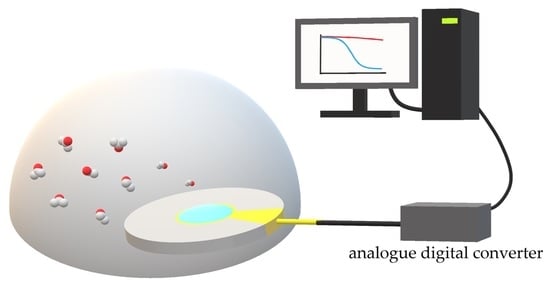
Affinity Ionic Liquids for Chemoselective Gas Sensing
Abstract
:1. Introduction
2. Gas Sensing Methods
2.1. Spectroscopic Methods
2.2. Spectrometric Methods
2.3. Conductivity Based Methods
2.3.1. Metal Organic Semiconductors
2.3.2. Metal Organic Framework
2.3.3. Organic Conducting Polymer
2.4. Piezoelectric Methods
2.4.1. Surface Acoustic Wave
2.4.2. Bulk Acoustic Wave
3. Ionic Liquids and Its Use for Adsorption Analysis of Gases on QCM
4. Ionic Liquid on QCM for Chemoselective Gas Sensing
4.1. Chemoselective Sensing of Aldehyde and Ketone Gases
4.2. Chemoselective Sensing of Amine Gases
4.3. Chemoselective Sensing of Azide Gases
4.4. Chemoselective Sensing of Alkene Gases
4.5. Chemoselective Sensing of Chemical Warfare Agent Mimics
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tassopoulos, C.N.; Barnett, D.; Russell Fraser, T. Breath-acetone and blood-sugar measurements in diabetes. Lancet 1969, 293, 1282–1286. [Google Scholar] [CrossRef]
- Teranishi, R.; Mon, T.R.; Robinson, A.B.; Cary, P.; Pauling, L. Gas chromatography of volatiles from breath and urine. Anal. Chem. 1972, 44, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Spanel, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, R.; Santonico, M.; Valentini, C.; Sedda, G.; Borri, A.; Petrella, F.; Maisonneuve, P.; Pennazza, G.; D’Amico, A.; Di Natale, C.; et al. Volatile signature for the early diagnosis of lung cancer. J. Breath Res. 2016, 10, 016007. [Google Scholar] [CrossRef] [PubMed]
- Nardi-Agmon, I.; Peled, N. Exhaled breath analysis for the early detection of lung cancer: Recent developments and future prospects. Lung Cancer Targets Ther. 2017, 8, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Zhu, Y.; Liu, H. Detection of volatile organic compounds in exhaled breath to screen lung cancer: A systematic review. Future Oncol. 2018, 14, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, R.; Romano, R.; Sedda, G.; Borri, A.; Petrella, F.; Galetta, D.; Casiraghi, M.; Spaggiari, L. Diagnostic biomarkers for lung cancer prevention. J. Breath Res. 2018, 12, 027111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaney, L.M.; Lindley, M.R. Translation of exhaled breath volatile analyses to sport and exercise applications. Metabolomics 2017, 13, 139. [Google Scholar] [CrossRef]
- Ali, S.B.; Ghatak, B.; Gupta, S.D.; Debabhuti, N.; Chakraborty, P.; Sharma, P.; Ghosh, A.; Tudu, B.; Mitra, S.; Sarkar, M.P.; et al. Detection of 3-carene in mango using a quartz crystal microbalance sensor. Sens. Actuator B 2016, 230, 791–800. [Google Scholar] [CrossRef]
- Ali, S.B.; Ghatak, B.; Debabhuti, N.; Sharma, P.; Ghosh, A.; Tudu, B.; Bhattacharya, N.; Bandyopadhyay, R. Detection of β-caryophyllene in mango using a quartz crystal microbalance sensor. Sens. Actuator B 2018, 255, 3064–3073. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses: Powerful tools in meat quality assessment. Meat Sci. 2017, 131, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Farneti, B.; Alarcón, A.A.; Cristescu, S.M.; Costa, G.; Harren, F.J.M.; Holthuysen, N.T.E.; Woltering, E.J. Aroma volatile release kinetics of tomato genotypes measured by ptr-ms following artificial chewing. Food Res. Int. 2013, 54, 1579–1588. [Google Scholar] [CrossRef]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuator B 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Fonollosa, J.; Solórzano, A.; Marco, S. Chemical sensor systems and associated algorithms for fire detection: A review. Sensors 2018, 18, 553. [Google Scholar] [CrossRef] [PubMed]
- Schütze, A.; Reimann, P. Fire detection in coal mines based on semiconductor gas sensors. Sens. Rev. 2012, 32, 47–58. [Google Scholar]
- Lefferts, M.J.; Castell, M.R. Vapour sensing of explosive materials. Anal. Methods 2015, 7, 9005–9017. [Google Scholar] [CrossRef]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dweik, R.A.; Amann, A. Exhaled breath analysis: The new frontier in medical testing. J. Breath Res. 2008, 2, 030301. [Google Scholar] [CrossRef] [PubMed]
- American Chemical Society National Historic Chemical Landmarks. The Keeling Curve. Available online: http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/keeling-curve.html (accessed on 14 August 2018).
- Pauling, L.; Robinson, A.B.; Teranishi, R.; Cary, P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc. Natl. Acad. Sci. USA 1971, 68, 2374. [Google Scholar] [CrossRef] [PubMed]
- Timofeyev, Y.; Virolainen, Y.; Makarova, M.; Poberovsky, A.; Polyakov, A.; Ionov, D.; Osipov, S.; Imhasin, H. Ground-based spectroscopic measurements of atmospheric gas composition near saint petersburg (russia). J. Mol. Spectrosc. 2016, 323, 2–14. [Google Scholar] [CrossRef]
- Stockwell, C.E.; Yokelson, R.J.; Kreidenweis, S.M.; Robinson, A.L.; DeMott, P.J.; Sullivan, R.C.; Reardon, J.; Ryan, K.C.; Griffith, D.W.T.; Stevens, L. Trace gas emissions from combustion of peat, crop residue, domestic biofuels, grasses and other fuels: Configuration and fourier transform infrared (FTIR) component of the fourth fire lab at missoula experiment (FLAME-4). Atmos. Chem. Phys. 2014, 14, 9727–9754. [Google Scholar] [CrossRef]
- Platt, U.; Stutz, J. Evaluation of DOAS Spectra, Sensitivity, and Detection Limits. In Differential Absorption Spectroscopy: Principles and Applications; Platt, U., Stutz, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 287–328. ISBN 978-3-540-21193-8. [Google Scholar]
- Xu, M.; Tang, Z.; Duan, Y.; Liu, Y. GC-based techniques for breath analysis: Current status, challenges and prospects. Crit. Rev. Anal. Chem. 2016, 46, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Belardi, R.P.; Pawliszyn, J.B. Application of chemically modified fused silica fibers in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Poll. Res. J. Can. 1989, 24, 179–191. [Google Scholar]
- Devasurendra, A.M.; Zhang, C.; Young, J.A.; Viranga Tillekeratne, L.M.; Anderson, J.L.; Kirchhoff, J.R. Electropolymerized pyrrole-based conductive polymeric ionic liquids and their application for solid-phase microextraction. ACS Appl. Mater. Interfaces 2017, 9, 24955–24963. [Google Scholar] [CrossRef] [PubMed]
- Young, J.A.; Zhang, C.; Devasurendra, A.M.; Viranga Tillekeratne, L.M.; Anderson, J.L.; Kirchhoff, J.R. Conductive polymeric ionic liquids for electroanalysis and solid-phase microextraction. Anal. Chim. Acta 2016, 910, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doran, G.S.; Deans, R.; De Filippis, C.; Kostakis, C.; Howitt, J.A. Air quality inside police drug safes and drug storage areas. J. Anal. Toxicol. 2018, 42, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.-P.; Mwakikunga, B. Sensing technologies for detection of acetone in human breath for diabetes diagnosis and monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Dryahina, K.; Smith, D.; Španěl, P. Quantification of volatile compounds released by roasted coffee by selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Spanel, P.; Smith, D. Quantification of trace levels of the potential cancer biomarkers formaldehyde, acetaldehyde and propanol in breath by sift-ms. J. Breath Res. 2008, 2, 046003. [Google Scholar] [CrossRef] [PubMed]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-transfer reaction mass spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, T.; Wojnowski, W.; Lubinska-Szczygeł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T. PTR-MS and GC-MS as complementary techniques for analysis of volatiles: A tutorial review. Anal. Chim. Acta 2018. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Liu, S.-B.; Meng, F.-L.; Liu, J.-Y.; Jin, Z.; Kong, L.-T.; Liu, J.-H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.; Bandyopadhyay, R.; Bhattacharyya, N.; Pandey, R.A.; Jana, A. Application of electronic nose for industrial odors and gaseous emissions measurement and monitoring—An overview. Talanta 2015, 144, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kanan, M.S.; El-Kadri, M.O.; Abu-Yousef, A.I.; Kanan, C.M. Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 2009, 9, 8158–8196. [Google Scholar] [CrossRef] [PubMed]
- Schaller, E.; Bosset, J.O.; Escher, F. ‘Electronic noses’ and their application to food. LWT-Food Sci. Technol. 1998, 31, 305–316. [Google Scholar] [CrossRef]
- Núñez Carmona, E.; Sberveglieri, V.; Ponzoni, A.; Galstyan, V.; Zappa, D.; Pulvirenti, A.; Comini, E. Detection of food and skin pathogen microbiota by means of an electronic nose based on metal oxide chemiresistors. Sens. Actuator B 2017, 238, 1224–1230. [Google Scholar] [CrossRef]
- Poloju, M.; Jayababu, N.; Ramana Reddy, M.V. Improved gas sensing performance of al doped zno/cuo nanocomposite based ammonia gas sensor. Mater. Sci. Eng. B 2018, 227, 61–67. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuator B 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal–organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Q.-L.; Zou, R.; Xu, Q. Metal-organic frameworks for energy applications. Chem 2017, 2, 52–80. [Google Scholar] [CrossRef]
- Xu, G.; Nie, P.; Dou, H.; Ding, B.; Li, L.; Zhang, X. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Yi, F.-Y.; Chen, D.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical sensors based on metal–organic frameworks. ChemPlusChem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal–organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef] [PubMed]
- Achmann, S.; Hagen, G.; Kita, J.; Malkowsky, M.I.; Kiener, C.; Moos, R. Metal-organic frameworks for sensing applications in the gas phase. Sensors 2009, 9, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Zhang, Y.; Cong, H.; Fu, B.; Wen, S.; Ruan, S. A novel humidity sensor based on NH2-MIL-125(Ti) metal organic framework with high responsiveness. J. Nanopart. Res. 2013, 15, 2014. [Google Scholar] [CrossRef]
- Guo, K.; Zhao, L.; Yu, S.; Zhou, W.; Li, Z.; Li, G. A water-stable proton-conductive barium(II)-organic framework for ammonia sensing at high humidity. Inorg. Chem. 2018, 57, 7104–7112. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yu, S.; Zhao, L.; Wang, J.; Li, Z.; Li, G. A highly stable two-dimensional copper(II)-organic framework for proton conduction and ammonia impedance sensing. Chem. Eur. J. 2018, 24, 10829–10839. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.-X.; Yang, H.; Zhang, J. Zeolitic imidazolate framework as formaldehyde gas sensor. Inorg. Chem. 2014, 53, 5411–5413. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Liu, S.F.; Swager, T.M.; Dincă, M. Chemiresistive sensor arrays from conductive 2D metal–organic frameworks. J. Am. Chem. Soc. 2015, 137, 13780–13783. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.K.; Jensen, K.E.; Pivak, P.A.; Mirica, K.A. Direct self-assembly of conductive nanorods of metal–organic frameworks into chemiresistive devices on shrinkable polymer films. Chem. Mater. 2016, 28, 5264–5268. [Google Scholar] [CrossRef]
- Campbell, G.M.; Dincă, M. Metal–organic frameworks as active materials in electronic sensor devices. Sensors 2017, 17, 1108. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-B.; Liu, S.-Y.; Ye, J.-W.; Li, X.-Y.; Zhang, J.-P. Photoluminescent metal–organic frameworks for gas sensing. Adv. Sci. 2016, 3, 1500434. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. Trends Anal. Chem. 2015, 73, 39–53. [Google Scholar] [CrossRef]
- Pandey, S. Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: A comprehensive review. J. Sci. Adv. Mater. Dev. 2016, 1, 431–453. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G.Q. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.W. Conducting polyaniline nanowire and its applications in chemiresistive sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, W.; Lieberzeit, P.A. Combining two selection principles: Sensor arrays based on both biomietic recognition and chemometrics. Front Chem. 2018, 6, 268. [Google Scholar] [CrossRef] [PubMed]
- Ballantine, D.S.; Martin, S.J.; Ricco, A.J.; Frye, G.C.; Wohltjen, H.; White, R.M.; Zellers, E.T. Fundamentals of acoustic waves. In Acoustic Wave Sensors; Ballantine, D.S., Martin, S.J., Ricco, A.J., Frye, G.C., Wohltjen, H., White, R.M., Zellers, E.T., Eds.; Academic Press: San Diego, CA, USA, 1997; Chapter 2; pp. 10–35. [Google Scholar]
- Wohltjen, H.; Dessy, R. Surface acoustic wave probe for chemical analysis. I. Introduction and instrument description. Anal. Chem. 1979, 51, 1458–1464. [Google Scholar] [CrossRef]
- Go, D.B.; Atashbar, M.Z.; Ramshani, Z.; Chang, H.-C. Surface acoustic wave devices for chemical sensing and microfluidics: A review and perspective. Anal. Methods 2017, 9, 4112–4134. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Dickert, L.F. Surface acoustic wave (SAW) for chemical sensing applications of recognition layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef] [PubMed]
- Devkota, J.; Ohodnicki, R.P.; Greve, W.D. SAW sensors for chemical vapors and gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, K.V.; Kumar, S.; Swaraj, S.; Neethirajan, S. Advances in biosensors and optical assays for diagnosis and detection of malaria. Biosens. Bioelectron. 2018, 105, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Ly, T.N.; Park, S.; Park, S.J. Detection of HIV-1 antigen by quartz crystal microbalance using gold nanoparticles. Sens. Actuator B 2016, 237, 452–458. [Google Scholar] [CrossRef]
- Arif, S.; Qudsia, S.; Urooj, S.; Chaudry, N.; Arshad, A.; Andleeb, S. Blueprint of quartz crystal microbalance biosensor for early detection of breast cancer through salivary autoantibodies against ATP6AP1. Biosens. Bioelectron. 2015, 65, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, N.L.; Amicizia, D.; Panatto, D.; Tramalloni, D.; Valle, I.; Gasparini, R. Quartz crystal microbalance (QCM) for public health: An overview of its applications. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: San Diego, CA, USA, 2015; Volume 101, pp. 149–211. [Google Scholar]
- Chen, J.Y.; Penn, L.S.; Xi, J. Quartz crystal microbalance: Sensing cell-substrate adhesion and beyond. Biosens. Bioelectron. 2018, 99, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Skládal, P. Piezoelectric biosensors. Trends Anal. Chem. 2016, 79, 127–133. [Google Scholar] [CrossRef]
- Kurosawa, S.; Park, J.-W.; Aizawa, H.; Wakida, S.-I.; Tao, H.; Ishihara, K. Quartz crystal microbalance immunosensors for environmental monitoring. Biosens. Bioelectron. 2006, 22, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Zhang, X.; Tian, Y.; Meng, Y. Progresses on the theory and application of quartz crystal microbalance. Appl. Phys. Rev. 2016, 3, 031106. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von schwingquarzen zur wägung dünner schichten und zur mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Gordon, J.G. Frequency of a quartz microbalance in contact with liquid. Anal. Chem. 1985, 57, 1770–1771. [Google Scholar] [CrossRef]
- Rodahl, M.; Kasemo, B. A simple setup to simultaneously measure the resonant frequency and the absolute dissipation factor of a quartz crystal microbalance. Rev. Sci. Instrum. 1996, 67, 3238–3241. [Google Scholar] [CrossRef]
- Johannsmann, D. Viscoelastic, mechanical and dielectric measurements on complex samples with the quartz crystal microbalance. Phys. Chem. Chem. Phys. 2008, 10, 4516–4534. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Rupp, F.; Wendel, H.P.; Gehring, F.K. Bioapplications of acoustic crystals, a review. Trends Anal. Chem. 2018, 102, 194–209. [Google Scholar] [CrossRef]
- Ma, F.; Rehman, A.; Sims, M.; Zeng, X. Antimicrobial susceptibility assays based on the quantification of bacterial lipopolysaccharides via a label free lectin biosensor. Anal. Chem. 2015, 87, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Shpigel, N.; Levi, M.D.; Sigalov, S.; Girshevitz, O.; Aurbach, D.; Daikhin, L.; Pikma, P.; Marandi, M.; Jänes, A.; Lust, E.; et al. In situ hydrodynamic spectroscopy for structure characterization of porous energy storage electrodes. Nat. Mater. 2016, 15, 570. [Google Scholar] [CrossRef] [PubMed]
- Dargel, V.; Shpigel, N.; Sigalov, S.; Nayak, P.; Levi, M.D.; Daikhin, L.; Aurbach, D. In situ real-time gravimetric and viscoelastic probing of surface films formation on lithium batteries electrodes. Nat. Commun. 2017, 8, 1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi, M.D.; Shpigel, N.; Sigalov, S.; Dargel, V.; Daikhin, L.; Aurbach, D. In situ porous structure characterization of electrodes for energy storage and conversion by EQCM-D: A review. Electrochim. Acta 2017, 232, 271–284. [Google Scholar] [CrossRef]
- Yu, L.; Huang, Y.; Jin, X.; Mason, A.J.; Zeng, X. Ionic liquid thin layer EQCM explosives sensor. Sens. Actuator B 2009, 140, 363–370. [Google Scholar] [CrossRef]
- Emir Diltemiz, S.; Keçili, R.; Ersöz, A.; Say, R. Molecular imprinting technology in quartz crystal microbalance (QCM). Sensors 2017, 17, 454. [Google Scholar] [CrossRef] [PubMed]
- Lay, B.; Kandjani, A.E.; Amin, M.H.; Kabir, K.M.M.; Ippolito, S.J.; Sabri, Y.M.; Bhargava, S.K. Galvanic replacement of colloidal monolayer crystal on a QCM device for selective detection of mercury vapor. Sens. Actuator B 2017, 250, 383–392. [Google Scholar] [CrossRef]
- Öztürk, S.; Kösemen, A.; Şen, Z.; Kılınç, N.; Harbeck, M. Poly(3-methylthiophene) thin films deposited electrochemically on QCMs for the sensing of volatile organic compounds. Sensors 2016, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Ruifen, H.; Kaihuan, Z.; Guokang, F.; Zhiyuan, L.; Guang, L. Development of a high-sensitivity plasticizer sensor based on a quartz crystal microbalance modified with a nanostructured nickel hydroxide film. Meas. Sci. Technol. 2015, 26, 055102. [Google Scholar] [Green Version]
- Deng, F.; Chen, W.; Wang, J.; Wei, Z. Fabrication of a sensor array based on quartz crystal microbalance and the application in egg shelf life evaluation. Sens. Actuator B 2018, 265, 394–402. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, H.; Zhang, Y.; Dong, F.; Li, Z. Cellulose acetate nanofibers coated layer-by-layer with polyethylenimine and graphene oxide on a quartz crystal microbalance for use as a highly sensitive ammonia sensor. Colloids Surf. B 2016, 148, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Niedermeyer, H.; Hallett, J.P.; Villar-Garcia, I.J.; Hunt, P.A.; Welton, T. Mixtures of ionic liquids. Chem. Soc. Rev. 2012, 41, 7780–7802. [Google Scholar] [CrossRef] [PubMed]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.E.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present and future trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Thomas, M.L.; Zhang, S.G.; Ueno, K.; Yasuda, T.; Dokko, K. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qu, J. Ionic liquids as lubricant additives: A review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Wishart, J.F. Energy applications of ionic liquids. Energy Environ. Sci. 2009, 2, 956–961. [Google Scholar] [CrossRef]
- Rehman, A.; Zeng, X. Methods and approaches of utilizing ionic liquids as gas sensing materials. RSC Adv. 2015, 5, 58371–58392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Yuan, C.-Y.; Warmack, R.J.; Barnes, C.E.; Dai, S. Ionic liquids: A new class of sensing materials for detection of organic vapors based on the use of a quartz crystal microbalance. Anal. Chem. 2002, 74, 2172–2176. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yu, L.; Garcia, D.; Ren, R.X.; Zeng, X. Ionic liquid high-temperature gas sensor array. Anal. Chem. 2006, 78, 6980–6989. [Google Scholar] [CrossRef] [PubMed]
- Behera, K.; Pandey, S.; Kadyan, A.; Pandey, S. Ionic liquid-based optical and electrochemical carbon dioxide sensors. Sensors 2015, 15, 30487–30503. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Dao, R.; Zhang, W.; Lv, X.; Li, H.; Wang, C. Designing an anion-functionalized fluorescent ionic liquid as an efficient and reversible turn-off sensor for detecting SO2. Chem. Commun. 2017, 53, 3862–3865. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, T.; Gębicki, J.; Kamysz, W. Prospects of ionic liquids application in electronic and bioelectronic nose instruments. Trends Anal. Chem. 2017, 93, 23–36. [Google Scholar] [CrossRef]
- Gebicki, J. Application of ionic liquids in electronic nose instruments. In Analytical Applications of Ionic Liquids; Koel, M., Ed.; World Scientific: London, UK, 2016; pp. 339–360. [Google Scholar]
- Tseng, M.-C.; Chu, Y.-H. Chemoselective gas sensing ionic liquids. Chem. Commun. 2010, 46, 2983–2985. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Hsu, T.-H.; Chen, C.-Y.; Tseng, M.-C.; Chu, Y.-H. Exploring silver ionic liquids for reaction-based gas sensing on a quartz crystal microbalance. Analyst 2015, 140, 6245–6249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-L.; Tseng, M.-C.; Chu, Y.-H. Sensing ionic liquids for chemoselective detection of acyclic and cyclic ketone gases. Chem. Commun. 2013, 49, 2560–2562. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Chu, Y.-H. Exploiting solvate ionic liquids for amine gas analysis on a quartz crystal microbalance. Anal. Chem. 2017, 89, 5186–5192. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.-C.; Chu, Y.-H. Reaction-based azide gas sensing with tailored ionic liquids measured by quartz crystal microbalance. Anal. Chem. 2014, 86, 1949–1952. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-H.; Chiang, S.-J.; Chu, Y.-H. Quartz crystal microbalance analysis of Diels-Alder reactions of alkene gases to functional ionic liquids on chips. Anal. Chem. 2016, 88, 10837–10841. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Li, K.-H.; Chu, Y.-H. Reaction-based detection of chemical warfare agent mimics with affinity ionic liquids. Anal. Chem. 2018, 90, 8320–8325. [Google Scholar] [CrossRef] [PubMed]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-P.; Liu, W.-C.; Tseng, M.-C.; Chu, Y.-H. Ionic liquids tailored for reaction-based gas sensing on quartz crystal microbalance. Rev. Anal. Chem. 2015, 34, 77–86. [Google Scholar] [CrossRef]






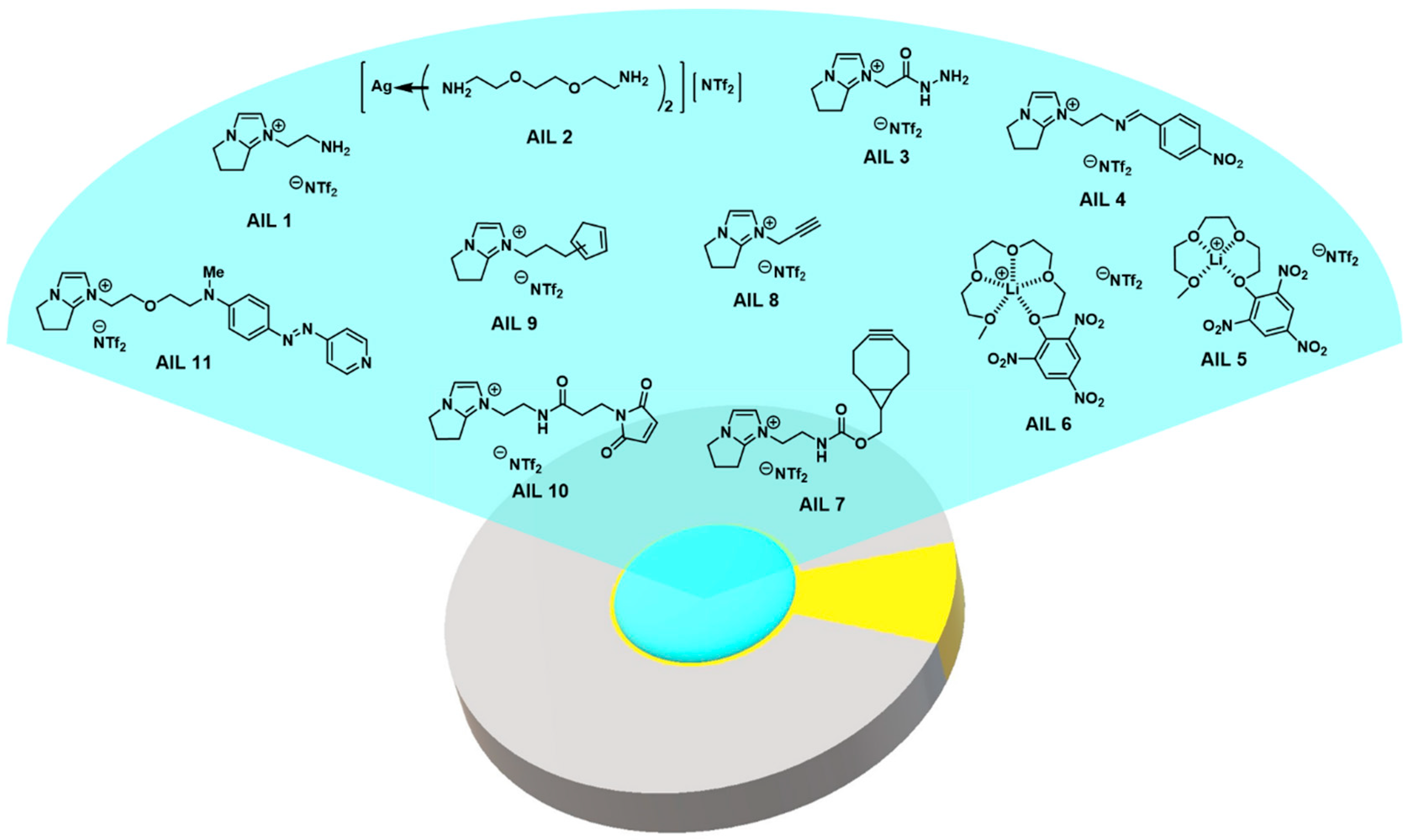






| Ionic Liquid | Target Gas Species | Sensing Mechanism | Sensitivity of Detection | Literature |
|---|---|---|---|---|
| AIL1 | aldehydes; ketones | imination | ΔF = −1.0 Hz: 4.5 ppb 1; 148 ppb 2 | [110] |
| AIL2 | aldehydes | imination | ΔF = −2.0 Hz: 4.6 ppb 3 | [111] |
| AIL3 | acyclic and cyclic ketones | hydrazone adduct formation | ΔF = −1.0 Hz: 0.6 ppb 4 | [112] |
| AIL4 | amines | transamination | ΔF = −1.0 Hz: 2.5 ppb 5 | [110] |
| AIL5 | amines | nucleophilic aromatic addition | ΔF = 10 Hz: 8.0 ppb 6 | [113] |
| AIL6 | amines | nucleophilic aromatic addition | ΔF = 10 Hz: 5.4 ppb 7 | [113] |
| AIL7 | azides | Huisgen 1,3-dipolar [3 + 2] cycloaddition | ΔF = 10 Hz: 5 ppb 8; 35 ppb 9 | [114] |
| AIL8 | control group | inert | inert | [114] |
| AIL9 | dienes | Diels-Alder [4 + 2] cycloaddition | N/A | [115] |
| AIL10 | dienes | Diels-Alder [4 + 2] cycloaddition | ΔF = −1 Hz: 1.5 ppb 10 | [115] |
| AIL11 | CWA mimics | nucleophilic substitution | ΔF = 5 Hz: 20 ppb 11 | [116] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, A.; Li, H.-Y.; Chang, I.-N.; Chu, Y.-H. Affinity Ionic Liquids for Chemoselective Gas Sensing. Molecules 2018, 23, 2380. https://doi.org/10.3390/molecules23092380
Chang A, Li H-Y, Chang I-N, Chu Y-H. Affinity Ionic Liquids for Chemoselective Gas Sensing. Molecules. 2018; 23(9):2380. https://doi.org/10.3390/molecules23092380
Chicago/Turabian StyleChang, Albert, Hsin-Yi Li, I-Nan Chang, and Yen-Ho Chu. 2018. "Affinity Ionic Liquids for Chemoselective Gas Sensing" Molecules 23, no. 9: 2380. https://doi.org/10.3390/molecules23092380