for chloro complex formation in YbCl
3(aq) from
(see ref [
17] for details) equal to ca. 0.06 ± 0.015 and a logK
1 value at ca. −1.22 follows at 22 °C. (Quantitative Raman spectroscopy applied to these solution spectra with weak and broad low frequency bands is not very precise and therefore a higher uncertainty results.) Data from thermodynamic and spectroscopic studies on YbCl
3(aq) solutions confirm the weak nature of the complex species [
29,
30,
31,
32]. The results on aqueous LuCl
3 solutions and similar rare earth systems [
17,
29,
30,
31,
32] confirm our findings on YbCl
3 solutions. The chloride ion substitutes a water molecule from the flexible first hydration shell of Lu
3+ and Yb
3+. With dilution, the weak chloro-complex species dissociates and fully hydrated Yb
3+(aq) ions detected. This is in contrast to AlCl
3(aq) solutions, even in concentrated AlCl
3(aq), Cl
− does not substitute water in the first hydration shell of Al
3+ and it is known that the hydration shell of [Al(OH
2)
6]
3+ is quite inert [
22,
23].
The results of an extensive EXAFS study by Allen and co-workers [
34] on 0.1 and 0.01 mol·L
−1 Lu
3+-, Yb
3+ and Tm
3+- solutions in 0.20 mol·L
−1 HCl and with 14 mol·L
−1 LiCl are worthwhile to consider. It could be shown that in solutions with low chloride concentrations, the Ln–O bond distance for Yb
3+ is consistent with the fully hydrated Yb
3+. In solutions with an excess of LiCl, it was demonstrated that inner sphere chloro-complexation takes place together with a loss of water [
34]. Furthermore, a current study in the terahertz frequency range of YbCl
3 solutions using FT-IR spectroscopy [
35] confirmed weak contact ion pairs as do our recent Raman results on LuCl
3(aq) [
17]. Choppin and Unrein [
36] claimed that only outer-sphere ions pairs exist in lanthanide chloride solutions, but such a view has been questioned [
17,
29,
30,
31,
32].
To summarize, the [Yb(OH
2)
7Cl]
2+ modes in chloride solutions could be detected and formation of weak chloro-complexes with Yb
3+ verified. In dilute solutions (C
T < 0.4 mol·L
−1) the chloro-complex species disappeared upon dilution and [Yb(OH
2)
8]
3+ and Cl
− (aq) formed. The chloro-complex formation may be one reason for the data scatter of the recently published Yb–O bond distances and coordination numbers presented for Yb
3+(aq) and other rare earth chloride systems [
35,
36]. In recent experimental structural studies, it was observed that inner-sphere chloro-complex species are formed in aqueous LnCl
3 solution (Ln = Lu and Yb) with high chloride concentrations while in dilute solutions, fully hydrated ions exist [
17,
34,
35].