Optimization of Transesterification Reactions with CLEA-Immobilized Feruloyl Esterases from Thermothelomyces thermophila and Talaromyces wortmannii
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Organic Solvents on the Transesterification Performance of FAEs CLEAs
2.2. Comparison of the Synthetic Performance of CLEAs with the Corresponding Free Enzymes
2.3. Operational Stability of CLEAs in the Synthesis of Prenyl Ferulate in Acetone
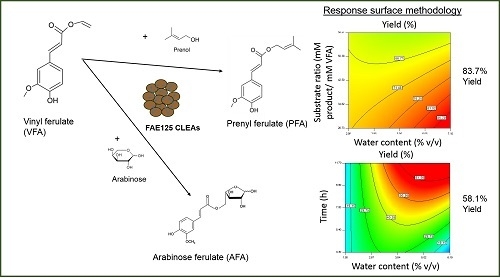
2.4. Response Surface Methodology for Optimization of Transesterification Yield and Selectivity
2.4.1. Prenyl Ferulate Synthesis
2.4.2. Arabinose Ferulate Synthesis
2.5. Operational Stability of CLEAs in the Optimum Transesterification Conditions
2.6. Effect of the Transesterification Reactions on the Structure of CLEAs
3. Materials and Methods
3.1. Materials
3.2. Enzymes
3.3. Enzyme Activities
3.4. Transesterification Reactions
3.5. CLEA Preparations
3.6. Central Composite Design
3.7. Statistical Analysis
3.8. Structure of CLEAs
3.9. Operational Stability of CLEAs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Topakas, E.; Vafiadi, C.; Christakopoulos, P. Microbial production, characterization and applications of feruloyl esterases. Process. Biochem. 2007, 42, 497–509. [Google Scholar] [CrossRef]
- Priefert, H.; Rabenhorst, J.; Steinbuchel, A. Biotechnological production of vanillin. Appl. Microbiol. Biotechnol. 2001, 56, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Rosazza, J.; Huang, Z.; Dostal, L.; Volm, T.; Rosseau, B. Review: Biocatalytic transformation of ferulic acid: An abundant aromatic natural product. J. Ind. Microbiol. 1995, 15, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, I.; Leonov, L.; Jütten, P.; Cerullo, G.; Faraco, V.; Papadopoulou, A.; Kletsas, D.; Ralli, M.; Rova, U.; Christakopoulos, P. Optimized synthesis of novel prenyl ferulate performed by feruloyl esterases from Myceliophthora thermophila in microemulsions. Appl. Microbiol. Biotechnol. 2017, 101, 3213–3226. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.; St-Louis, R.; Karboune, S. Optimization of feruloyl esterase-catalyzed synthesis of feruloylated oligosaccharides by response surface methodology. J. Mol. Catal. B Enzym. 2011, 73, 53–62. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Papadopoulou, A.; Iancu, L.; Cerullo, G.; Ralli, M.; Jütten, P.; Piechot, A.; Faraco, V.; Kletsas, D.; Rova, U.; et al. Optimization of enzymatic synthesis of l-arabinose ferulate catalyzed by feruloyl esterases from Myceliophthora thermophila in detergentless microemulsions and assessment of its antioxidant and cytotoxicity activities. Process. Biochem. 2017, 65, 100–108. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, H.H.; Chen, Y.K.; Chang, H.C.; Lin, P.Y.; Pan, I.H.; Chen, D.Y.; Chen, C.M.; Lin, S.Y. Rice bran feruloylated oligosaccharides activate dendritic cells via Toll-like receptor 2 and 4 signaling. Molecules 2014, 19, 5325–5347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Yao, S.; Ou, S.Y. Maillard volatiles in baked products as affected by feruloylated oligosaccharides from maize bran. Int J. Food Prop. 2017, 20, 3266–3273. [Google Scholar] [CrossRef] [Green Version]
- Mastihubova, M.; Mastihuba, V.; Bilanicova, D.; Borekova, M. Commercial enzyme preparations catalyse feruloylation of glycosides. J. Mol. Catal. B Enzym. 2006, 38, 54–57. [Google Scholar] [CrossRef]
- Vafiadi, C.; Topakas, E.; Christakopoulos, P. Preparation of multipurpose cross-linked enzyme aggregates and their application to production of alkyl ferulates. J. Mol. Catal. B Enzym. 2008, 54, 35–41. [Google Scholar] [CrossRef]
- Thörn, C.; Gustafsson, H.; Olsson, L. Immobilization of feruloyl esterases in mesoporous materials leads to improved transesterification yield. J. Mol. Catal. B Enzym. 2011, 72, 57–64. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEAs): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Cross-Linked Enzyme Aggregates as Industrial Biocatalysts. Org. Process. Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Yan, J.; Gui, X.; Wang, G.; Yan, Y. Improving stability and activity of cross-linked enzyme aggregates based on polyethylenimine in hydrolysis of fish oil for enrichment of polyunsaturated fatty acids. Appl. Biochem. Biotechnol. 2012, 166, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Bhatti, H.N.; Bilal, M.; Asgher, M. Cross-linked enzyme aggregates (CLEAs) of Pencillium notatum lipase enzyme with improved activity, stability and reusability characteristics. Int. J. Biol. Macromol. 2016, 91, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- De Winter, K.; Soetaert, W.; Desmet, T. An imprinted cross-linked enzyme aggregate (iCLEA) of sucrose phosphorylase: Combining improved stability with altered specificity. Int. J. Mol. Sci. 2012, 13, 11333–11342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerva, A.; Antonopoulou, I.; Enman, J.; Iancu, L.; Rova, U.; Christakopoulos, P. Cross-Linked Enzyme Aggregates of Feruloyl Esterase Preparations from Thermothelomyces thermophila and Talaromyces wortmannii. Catalysts 2018, 8, 208. [Google Scholar] [CrossRef]
- Furutani, T.; Su, R.; Ooshima, H.; Kato, J. Simple screening method for lipase for transesterification in organic solvent. Enzyme Microb. Technol. 1995, 17, 1067–1072. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Iancu, L.; Jütten, P.; Piechot, A.; Rova, U.; Christakopoulos, P. Screening of novel feruloyl esterases from Talaromyces wortmannii for the development of efficient and sustainable syntheses of feruloyl derivatives. Enzyme Microb. Technol. 2018, in press. [Google Scholar] [CrossRef]
- Shah, S.; Sharma, A.; Gupta, M.N. Preparation of cross-linked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal. Biochem. 2006, 351, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Kim, H.K. Transesterification using the cross-linked enzyme aggregate of Photobacterium lipolyticum lipase M37. J. Microbiol. Biotechnol. 2011, 21, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Schroeck, A.M.; Schober, S.; Mittelbach, M. Highly active biocatalyst for transesterification: Cross linked enzyme aggregates of Thermomyces lanuginosus and Candida antarctica B. Eur. J. Lipid Sci. Technol. 2013, 115, 1164–1172. [Google Scholar] [CrossRef]
- Vafiadi, C.; Topakas, E.; Nahmias, V.R.; Faulds, C.B.; Christakopoulos, P. Feruloyl esterase-catalysed synthesis of glycerol sinapate using ionic liquids mixtures. J. Biotechnol. 2009, 139, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Topakas, E.; Vafiadi, C.; Stamatis, H.; Christakopoulos, P. Sporotrichum thermophile type C feruloyl esterase (StFaeC): Purification, characterization, and its use for phenolic acid (sugar) ester synthesis. Enzyme Microb. Technol. 2005, 36, 729–736. [Google Scholar] [CrossRef]
- Giuliani, S.; Piana, C.; Setti, L.; Hochkoeppler, A.; Pifferi, P.G.; Williamson, G.; Faulds, C.B. Synthesis of pentylferulate by a feruloyl esterase from Aspergillus niger using water-in-oil microemulsions. Biotechnol. Lett. 2001, 23, 325–330. [Google Scholar] [CrossRef]
- Kühnel, S.; Pouvreau, L.; Appeldoorn, M.M.; Hinz, S.W.; Schols, H.A.; Gruppen, H. The ferulic acid esterases of Chrysosporium lucknowense C1: Purification, characterization and their potential application in biorefinery. Enzyme Microb. Technol. 2012, 50, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Visser, H.; Joosten, V.; Punt, P.J.; Gusakov, A.V.; Olson, P.T.; Joosten, R.; Bartels, J.; Visser, J.; Sinitsyn, A.P.; Emalfarb, M.A.; et al. RESEARCH: Development of a mature fungal technology and production platform for industrial enzymes based on a Myceliophthora thermophila isolate, previously known as Chrysosporium lucknowense C1. Ind. Biotechnol. 2011, 7, 214–223. [Google Scholar] [CrossRef]
- Verdoes, J.C.; Punt, P.J.; Burlingame, R.; Bartels, J.; van Dijk, R.; Slump, E.; Meens, M.; Joosten, R.; Emalfarb, M. ORIGINAL RESEARCH: A dedicated vector for efficient library construction and high throughput screening in the hyphal fungus Chrysosporium lucknowense. Ind. Biotechnol. 2007, 3, 48–57. [Google Scholar] [CrossRef]
- Topakas, E.; Moukouli, M.; Dimarogona, M.; Christakopoulos, P. Expression, characterization and structural modelling of a feruloyl esterase from the thermophilic fungus Myceliophthora thermophila. Appl. Microbiol. Biotechnol. 2012, 94, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Topakas, E.; Stamatis, H.; Biely, P.; Kekos, D.; Macris, B.J.; Christakopoulos, P. Purification and characterization of a feruloyl esterase from Fusarium oxysporum catalyzing esterification of phenolic acids in ternary water-organic solvent mixtures. J. Biotechnol. 2003, 102, 33–44. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Dilokpimol, A.; Iancu, L.; Mäkelä, M.R.; Varriale, S.; Cerullo, G.; Huttner, S.; Uthoff, S.; Jutten, P.; Piechot, A.; et al. The synthetic potential of fungal feruloyl esterase: A correlation with current classification systems and predicted structural properties. Catalysts 2018, 8, 242. [Google Scholar] [CrossRef]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.; van Rantwijk, F.; van der Wielen, L.A.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |








| Prenyl ferulate synthesis | Factor | Name | Units | Minimum | Maximum | −1 | +1 | Mean | Std. Dev. |
| A | Water content | % v/v | 0.00 | 10.00 | 2.90 | 7.10 | 5.00 | 1.98 | |
| B | Substrate ratio | mM prenol/mM VFA | 5.00 | 80.00 | 26.73 | 58.27 | 42.50 | 14.82 | |
| C | Time | h | 1.00 | 48.00 | 14.62 | 34.38 | 24.50 | 9.29 | |
| D | Enzyme load | U/mL | 0.00 | 0.40 | 0.12 | 0.28 | 0.20 | 0.08 | |
| E | Temperature | °C | 25.00 | 50.00 | 32.24 | 42.76 | 37.50 | 4.94 | |
| Arabinose ferulate synthesis | Factor | Name | Units | Minimum | Maximum | −1 | +1 | Mean | Std. Dev. |
| A | Water content | % v/v | 0.31 | 6.19 | 1.50 | 5.00 | 3.25 | 1.52 | |
| B | Substrate ratio | mM arabinose/mM VFA | 1.65 | 5.85 | 2.50 | 5.00 | 3.75 | 1.09 | |
| C | Time | h | 3.30 | 11.70 | 5.00 | 10.00 | 7.50 | 2.18 |
| Source | Response 1: Yield | Response 2: Selectivity | ||||
|---|---|---|---|---|---|---|
| Mean Square | F Value | Probability > F | Mean Square | F Value | Probability > F | |
| Model | 830.41 | 11.95 | <0.0001 | 2.87 | 10.00 | <0.0001 |
| A-Water content | 24.88 | 0.36 | 0.5543 | 0.03 | 0.10 | 0.7574 |
| B-Substrate ratio | 2801.62 | 40.33 | <0.0001 | 11.19 | 39.02 | <0.0001 |
| C-Time | 956.78 | 13.77 | 0.0009 | 3.16 | 11.02 | 0.0025 |
| D-Enzyme load | 1443.55 | 20.78 | <0.0001 | 5.19 | 18.09 | 0.0002 |
| E-Temperature | 5578.21 | 80.30 | <0.0001 | 21.72 | 75.74 | <0.0001 |
| AB | 913.54 | 13.15 | 0.0011 | 1.63 | 5.67 | 0.0243 |
| AC | 6.49 | 0.09 | 0.7622 | 0.03 | 0.10 | 0.7491 |
| AD | 934.61 | 13.45 | 0.0010 | 0.38 | 1.31 | 0.2619 |
| AE | 1.34 | 0.02 | 0.8904 | 0.00 | 0.00 | 0.9663 |
| BC | 8.68 | 0.12 | 0.7264 | 0.19 | 0.65 | 0.4257 |
| BD | 1.22 | 0.02 | 0.8953 | 0.01 | 0.04 | 0.8509 |
| BE | 80.35 | 1.16 | 0.2914 | 0.49 | 1.73 | 0.1996 |
| CD | 12.55 | 0.18 | 0.6740 | 0.08 | 0.29 | 0.5953 |
| CE | 129.43 | 1.86 | 0.1831 | 1.43 | 4.99 | 0.0336 |
| DE | 1187.75 | 17.10 | 0.0003 | 1.90 | 6.62 | 0.0157 |
| A2 | 495.59 | 7.13 | 0.0125 | 1.35 | 4.70 | 0.0388 |
| B2 | 131.07 | 1.89 | 0.1805 | 0.12 | 0.42 | 0.5237 |
| C2 | 399.16 | 5.75 | 0.0234 | 2.79 | 9.72 | 0.0042 |
| D2 | 218.38 | 3.14 | 0.0871 | 0.48 | 1.68 | 0.2053 |
| E2 | 409.62 | 5.90 | 0.0218 | 2.14 | 7.45 | 0.0108 |
| Lack of Fit | 89.19 | 8.65 | 0.0035 | 0.35 | 3.91 | 0.0355 |
| Source | Response 1: Yield | Response 2: Selectivity | ||||
|---|---|---|---|---|---|---|
| Mean Square | F Value | Probability > F | Mean Square | F Value | Probability > F | |
| Model | 653.14 | 7.65 | 0.0029 | 0.75 | 22.52 | <0.0001 |
| A-Water content | 3466.99 | 40.58 | 0.0001 | 1.98 | 59.53 | <0.0001 |
| B-Substrate ratio | 9.75 | 0.11 | 0.7432 | 0.00 | 0.06 | 0.8107 |
| C-Time | 599.00 | 7.01 | 0.0266 | 0.00 | 0.01 | 0.9196 |
| AB | 79.66 | 0.93 | 0.3595 | 1.67 | 50.19 | <0.0001 |
| AC | 446.82 | 5.23 | 0.0480 | 1.72 | 51.60 | <0.0001 |
| BC | 119.53 | 1.40 | 0.2672 | 1.88 | 56.57 | <0.0001 |
| A2 | 1503.48 | 17.60 | 0.0023 | 1.08 | 32.28 | 0.0003 |
| B2 | 5.36 | 0.06 | 0.8078 | 0.01 | 0.16 | 0.7018 |
| C2 | 79.55 | 0.93 | 0.3598 | 0.02 | 0.54 | 0.4830 |
| Lack of Fit | 175.59 | 13.20 | 0.01 | 0.07 | 111.54 | <0.0001 |
| Summary of the Obtained Results | PFA Synthesis | AFA Synthesis |
|---|---|---|
| Optimized conditions | ||
| Water content (%) | 7.1 | 5.0 |
| Substrate ratio (mM acceptor/mM VFA) | 26.7 | 5.0 |
| Enzyme concentration (U/mL) | 0.116 | 0.04 |
| Temperature (°C) | 32 | 32 |
| Time (h) | 34.38 | 10.0 |
| Predicted responses | ||
| Product yield (%) | 83.1 | 60.09 |
| Selectivity (mM product/mM FA) | 5.6 | 2.83 |
| Obtained parameters | ||
| Product concentration (mM) | 50.25 ± 0.48 | 46.15 ± 1.4 |
| Product yield (%) | 83.74 ± 0.8 | 58.14 ± 1.75 |
| Overall yield (%) | 99.32 ± 0.1 | 97.47 ± 0.9 |
| Rate (mol product/ U FAE L h) | 74.46 ± 0.7 | 140.95 ± 4.24 |
| Selectivity (mM product /mM FA) | 5.38 ± 0.29 | 1.48 ± 0.08 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerva, A.; Antonopoulou, I.; Enman, J.; Iancu, L.; Jütten, P.; Rova, U.; Christakopoulos, P. Optimization of Transesterification Reactions with CLEA-Immobilized Feruloyl Esterases from Thermothelomyces thermophila and Talaromyces wortmannii. Molecules 2018, 23, 2403. https://doi.org/10.3390/molecules23092403
Zerva A, Antonopoulou I, Enman J, Iancu L, Jütten P, Rova U, Christakopoulos P. Optimization of Transesterification Reactions with CLEA-Immobilized Feruloyl Esterases from Thermothelomyces thermophila and Talaromyces wortmannii. Molecules. 2018; 23(9):2403. https://doi.org/10.3390/molecules23092403
Chicago/Turabian StyleZerva, Anastasia, Io Antonopoulou, Josefine Enman, Laura Iancu, Peter Jütten, Ulrika Rova, and Paul Christakopoulos. 2018. "Optimization of Transesterification Reactions with CLEA-Immobilized Feruloyl Esterases from Thermothelomyces thermophila and Talaromyces wortmannii" Molecules 23, no. 9: 2403. https://doi.org/10.3390/molecules23092403