Chemical Constituents from Croton Species and Their Biological Activities
Abstract
:1. Introduction
2. Chemical Constituents
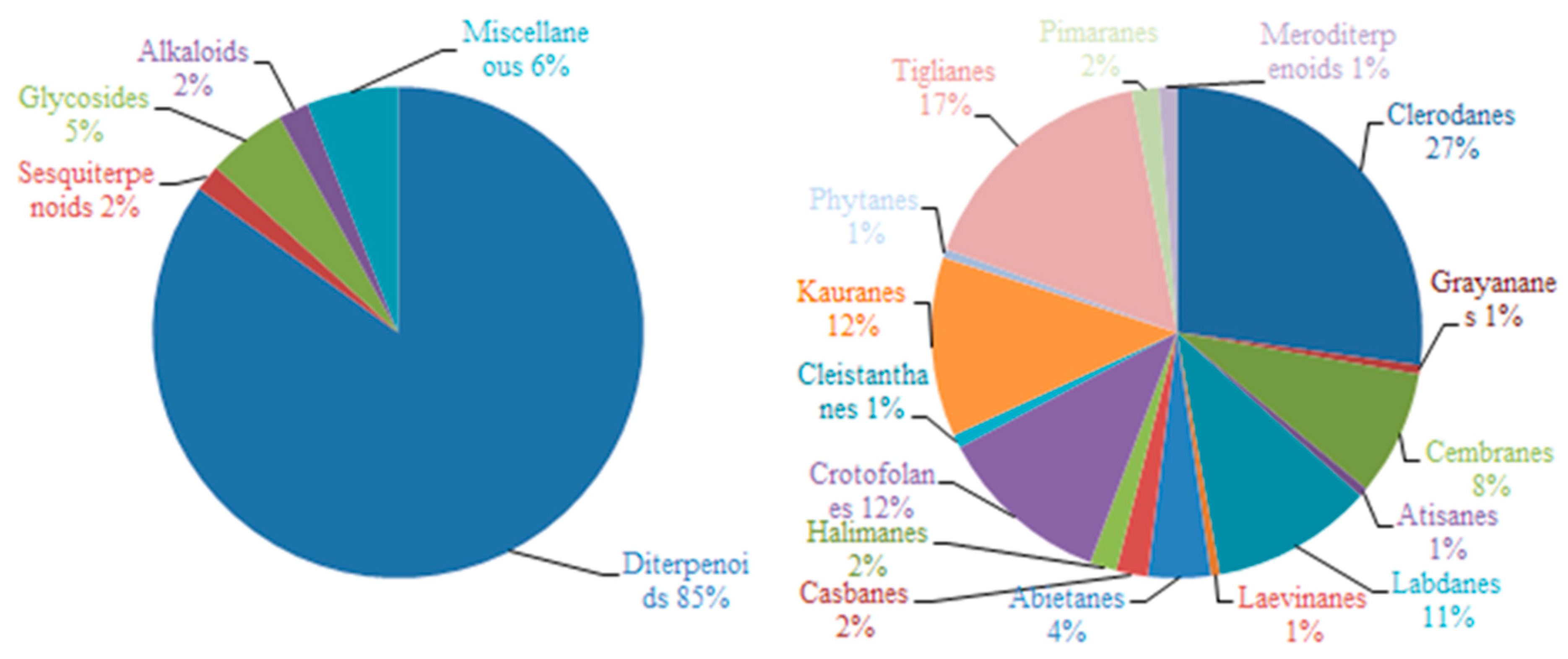
2.1. Diterpenoids
2.1.1. Clerodanes
2.1.2. Tiglianes
2.1.3. Kauranes
2.1.4. Crotofolanes
2.1.5. Labdanes
2.1.6. Cembranes
2.1.7. Abietanes
2.1.8. Casbanes
2.1.9. Halimanes
2.1.10. Pimaranes
2.1.11. Cleistanthanes
2.1.12. Grayananes, Atisanes, Phytanes, Laevinanes and Meroditerpenoids
2.2. Sesquiterpenoids, Sesterterpenoids and Triterpenoids
2.3. Glycosides
2.4. Alkaloids
2.5. Benzoate Derivatives, Pyran-2-One Derivatives, Cyclicpeptides, Tropone Derivatives and Limonoids
2.6. Miscellaneous Compounds
3. Biological Activities
3.1. Cytotoxic Activity
3.2. Anti-Inflammatory Activity
3.3. Antifungal Activity
3.4. Acetylcholinesterase Inhibitory Activity
3.5. Neurite Outgrowth-Promoting Activity
3.6. Other Activities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Júnior, S.F.P.; Conserva, L.M.; Filho, J.M.B. Clerodane diterpenes from Croton species: Distribution and a compilation of their 13C-NMR spectral data. Nat. Prod. Commun. 2006, 1, 319–344. [Google Scholar]
- Wu, X.A.; Zhao, Y.M. Advance on chemical composition and pharmacological action of Croton L. Nat. Prod. Res. Dev. 2004, 16, 467–472. [Google Scholar]
- Nath, R.; Roy, S.; De, B.; Choudhury, M.D. Anticancer and antioxidant activity of Croton: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 63–70. [Google Scholar]
- Premprasert, C.; Tewtrakul, S.; Plubrukarn, A.; Wungsintaweekul, J. Anti-inflammatory activity of diterpenes from Croton stellatopilosus on LPS-induced RAW264. 7 cells. J. Nat. Med. 2013, 67, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. Ethnopharmacological uses, phytochemistry, and pharmacological properties of Croton macrostachyus Hochst. Ex Delile: A Comprehensive Review. Evid Based. Compl. Alt. 2017, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. Ethnomedicinal uses and pharmacological activities of Croton megalobotrys Müll Arg: A systematic review. Trop. J. Pharm. Res. 2017, 16, 2535–2543. [Google Scholar]
- Maroyi, A. Traditional usage, phytochemistry and pharmacology of Croton sylvaticus Hochst. ex C. Krauss. Asian Pac. J. Trop. Med. 2017, 10, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Chaudhuri, T.K. Pharmacological aspect of Croton bonplandianus Baill: A comprehensive review. J. Pharmacogn. Phytochem. 2018, 7, 811–813. [Google Scholar]
- Ghosh, T.; Biswas, M.K.; Roy, P.; Guin, C. A review on traditional and pharmacological uses of Croton bonplandianum with special reference to phytochemical aspect. Eur. J. Med. Plant. 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschke, S.L.; Lee, K.H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef] [PubMed]
- Ndunda, B.; Langat, M.K.; Midiwo, J.O.; Omosa, L.K. Diterpenoid derivatives of Kenyan croton sylvaticus. Nat. Prod. Commun. 2015, 10, 557–578. [Google Scholar] [PubMed]
- Zou, G.A.; Zhang, H.W.; Aisa, H.A.; Yang, J.S.; Peng, C.Z.; Zou, Z.M. Laevigatbenzoate from Croton laevigatus vahl. J. Nat. Med. 2011, 65, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; Wen, X.Q.; Zeng, X.J.; Rui, W.; Cen, Y.Z. Two new diterpenoids from croton crassifolius. J. Asian. Nat. Prod. Res. 2012, 14, 785–788. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Ramírez-Apan, T.; Cogordan, J.A.; Delgado, G. Absolute configuration assignments by experimental and theoretical approaches of ent-labdane-and cis-ent-clerodane-type diterpenes isolated from Croton glabellus. Can. J. Chem. 2006, 84, 1593–1602. [Google Scholar] [CrossRef]
- Wang, G.C.; Li, J.G.; Li, G.Q.; Xu, J.J.; Wu, X.; Ye, W.C.; Li, Y.L. Clerodane diterpenoids from Croton crassifolius. J. Nat. Prod. 2012, 75, 2188–2192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.Q.; Tang, S.; Song, W.B.; Wang, W.Q.; Huang, M.; Xuan, L.J. Crassins A-H, diterpenoids from the roots of Croton crassifolius. J. Nat. Prod. 2017, 80, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Guetchueng, S.T.; Nahar, L.; Ritchie, K.J.; Ismail, F.M.D.; Evans, A.R.; Sarker, S.D. Ent-clerodane diterpenes from the bark of Croton oligandrus Pierre ex Hutch. and assessment of their cytotoxicity against human cancer cell lines. Molecules 2018, 23, 410. [Google Scholar] [CrossRef] [PubMed]
- Yamale, S.C.; Koudou, J.; Samb, A.; Heitz, A.; Teulade, J.C. Structural elucidation of a new furoclerodane from stem barks of croton mayumbensis J. Leonard extracts. Int. J. Phys. Sci. 2009, 4, 96–100. [Google Scholar]
- Wang, G.C.; Zhang, H.; Liu, H.B.; Yue, J.M. Laevinoids A and B: Two diterpenoids with an unprecedented backbone from Croton laevigatus. Org. Lett. 2013, 15, 4880–4883. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.P.; Xu, J.B.; Zhao, J.X.; Xu, C.H.; Dong, L.; Ding, J.; Yue, J.M. Diterpenoids from Croton laui and their cytotoxic and antimicrobial activities. J. Nat. Prod. 2014, 77, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Pudhom, K.; Sommit, D. Clerodane diterpenoids and a trisubstituted furan from Croton oblongifolius. Phytochem. Lett. 2011, 4, 147–150. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Ren, Q.; Li, S.; Xu, J.; Ohizumi, Y.; Xie, C.; Jin, D.Q.; Guo, Y. Two novel clerodane diterpenenes with NGF-potentiating activities from the twigs of Croton yanhuii. Fitoterapia 2014, 95, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Jiménez, R.; Vargas-Mendoza, D.; Gayosso-de-Lucio, J.A.; González-Montiel, S.; Villagómez-Ibarra, J.R. Three novel epoxy-clerodanes bearing a furan ring from Croton hypoleucus. Phytochem. Lett. 2018, 24, 21–26. [Google Scholar] [CrossRef]
- Pan, Z.; Ning, D.; Wu, X.; Huang, S.; Li, D.; Lv, S. New clerodane diterpenoids from the twigs and leaves of Croton euryphyllus. Bioorg. Med. Chem. Lett. 2015, 46, 329–1332. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Cao, D.; Gao, Y.; Li, S.; Zhu, J.; Yang, B.; Zhou, L.; Zhou, Y.; Jin, J.; Zhao, Z. New clerodane diterpenoids from Croton crassifolius. Fitoterapia 2016, 108, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ndunda, B.; Langat, M.K.; Mulholland, D.A.; Eastman, H.; Jacob, M.R.; Khan, S.I.; Walker, L.A.; Muhammad, I.; Kerubo, L.O.; Midiwo, J.O. New ent-Clerodane and abietane diterpenoids from the Roots of Kenyan Croton megalocarpoides Friis & M. G. Gilbert. Planta Med. 2016, 82, 1079–1086. [Google Scholar] [PubMed]
- Zhang, Z.X.; Li, H.H.; Fan, G.X.; Li, Z.Y.; Dong, L.L.; Li, H.Y.; Fei, D.Q. A novel norclerodane diterpenoid from the roots of Croton crassifolius. Nat. Prod. Commun. 2015, 10, 1917–1918. [Google Scholar] [PubMed]
- Youngsa-ad, W.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Prawat, H.; Kittakoop, P. Diterpenoids from the roots of Croton oblongifolius. Planta Med. 2007, 73, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Mbwambo, Z.; Foubert, K.M.; Kapingu, M.; Magadula, J.; Moshi, M.; Lemiere, F.; Goubitz, K.; Fraanje, J.; Peschar, R.; Vlietinck, A.; et al. New furanoditerpenoids from croton jatrophoides. Planta Med. 2008, 75, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Brasil, D.S.B.; Müller, A.H.; Guilhon, G.M.S.P.; Alves, C.N.; Peris, G.; Llusard, R.; Moliner, V. Isolation, X-ray crystal structure and theoretical calculations of the new compound 8-Epicordatin and identification of others terpenes and steroids from the bark and leaves of Croton palanostigma klotzsch. J. Braz. Chem. Soc. 2010, 21, 731–739. [Google Scholar] [CrossRef]
- Pizzolatti, M.G.; Bortoluzzi, A.J.; Brighente, I.M.C.; Zuchinalli, A.; Carvalho, F.K.; Candido, A.C.S.; Peresb, M.T.L.P. Clerodane diterpenes from bark of Croton urucurana Baillon. J. Braz. Chem. Soc. 2013, 24, 609–614. [Google Scholar]
- Abega, D.F.; Kapche, D.W.; Ango, P.Y.; Mapitse, R.; Yeboah, S.O.; Ngadjui, B.T. Chemical constituents of croton oligandrum (euphorbiaceae). Z. Naturforsch. C 2014, 69, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Li, G.Q.; Li, J.G.; Wu, X.; Ge, W.; Chung, H.Y.; Ye, W.C.; Li, Y.L.; Wang, G.C. Two new clerodane diterpenoids from Croton crassifolius. Heterocycles 2014, 89, 1585–1593. [Google Scholar]
- Sousa, A.H.; Junior, J.N.S.; Guedes, M.L.S.; Braz-Filho, R.; Costa-Lotufo, L.V.; Araujo, A.J.; Silveira, E.R.; Lima, M.A.S. New terpenoids from Croton limae (Euphorbiaceae). J. Braz. Chem. Soc. 2015, 26, 1565–1572. [Google Scholar]
- Wang, J.J.; Chung, H.Y.; Zhang, Y.B.; Li, G.Q.; Li, Y.L.; Huang, W.H.; Wang, G.C. Diterpenoids from the roots of Croton crassifolius and their anti-angiogenic activity. Phytochemistry 2016, 12, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.-B.; Wu, Z.-N.; Chen, N.-H.; Jiang, S.-Q.; Jiang, L.; Li, Y.-L.; Wang, G.-C. Three new diterpenoids from Croton laui. Chem. Lett. 2016, 45, 1235–1237. [Google Scholar] [CrossRef]
- Tian, J.L.; Yao, G.D.; Wang, Y.X.; Gao, P.Y.; Wang, D.; Li, L.Z.; Lin, B.; Huang, X.X.; Song, S.J. Cytotoxic clerodane diterpenoids from Croton crassifolius. Bioorg. Med. Chem. Lett. 2017, 27, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Tang, Y.Q.; Huang, J.L.; Li, W.; Zou, Y.H.; Tang, G.H.; Liu, B.; Yin, S. Bioactive diterpenoids from Croton laevigatus. Phytochemistry 2017, 144, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Jin, J.; Zhou, L.; Zhou, W.; Liu, Y.; Tan, Q.; Cao, D.; Zhao, Z. Diterpenoids from Croton crassifolius include a novel skeleton possibly generated via an intramolecular [2 + 2]-photocycloaddition reaction. Phytochemistry 2018, 145, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.Y.; Aguilar-Guadarrama, A.B. Nitrogen-containing phorbol esters from Croton ciliatoglandulifer and their effects on cyclooxygenases-1 and-2. J. Nat. Prod. 2006, 69, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Ndunda, B.; Langat, M.K.; Wanjohi, J.M.; Midiwo, J.O.; Kerubo, L.O. Alienusolin, a new 4α-deoxyphorbol ester derivative, and crotonimide C, a new glutarimide alkaloid from the Kenyan Croton alienus. Planta Med. 2013, 79, 1762–1766. [Google Scholar] [CrossRef] [PubMed]
- Corlay, N.; Delang, L.; Girard-Valenciennes, E.; Neyts, J.; Clerc, P.; Smadja, J.; Gueritte, F.; Leyssen, P.; Litaudon, M. Tigliane diterpenes from Croton mauritianus as inhibitors of chikungunya virus replication. Fitoterapia 2014, 97, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Yang, K.X.; Yang, X.W.; Khan, A.; Liu, L.; Wang, B.; Zhao, Y.L.; Liu, Y.P.; Li, Y.; Luo, X.D. New cytotoxic tigliane diterpenoids from Croton caudatus. Planta Med. 2016, 82, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, Y.B.; Jiang, S.Q.; Zhou, Y.D.; Luo, D.; Niu, Q.W.; Qian, Y.R.; Li, Y.L.; Wang, G.C. Phorbol ester-type diterpenoids from the twigs and leaves of Croton tiglium. J. Asian Nat. Prod. Res. 2017, 19, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Wang, L.; Li, F.; Yu, K.; Wang, M.K. Cytotoxic phorbol esters of Croton tiglium. J. Nat. Prod. 2013, 76, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.X.; Ren, F.Z.; Yang, Y.; Yu, N.J.; Zhang, Y.; Zhao, Y.M. Tigliane diterpene esters from the leaves of Croton tiglium. Helv. Chim. Acta 2014, 97, 1014–1019. [Google Scholar] [CrossRef]
- Wang, J.F.; Yang, S.H.; Liu, Y.Q.; Li, D.X.; He, W.J.; Zhang, X.X.; Liu, Y.H.; Zhou, X.J. Five new phorbol esters with cytotoxic and selective anti-inflammatory activities from Croton tiglium. Bioorg. Med. Chem. Lett. 2015, 25, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-D.; Zhou, B.; Yu, J.-H.; Xu, C.-H.; Ding, J.; Zhang, H.; Yue, J.-M. Cytotoxic tigliane-type diterpenoids from Croton tiglium. Tetrahedron 2015, 71, 9638–9644. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Khan, A.-A.; Wang, L.; Yu, K.; Li, F.; Wang, M.-K. Four new phorbol diesters from Croton tiglium and their cytotoxic activities. Phytochem. Lett. 2016, 16, 82–86. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Peng, S.; He, W.J.; Liu, Y.H.; Wang, J.F.; Zhou, X.J. Antitubercular and cytotoxic tigliane-type diterpenoids from Croton tiglium. Bioorg. Med. Chem. Lett. 2016, 26, 4996–4999. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhao, Y.; Liu, H.; Tang, C.; Zhang, M.; Ke, C.; Ye, Y. Isolation and structure characterization of cytotoxic phorbol esters from the seeds of Croton tiglium. Planta Med. 2017, 83, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Suirez, A.I.; Chavez, K.; Monache, F.D.; Vasquez, L.; Delannoy, D.M.; Orsini, G.; Compagnone, R.S. New 3,4-seco ent-kaurenes from Croton caracasana flowers. Nat. Prod. Commun. 2008, 3, 319–322. [Google Scholar]
- Mora, S.; Castro, V.; Poveda, L.; Chavarría, M.; Murillo, R. Two new 3,4-seco-ent-kaurenes and other constituents from the costa rican endemic species Croton megistocarpus. Helv. Chim. Acta 2011, 94, 1888–1892. [Google Scholar] [CrossRef]
- Suwancharoen, S.; Chonvanich, O.; Roengsumran, S.; Pornpakakul, S. Seco-kaurane skeleton diterpenoids from Croton oblongifolius. Chem. Nat. Compd. 2012, 48, 583–586. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.D.; Zhao, J.F.; Yang, J.H.; Zhang, H.B.; Li, Z.Y.; Li, L. Three new, 1-oxygenated ent-8,9-secokaurane diterpenes from Croton kongensis. Helv. Chim. Acta 2006, 89, 537–541. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.D.; Zhao, J.F.; Zhang, H.B.; Li, L. Two new, 1-oxygenated ent-kaurane-type diterpenes from Croton kongensis. Helv. Chim. Acta 2007, 90, 1554–1558. [Google Scholar] [CrossRef]
- Yang, X.-D.; Chen, W.; Zhao, J.-F.; Yang, L.-J.; Zhang, H.-B.; Li, L. Ent-kaurane diterpenes and phenolic compounds from Croton kongensis (Euphorbiaceae). Biochem. Syst. Ecol. 2009, 37, 237–240. [Google Scholar] [CrossRef]
- Shi, S.Q.; Fan, Y.Y.; Xu, C.H.; Ding, J.; Wang, G.W.; Yue, J.M. Cytotoxic 8,9-seco-ent-kaurane diterpenoids from Croton kongensis. J. Asian Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mateu, E.; Chavez, K.; Riina, R.S.; Compagnone, R.; Monache, F.D.; Suárez, A.I. New 3,4-seco-ent-kaurene dimers from Croton micans. Nat. Prod. Commun. 2012, 7, 5–8. [Google Scholar] [PubMed]
- Thuong, P.T.; Pham, T.H.; Le, T.V.; Dao, T.T.; Dang, T.T.; Nguyen, Q.T.; Oh, W.K. Symmetric dimers of ent-kaurane diterpenoids with cytotoxic activity from Croton tonkinensis. Bioorg. Med. Chem. Lett. 2012, 22, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Shen, Y.C.; Yang, M.L.; Wang, S.H.; Thang, T.D.; Dung, N.X.; Chiang, P.-C.; Lee, K.-H.; Lee, E.-J.; Wu, T.-S. Crotonkinins A and B and related diterpenoids from Croton tonkinensis as anti-inflammatory and antitumor agents. J. Nat. Prod. 2007, 70, 1906–1909. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.S.; Barros, F.W.; Albuquerque, M.R.; Bandeira, P.N.; Pessoa, C.; Braz-Filho, R.; Monte, F.J.Q.; Leal-Cardoso, J.H.; Lemos, T.L.G. Cytotoxic diterpenoids from croton argyrophylloides. J. Nat. Prod. 2009, 72, 1884–1887. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.T.; Lee, K.Y.; Jeong, H.M.; Nguyen, P.H.; Tran, T.L.; Thuong, P.T.; Nguyen, B.T.; Oh, W.K. Ent-kaurane diterpenoids from Croton tonkinensis stimulate osteoblast differentiation. J. Nat. Prod. 2011, 74, 2526–2531. [Google Scholar] [CrossRef] [PubMed]
- Langat, M.K.; Crouch, N.R.; Pohjala, L.; Tammela, P.; Smith, P.J.; Mulholland, D.A. Ent-kauren-19-oic acid derivatives from the stem bark of Croton pseudopulchellus Pax. Phytochem. Lett. 2012, 5, 414–418. [Google Scholar] [CrossRef]
- Kuo, P.C.; Yang, M.L.; Hwang, T.L.; Lai, Y.Y.; Li, Y.C.; Thang, T.D.; Wu, T.S. Anti-inflammatory diterpenoids from Croton tonkinensis. J. Nat. Prod. 2013, 76, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Meng, Z.; Li, Z.; Yang, B.; Wang, Z.; Ding, G.; Xiao, W. Two new natural products from Croton kongensis Gagnep. Nat. Prod. Res. 2014, 28, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Toyoda, H.; Harinantenaina, L.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Takeda, Y.; Kawahata, M.; Yamaguchid, K. Eight new diterpenoids and two new nor-diterpenoids from the stems of Croton cascarilloides. Chem. Pharm. Bull. 2013, 61, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Matsunami, K.; Otsuka, H.; Inagaki, M.; Takeda, Y.; Kawahata, M.; Yamaguchic, K. Crotocascarins I-K: Crotofolane-type diterpenoids, crotocascarin γ, isocrotofolane glucoside and phenolic glycoside from the leaves of Croton cascarilloides. Chem. Pharm. Bull. 2015, 63, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Inagaki, M.; Matsunami, K.; Otsuka, H.; Kawahata, M.; Yamaguchi, K. Crotofolane-type diterpenoids, crotocascarins L–Q, and a rearranged crotofolane-type diterpenoid, neocrotocascarin, from the Stems of Croton cascarilloides. Chem. Pharm. Bull. 2016, 64, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Xu, Y.S.; Fan, Y.Y.; Gan, L.S.; Zuo, J.P.; Yue, J.M. Cascarinoids A-C, a class of diterpenoid alkaloids with unpredicted conformations from Croton cascarilloides. Org. Lett. 2018, 20, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Filho, F.A.S.; Junior, J.N.S.; Braz-Filho, R.; Simone, C.A.; Silveira, E.R.; Lima, M.A.S. Crotofolane-and casbane-type diterpenes from Croton argyrophyllus. Helv. Chim. Acta 2013, 96, 1146–1154. [Google Scholar] [CrossRef]
- Chávez, K.; Compagnoneb, R.S.; Riina, R.; Briceño, A.; González, T.; Squitieri, E.; Landaetab, C.; Soscúnd, H.; Suáreza, A.I. Crotofolane diterpenoids from Croton caracasanus. Nat. Prod. Commun. 2013, 8, 1679–1682. [Google Scholar] [PubMed]
- Maslovskaya, L.A.; Savchenko, A.I.; Pierce, C.J.; Gordon, V.A.; Reddell, P.W.; Parsons, P.G.; Williams, C.M. Unprecedented 1,14-seco-crotofolanes from Croton insularis: Oxidative cleavage of crotofolin C by a putative homo-baeyer-villiger rearrangement. Chem. Eur. J. 2014, 20, 14226–14230. [Google Scholar] [CrossRef] [PubMed]
- Aldhaher, A.; Langat, M.; Ndunda, B.; Chirchir, D.; Midiwo, J.O.; Njue, A.; Schwikkard, S.; Carew, M.; Mulholland, D. Diterpenoids from the roots of Croton dichogamus Pax. Phytochemistry 2017, 144, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, Z.N.; Zhang, Y.B.; Chen, N.H.; Zhuang, L.; Li, Y.L.; Wang, G.C. Three new diterpenoids from Croton laui Merr. et Metc. Nat. Prod. Res. 2017, 31, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, A.C.S.S.; Teixeira, A.M.R.; de Menezes, J.E.S.A.; Pinto, C.C.C.; Santos, H.S.; Freire, P.T.C.; Coutinho, H.D.M.; Sena Junior, D.M.; Bandeira, P.N.; Braz-Filho, R. Spectroscopic and microbiological characterization of labdane diterpene 15,16-epoxy-4-hydroxy-labda-13(16),14-dien-3,12-dione isolated from the stems of Croton jacobinensis. J. Mol. Struct. 2017, 1147, 335–344. [Google Scholar] [CrossRef]
- Ramos, F.; Takaishi, Y.; Kashiwada, Y.; Osorio, C.; Duque, C.; Acuna, R.; Fujimoto, Y. Ent-3,4-seco-labdane and ent-labdane diterpenoids from Croton stipuliformis (Euphorbiaceae). Phytochemistry 2008, 69, 2406–2410. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Qi, F.-M.; Yuan, J.-C.; Zhao, C.-G.; Yang, J.-W.; Fang, F.-H.; Wu, Q.-X.; Gao, K.; Yuan, C.-S. Labdane-type diterpenoids from Croton laevigatus. RSC Adv. 2014, 4, 39530–39540. [Google Scholar] [CrossRef]
- Pompimon, W.; Udomputtimekakul, P.; Apisantiyakom, S.; Baison, W.; Penlap, N.; Chaibun, S.; Nuntasaen, N. Two new labdane-type diterpenoids cinnamate from Croton decalvatus Esser. Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Wu, P.Q.; Li, H.H.; Qi, F.M.; Fei, D.Q.; Hu, Q.L.; Liu, Y.H.; Huang, X.L. Norcrassin A, a novel C16 tetranorditerpenoid, and bicrotonol A, an unusual dimeric labdane-type diterpenoid, from the roots of Croton crassifolius. Org. Biomol. Chem. 2018, 16, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.B.; Chen, L.F.; Chen, N.H.; Wu, Z.N.; Jiang, S.Q.; Jiang, L.; Li, G.Q.; Li, Y.L.; Wang, G.C. New labdane diterpenoids from Croton laui and their anti-inflammatory activities. Bioorg. Med. Chem. Lett. 2016, 26, 4687–4691. [Google Scholar] [CrossRef] [PubMed]
- Pudhom, K.; Vilaivan, T.; Ngamrojanavanich, N.; Dechangvipart, S.; Sommit, D.; Petsom, A.; Roengsumran, S. Furanocembranoids from the stem bark of Croton oblongifolius. J. Nat. Prod. 2007, 70, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.A.; Gang, D.; Su, Z.-H.; Yang, J.-S.; Zhang, H.-W.; Peng, C.-Z.; Aisa, H.A.; Zou, Z.-M. Lactonecembranoids from Croton laewigatus. J. Nat. Prod. 2010, 73, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.A.; Langat, M.K.; Crouch, N.R.; Coley, H.M.; Mutambi, E.M.; Nuzillard, J.M. Cembranolides from the stem bark of the southern african medicinal plant, Croton gratissimus (Euphorbiaceae). Phytochemistry 2010, 71, 1381–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langat, M.K.; Crouch, N.R.; Smith, P.J.; Mulholland, D.A. Cembranolides from the leaves of Croton gratissimus. J. Nat. Prod. 2011, 74, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Matsunami, K.; Otsuka, H.; Lhieochaiphant, D.; Lhieochaiphant, S. Two new cembranoids from the leaves of Croton longissimus Airy Shaw. J. Nat. Med. 2013, 67, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Song, J.T.; Han, Y.; Wang, X.L.; Shen, T.; Lou, H.X.; Wang, X.N. Diterpenoids from the twigs and leaves of Croton caudatus var. tomentosus. Fitoterapia 2015, 107, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-T.; Liu, X.-Y.; Li, A.-L.; Wang, X.-L.; Shen, T.; Ren, D.-M.; Lou, H.-X.; Wang, X.-N. Cytotoxic abietane-type diterpenoids from twigs and leaves of Croton laevigatus. Phytochem. Lett. 2017, 22, 241–246. [Google Scholar] [CrossRef]
- Santos, H.S.; Mesquita, F.M.R.; Lemos, T.L.G.; Monte, F.J.Q.; Braz-Filho, R. Diterpenos casbanos e acetofenonas de Croton nepetaefolius (Euphorbiaceae). Quim. Nova 2008, 31, 601–604. [Google Scholar] [CrossRef]
- e Silva-Filho, F.A.; Braz-Filho, R.; Silveira, E.R.; Lima, M.A. Structure elucidation of casbane diterpenes from Croton argyrophyllus. Magn. Reson. Chem. 2011, 49, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Maslovskaya, L.A.; Savchenko, A.I.; Krenske, E.H.; Gordon, V.A.; Reddell, P.W.; Pierce, C.J.; Parsons, P.G.; Williams, C.M. Croton insularis introduces the seco-casbane class with EBC-329 and the first casbane endoperoxide EBC-324. Chem. Commun. 2014, 50, 12315–12317. [Google Scholar] [CrossRef] [PubMed]
- Roengsumran, S.; Pata, P.; Ruengraweewat, N.; Tummatorn, J.; Pornpakakul, S.; Sangvanich, P.; Puthong, S.; Petsom, A. New cleistanthane diterpenoids and 3,4-seco-cleistanthane diterpenoids from croton oblongifolius. Chem. Nat. Compd. 2009, 45, 641–646. [Google Scholar] [CrossRef]
- Torres, M.C.M.; Braz-Filho, R.; Silveira, E.R.; Diniz, J.C.; Viana, F.A.; Pessoa, O.D.L. Terpenoids from Croton regelianus. Helv. Chim. Acta 2010, 93, 375–381. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Li, H.-H.; Qi, F.-M.; Xiong, H.-Y.; Dong, L.-L.; Fan, G.-X.; Fei, D.-Q. A new halimane diterpenoid from Croton crassifolius. Bull. Korean Chem. Soc. 2014, 35, 1556–1558. [Google Scholar] [CrossRef]
- Maslovskaya, L.A.; Savchenko, A.I.; Gordon, V.A.; Reddell, P.W.; Pierce, C.J.; Parsons, P.G.; Williams, C.M. Isolation and confirmation of the proposed cleistanthol biogentic link from Croton insularis. Org. Lett. 2011, 13, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Maslovskaya, L.A.; Savchenko, A.I.; Gordon, V.A.; Reddell, P.W.; Pierce, C.J.; Parsons, P.G.; Williams, C.M. EBC-316, 325–327, and 345: New pimarane diterpenes from Croton insularis found in the Australian rainforest. Aust. J. Chem. 2015, 68, 652–659. [Google Scholar] [CrossRef]
- Thuong, P.T.; Dao, T.T.; Pham, T.H.; Nguyen, P.H.; Le, T.V.; Lee, K.Y.; Oh, W.-K. Crotonkinensins A and B, diterpenoids from the Vietnamese medicinal plant Croton tonkinensis. J. Nat. Prod. 2009, 72, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Rakotonandrasana, O.L.; Raharinjato, F.H.; Rajaonarivelo, M.; Dumontet, V.; Martin, M.-T.; Bignon, J.; Rasoanaivo, P. Cytotoxic 3,4-seco-atisane diterpenoids from Croton barorum and Croton goudotii. J. Nat. Prod. 2010, 73, 1730–1733. [Google Scholar] [CrossRef] [PubMed]
- Adelekan, A.M.; Prozesky, E.A.; Hussein, A.A.; Urena, L.D.; Rooyen, P.H.; Liles, D.C.; Meyer, J.J.M.; Rodrfguez, B. Bioactive diterpenes and other constituents of Croton steenkampianus. J. Nat. Prod. 2008, 71, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.B.; Gomes, C.L.; Freitas, J.V.B.D.; Pinto, F.D.C.L.; Silveira, E.R.; Gramosa, N.V.; Torres, D.S.C. Flavonoides e terpenoides de Croton muscicarpa (euphorbiaceae). Quim. Nova 2013, 36, 675–679. [Google Scholar] [CrossRef]
- Lopes, E.L.; Neto, M.A.; Silveira, E.R.; Pessoa, O.D.L.; Braz-Filho, R. Flavonoides e sesquiterpenos de Croton pedicellatus kunth. Quim. Nova 2012, 35, 2169–2172. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Li, H.-H.; Qi, F.-M.; Dong, L.-L.; Hai, Y.; Fan, G.-X.; Fei, D.-Q. Crocrassins A and B: Two novel sesquiterpenoids with an unprecedented carbon skeleton from Croton crassifolius. RSC Adv. 2014, 4, 30059–30061. [Google Scholar] [CrossRef]
- Langat, M.K.; Crouch, N.R.; Nuzillard, J.-M.; Mulholland, D.A. Pseudopulchellol: A unique sesquiterpene-monoterpene derived C-25 terpenoid from the leaves of Croton pseudopulchellus Pax (Euphorbiaceae). Phytochem. Lett. 2018, 23, 38–40. [Google Scholar] [CrossRef]
- Ghosh, P.; Mandal, A.; Rasul, M.G. A new bioactive ursane-type triterpenoid from Croton bonplandianum Bail. J. Chem. Sci. 2013, 125, 359–364. [Google Scholar] [CrossRef]
- Yuan, Q.Q.; Song, W.B.; Wang, W.Q.; Xuan, L.J. A new patchoulane-type sesquiterpenoid glycoside from the roots of Croton crassifolius. Nat. Prod. Res. 2017, 31, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.H.; Ning, D.S.; Liu, J.L.; Pan, B.; Li, D.P. A new triterpenoid saponin from the root of Croton lachnocarpus Benth. Nat. Prod. Res. 2014, 28, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Aderogba, M.A.; McGaw, L.J.; Bezabih, M.; Abegaz, B.M. Isolation and characterisation of novel antioxidant constituents of Croton zambesicus leaf extract. Nat. Prod. Res. 2011, 25, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Takeda, Y. Crotonionosides A-G: Megastigmane glycosides from leaves of Croton cascarilloides Rauschel. Phytochemistry 2011, 72, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Takeshige, Y.; Kawakami, S.; Matsunami, K.; Otsuka, H.; Lhieochaiphant, D.; Lhieochaiphant, S. Oblongionosides A-F, megastigmane glycosides from the leaves of Croton oblongifolius Roxburgh. Phytochemistry 2012, 80, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, R.; Bibi, A.; Malik, A. New secondary metabolites from Croton sparsiflorus Morong. Turk. J. Chem. 2013, 37, 111–118. [Google Scholar]
- Mehmood, R.; Imran, M.; Safder, M.; Anjum, S.; Malik, A. Structural determination of crotamides A and B, the new amides from Croton sparsiflorus. J. Asian Nat. Prod. Res. 2010, 12, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, R.; Malik, A. New secondary metabolites from Croton sparsiflorus. Z. Naturforsch. B 2011, 66, 857–860. [Google Scholar] [CrossRef]
- Wu, X.A.; Zhao, Y.M.; Yu, N.J. A novel analgesic pyrazine derivative from the leaves of Croton tiglium L. J. Asian Nat. Prod. Res. 2007, 9, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.S.; Abreu, A.d.S.; Batista, E.F.; Guilhon, G.M.S.P.; Müller, A.H.; Arruda, M.S.P.; Santos, L.S.; Arruda, A.C.; Secco, R.S. Glutarimide alkaloids and terpenoids from Croton pullei var. glabrior Lanj. Biochem. Syst. Ecol. 2007, 35, 887–890. [Google Scholar] [CrossRef]
- Queiroz, M.M.F.; Queiroz, E.F.; Zeraik, M.L.; Marti, G.; Favre-Godal, Q.; Simões-Pires, C.; Marcourt, L.; Carrupt, P.A.; Cuendet, M.; Paulo, M.Q.; et al. Antifungals and acetylcholinesterase inhibitors from the stem bark of Croton heliotropiifolius. Phytochem. Lett. 2014, 10. [Google Scholar] [CrossRef]
- Novello, C.R.; Marques, L.C.; Pires, M.E.; Kutschenco, A.P.; Nakamura, C.V.; Nocchi, S.; Sarragiotto, M.H.; Mello, J.C.P. Bioactive indole alkaloids from Croton echioides. J. Braz. Chem. Soc. 2016, 27, 2203–2209. [Google Scholar]
- Aderogba, M.; Ndhlala, A.R.; Staden, J.V. Acetylcholinesterase inhibitors from Croton sylvaticus ethyl acetate leaf extract and their mutagenic effects. Nat. Prod. Commun. 2013, 8, 795–798. [Google Scholar]
- Athikomkulchai, S.; Prawat, H.; Thasana, N.; Ruangrungsi, N.; Ruchirawat, S. Cox-1, Cox-2 inhibitors and antifungal agents from Croton hutchinsonianus. Chem. Pharm. Bull. 2006, 54, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Qi, F.-M.; Dong, L.-L.; Fan, G.-X.; Che, J.-M.; Guo, D.-D.; Zhang, Z.-X.; Fei, D.-Q. Cytotoxic and antibacterial pyran-2-one derivatives from Croton crassifolius. Phytochem. Lett. 2014, 10, 304–308. [Google Scholar] [CrossRef]
- Huang, W.; Wang, J.; Liang, Y.; Li, Y.; Wang, G. Pyran-2-one derivatives from the roots of Croton crassifolius. Nat. Prod. Commun. 2016, 11, 803–804. [Google Scholar] [PubMed]
- Quintyne-Walcott, S.; Maxwell, A.R.; Reynolds, W.F. Crotogossamide, a cyclic nonapeptide from the latex of Croton gossypifolius. J. Nat. Prod. 2007, 70, 1374–1376. [Google Scholar] [CrossRef] [PubMed]
- Candido-Bacani Pde, M.; Figueiredo Pde, O.; Matos Mde, F.; Garcez, F.R.; Garcez, W.S. Cytotoxic orbitide from the latex of Croton urucurana. J. Nat. Prod. 2015, 78, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Bracher, F.; Randau, K.P.; Lerche, H. Crototropone, a new tropone derivative from Croton zehntneri. Fitoterapia 2008, 79, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Randau, K.P.; Sproll, S.; Lerche, H.; Bracher, F. Pernambucone, a new tropone derivative from Croton argyroglossum. Pharmazie 2009, 64, 350–351. [Google Scholar] [PubMed]
- Nihei, K.; Asaka, Y.; Mine, Y.; Yamada, Y.; Iigo, M.; Yanagisawa, T. Musidunin and musiduol, insect antifeedants from Croton jatrophoides. J. Nat. Prod. 2006, 69, 975–977. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.A.; Su, Z.H.; Zhang, H.W.; Wang, Y.; Yang, J.S.; Zou, Z.M. Flavonoids from the stems of Croton caudatus Geisel. var. tomentosus Hook. Molecules 2010, 15, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Ahmat, N.; Said, I.M.; Latip, J.; Din, L.B.; Syah, Y.M.; Hakim, E.H. New prenylated dihydrostilbenes from Croton laevifolius. Nat. Prod. Commun. 2007, 2, 1137–1140. [Google Scholar]
- Attioua, B.; Weniger, B.; Chabert, P. Antiplasmodial activity of constituents isolated from Croton lobatus. Pharm. Biol. 2007, 45, 263–266. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


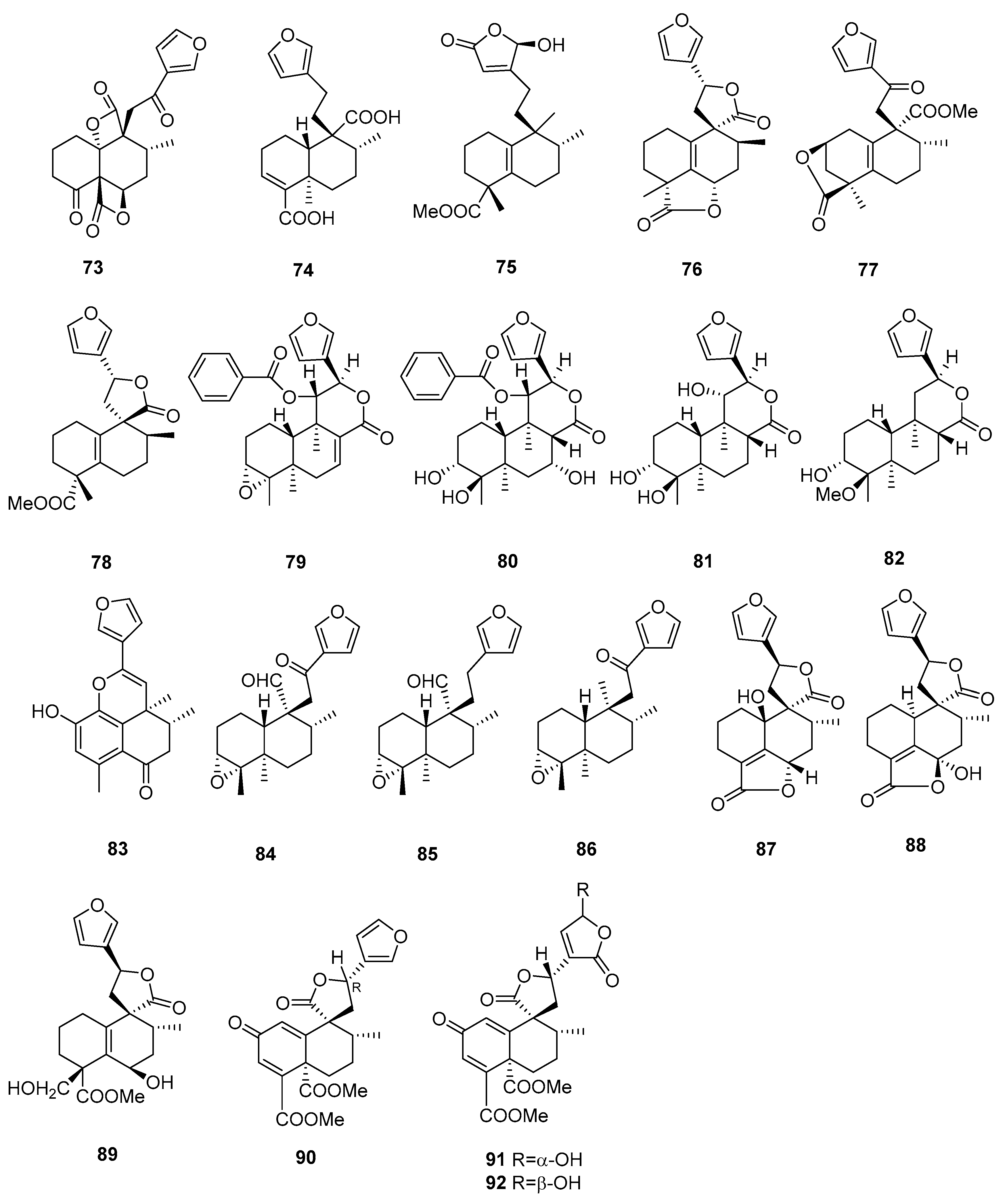
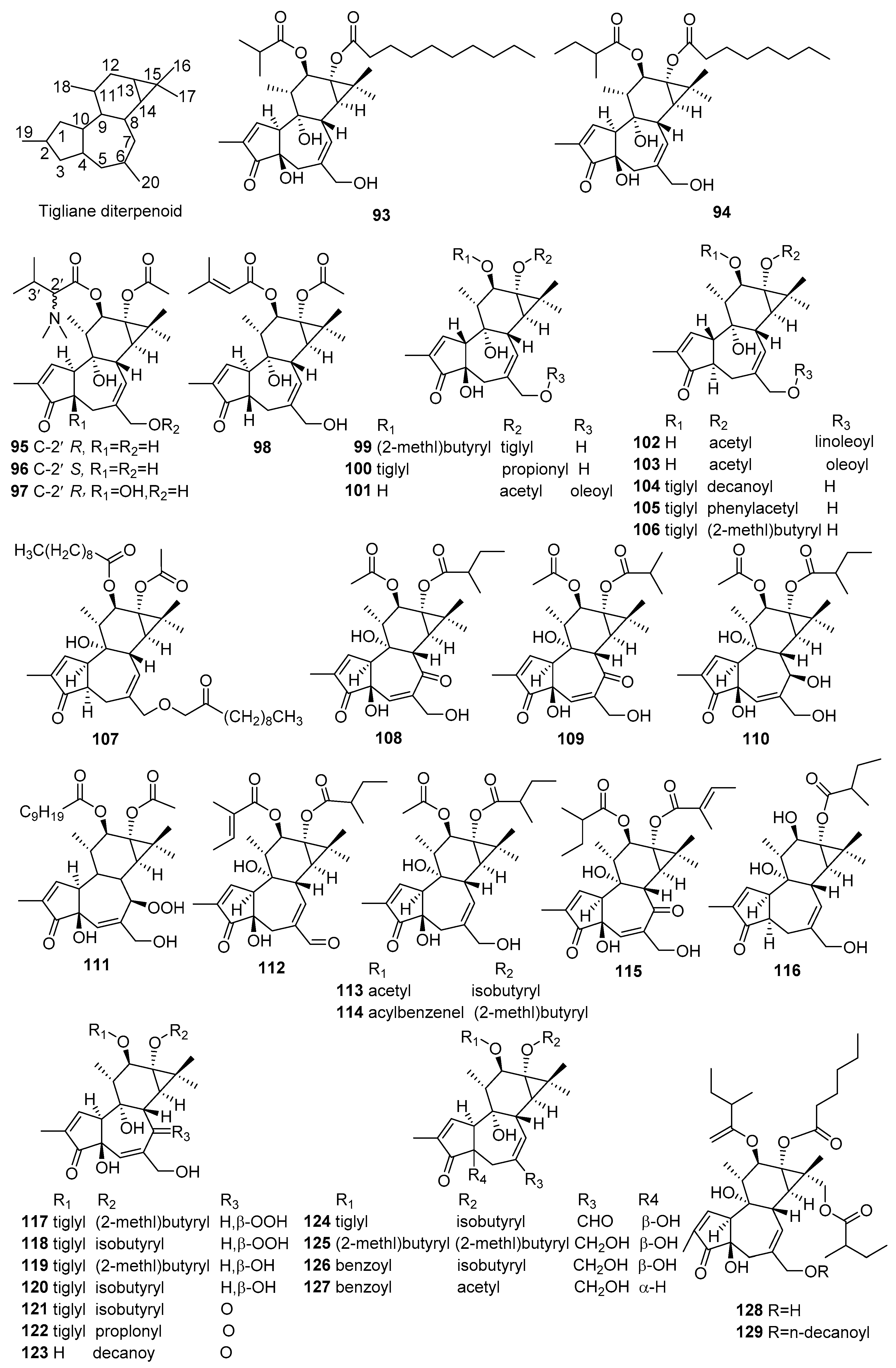
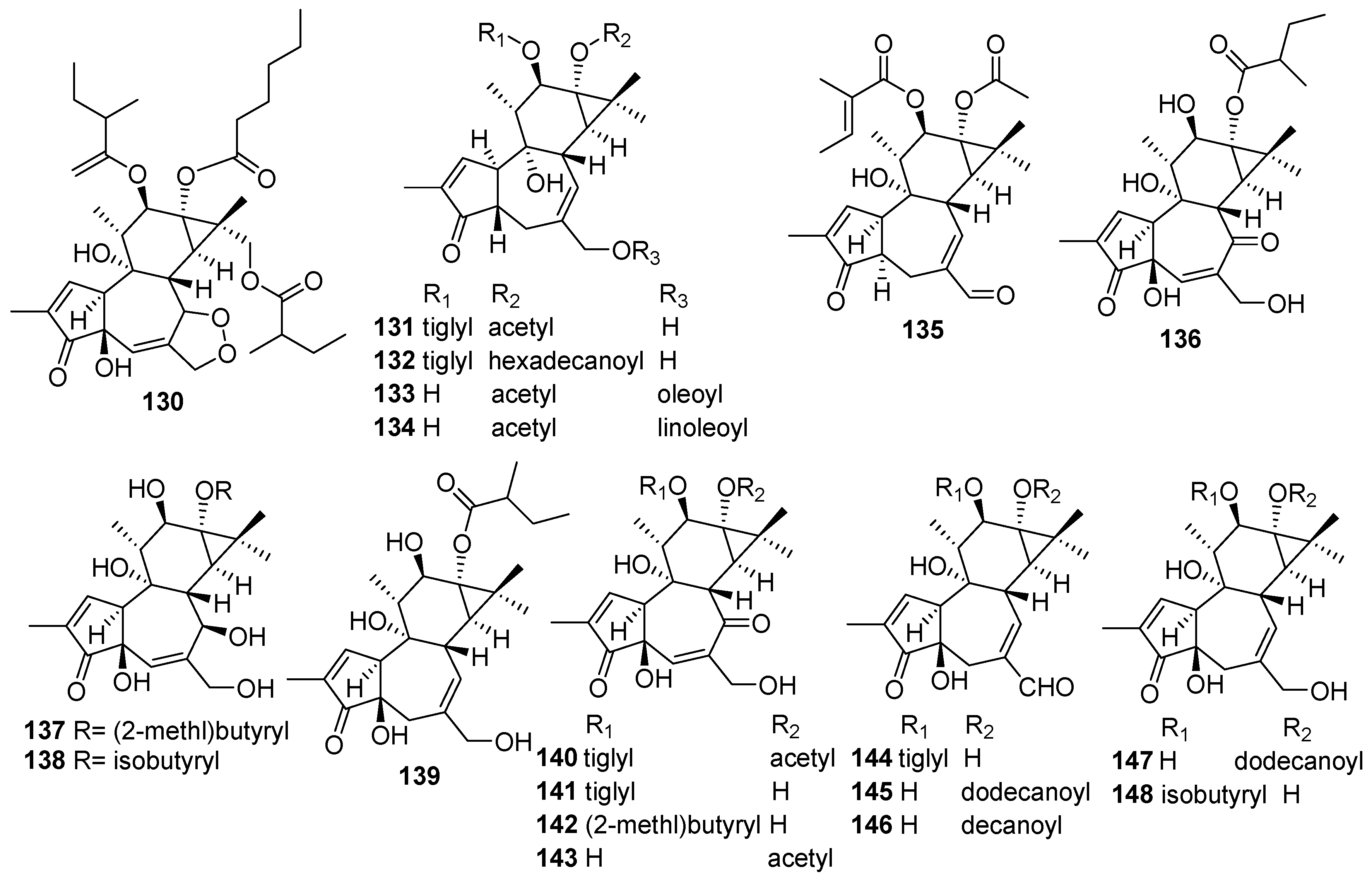
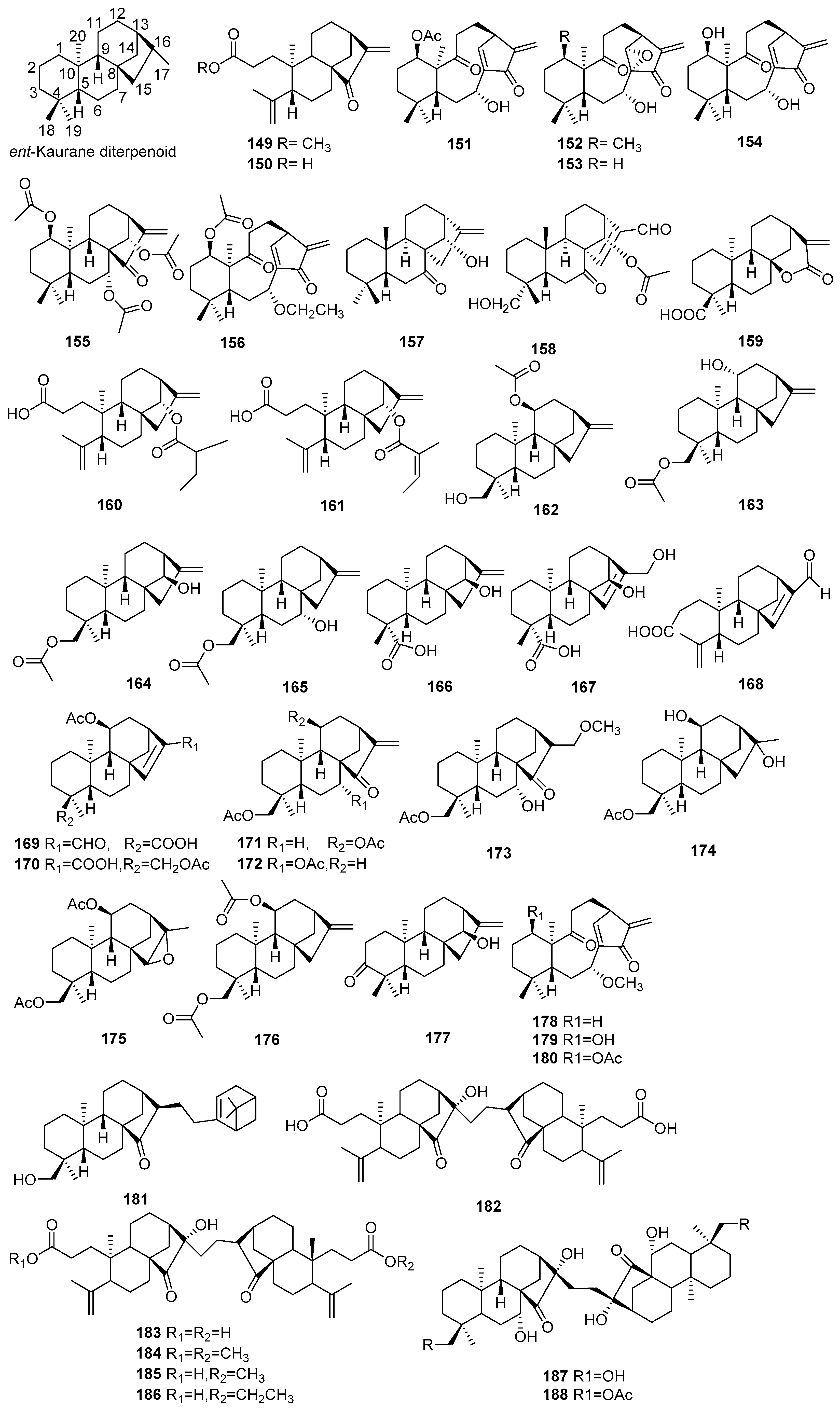

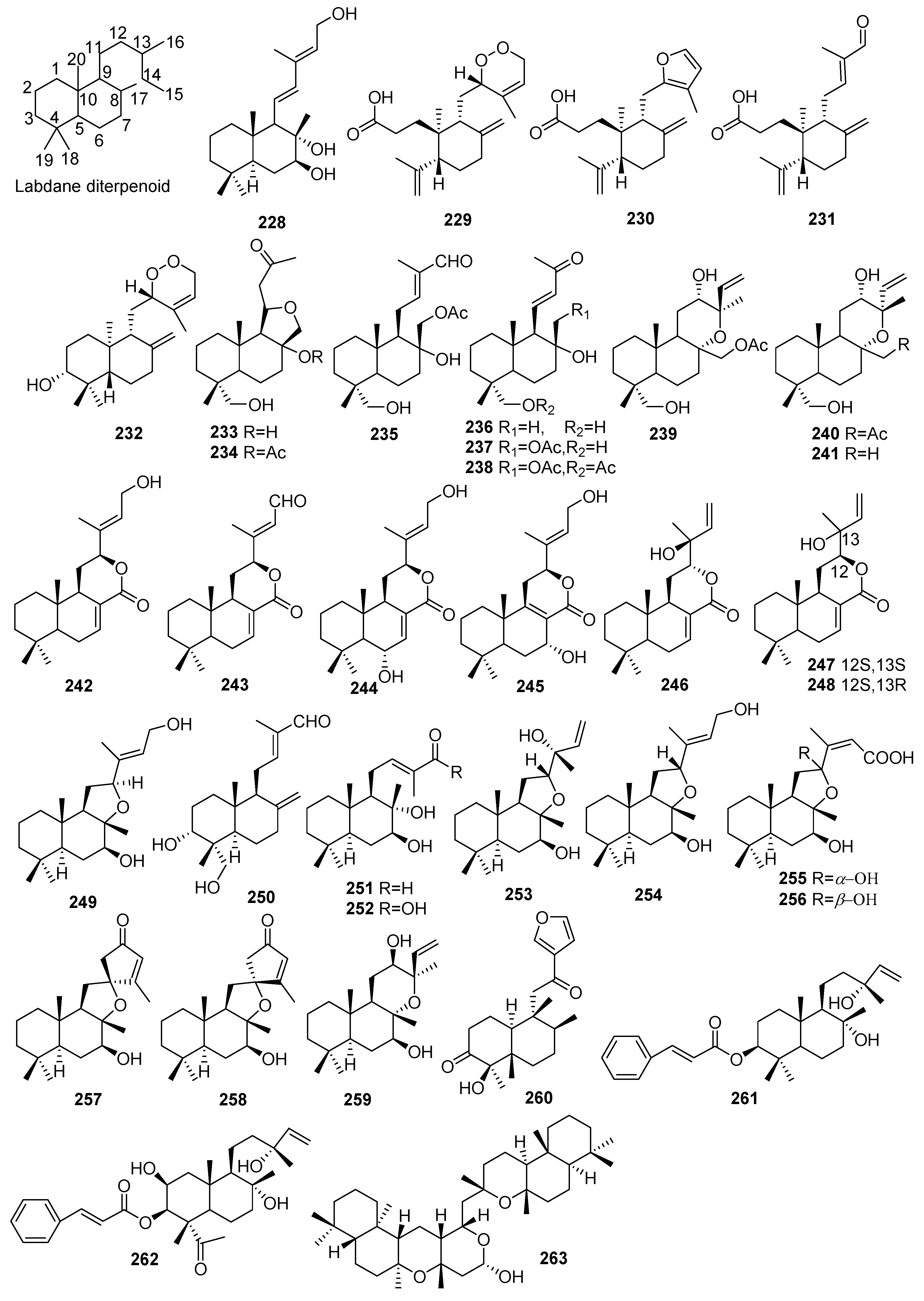

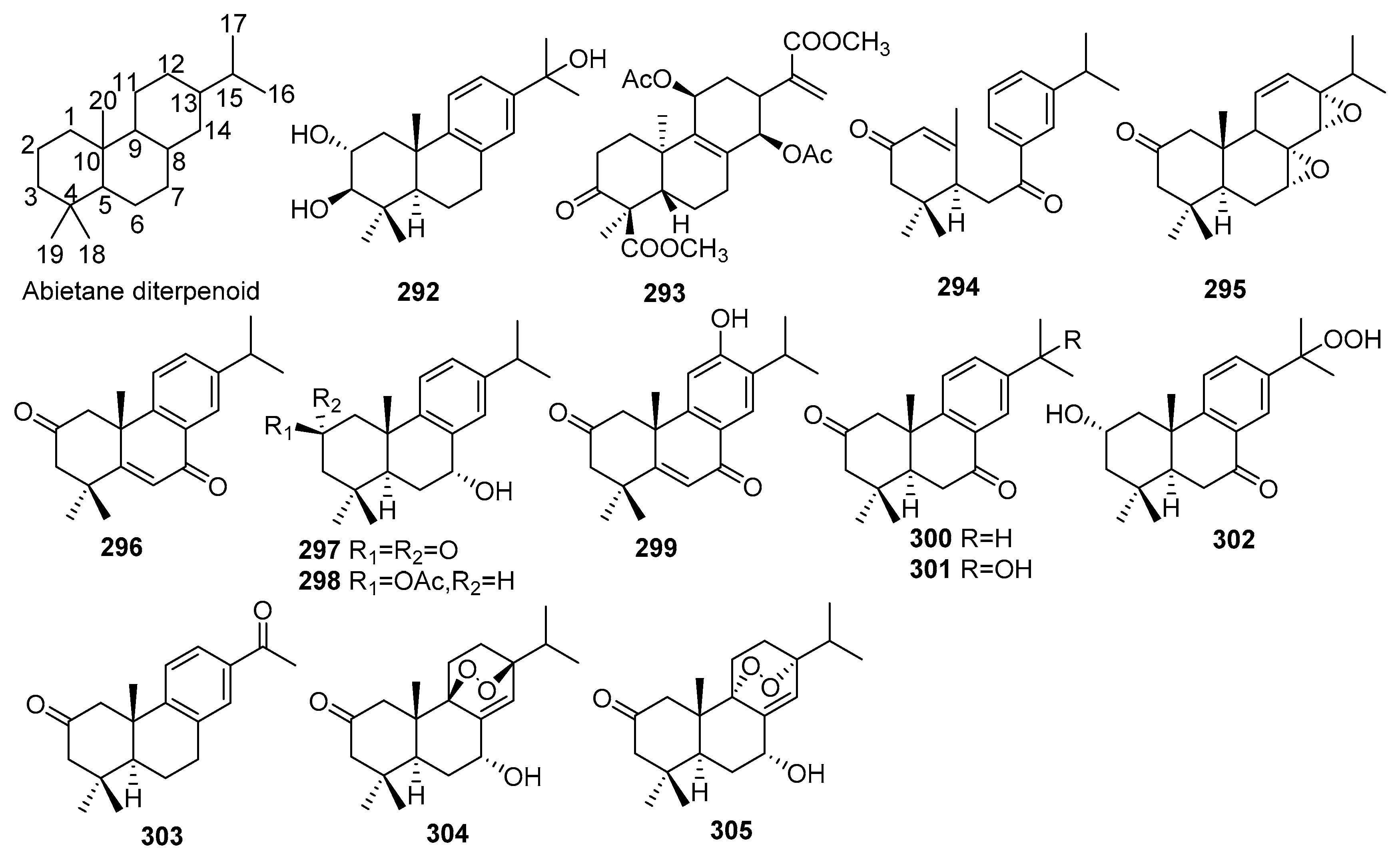
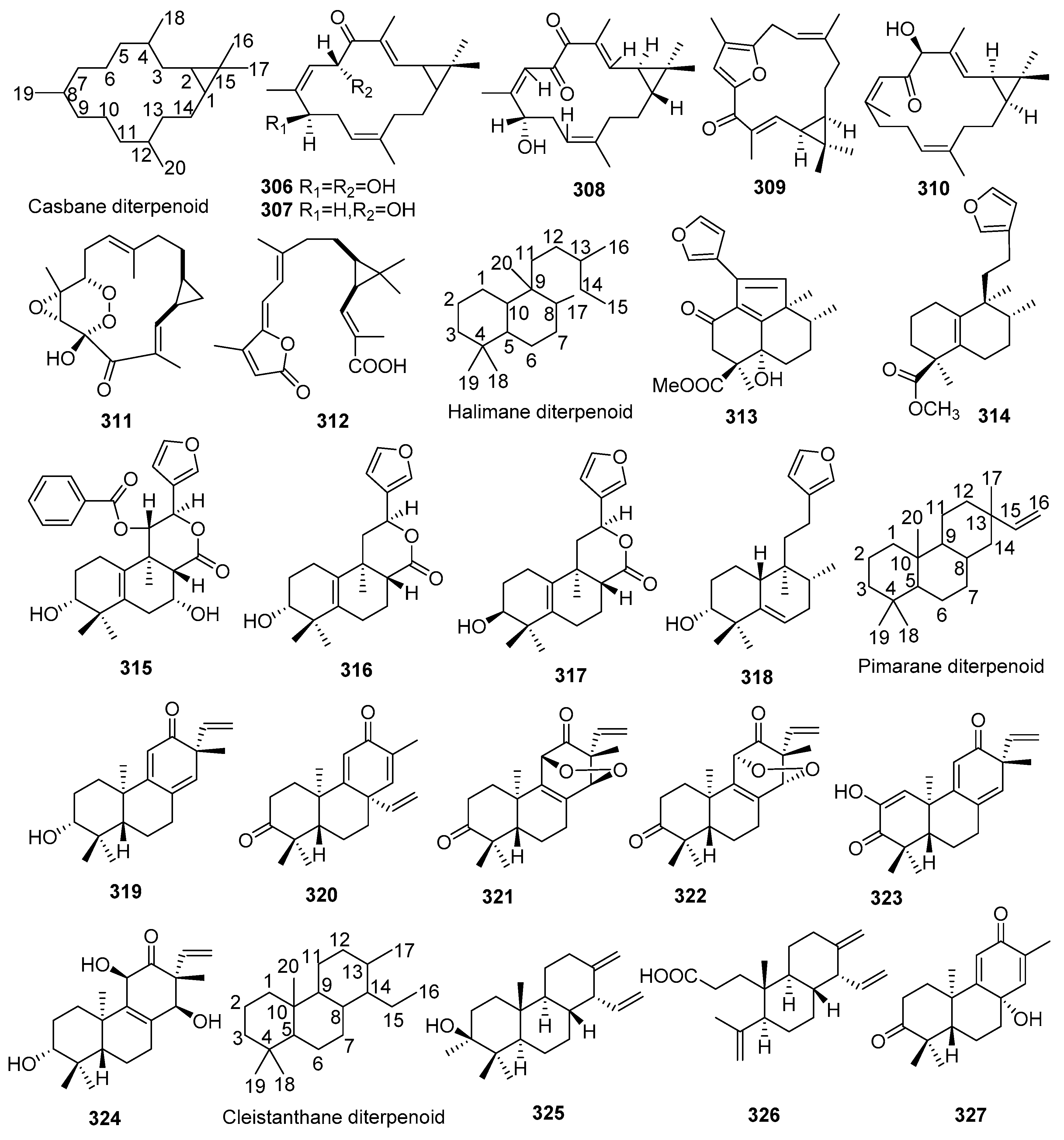
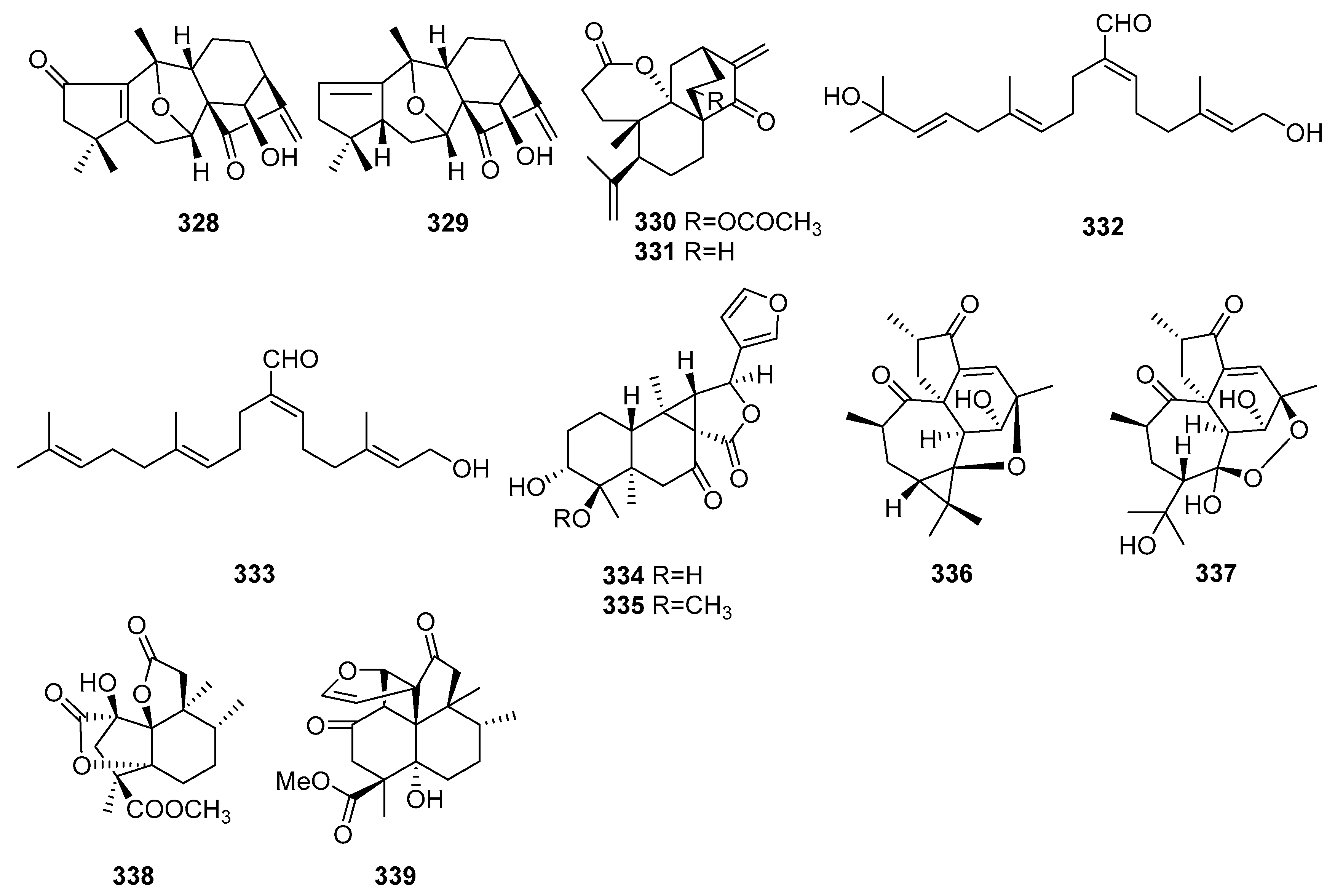

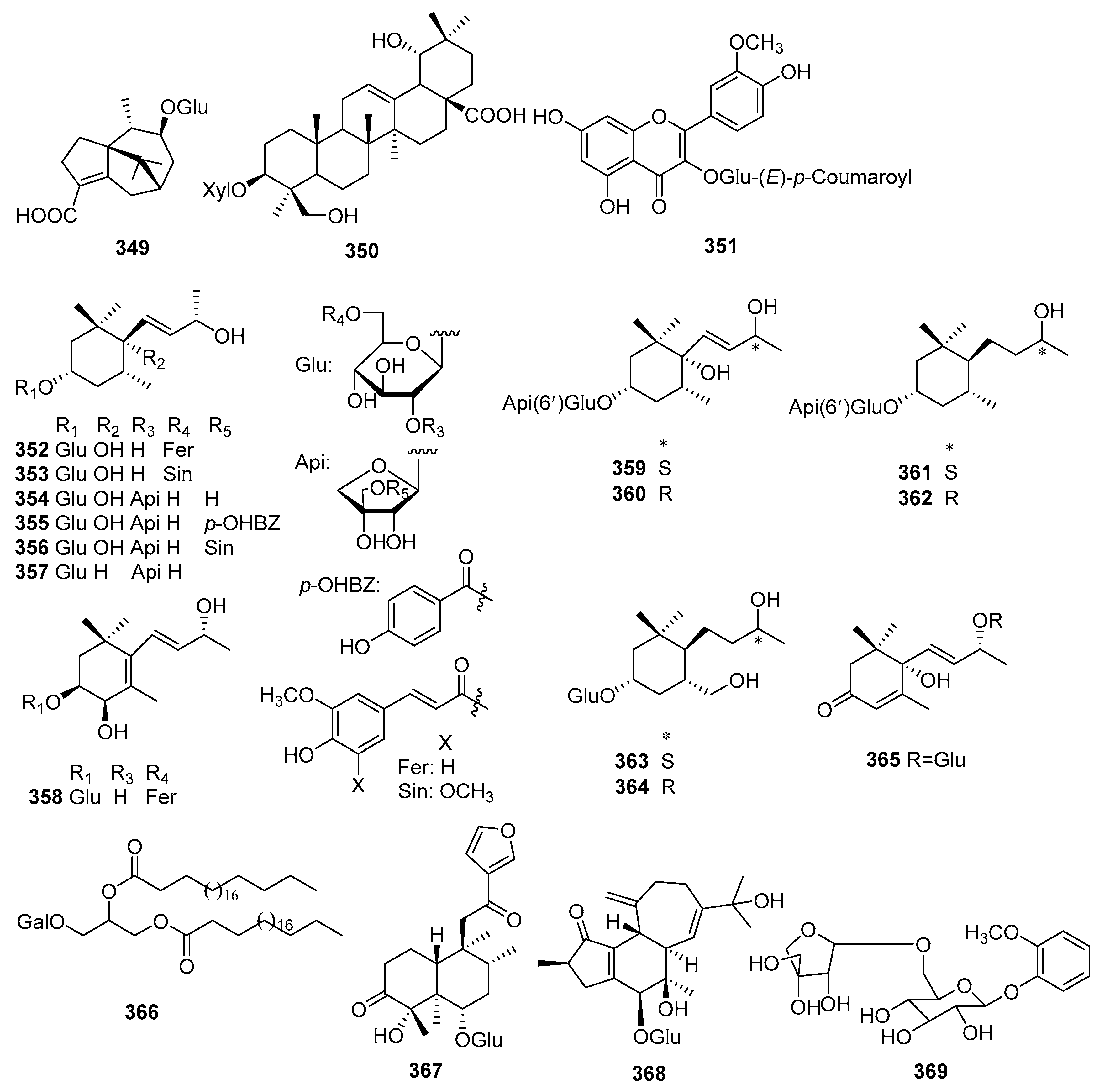
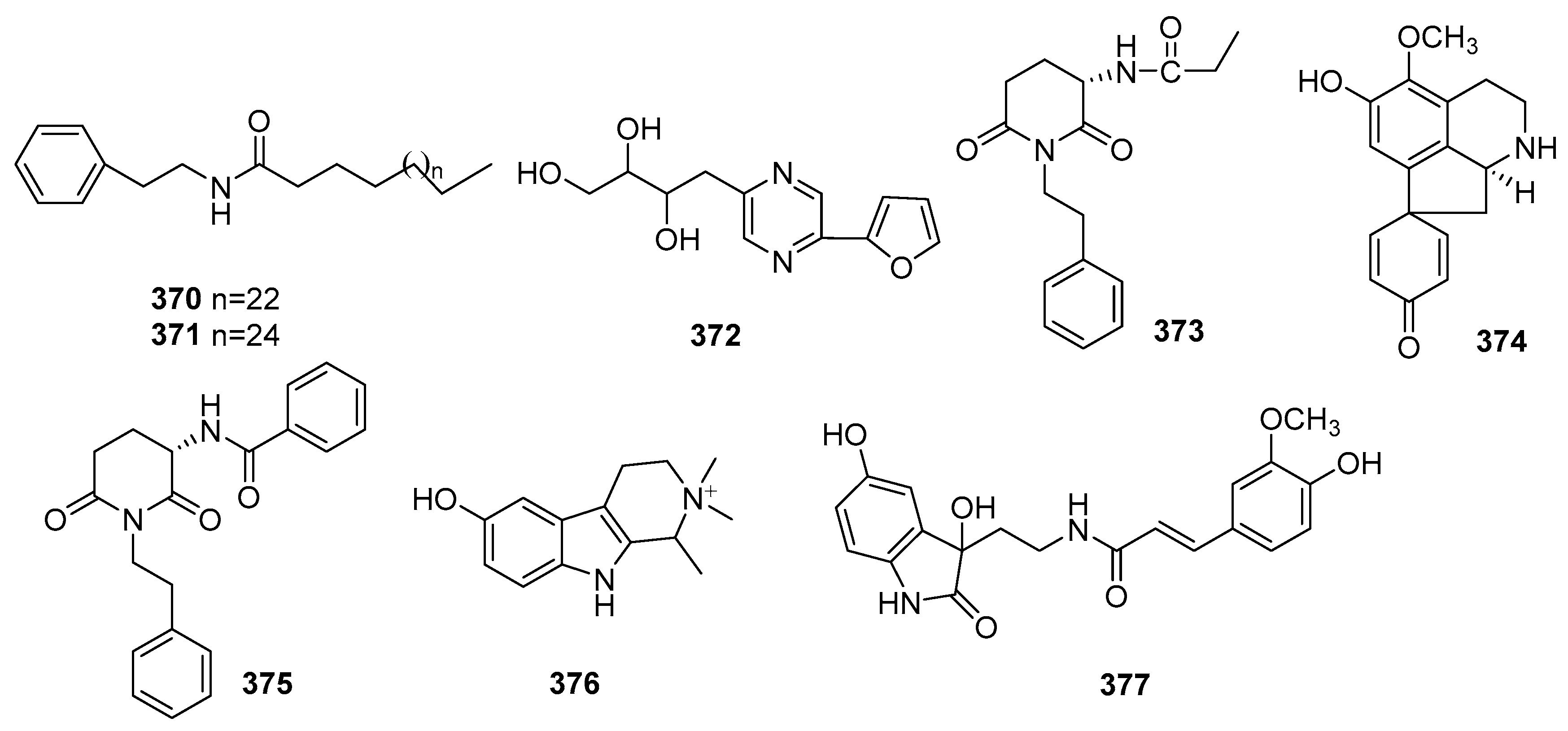
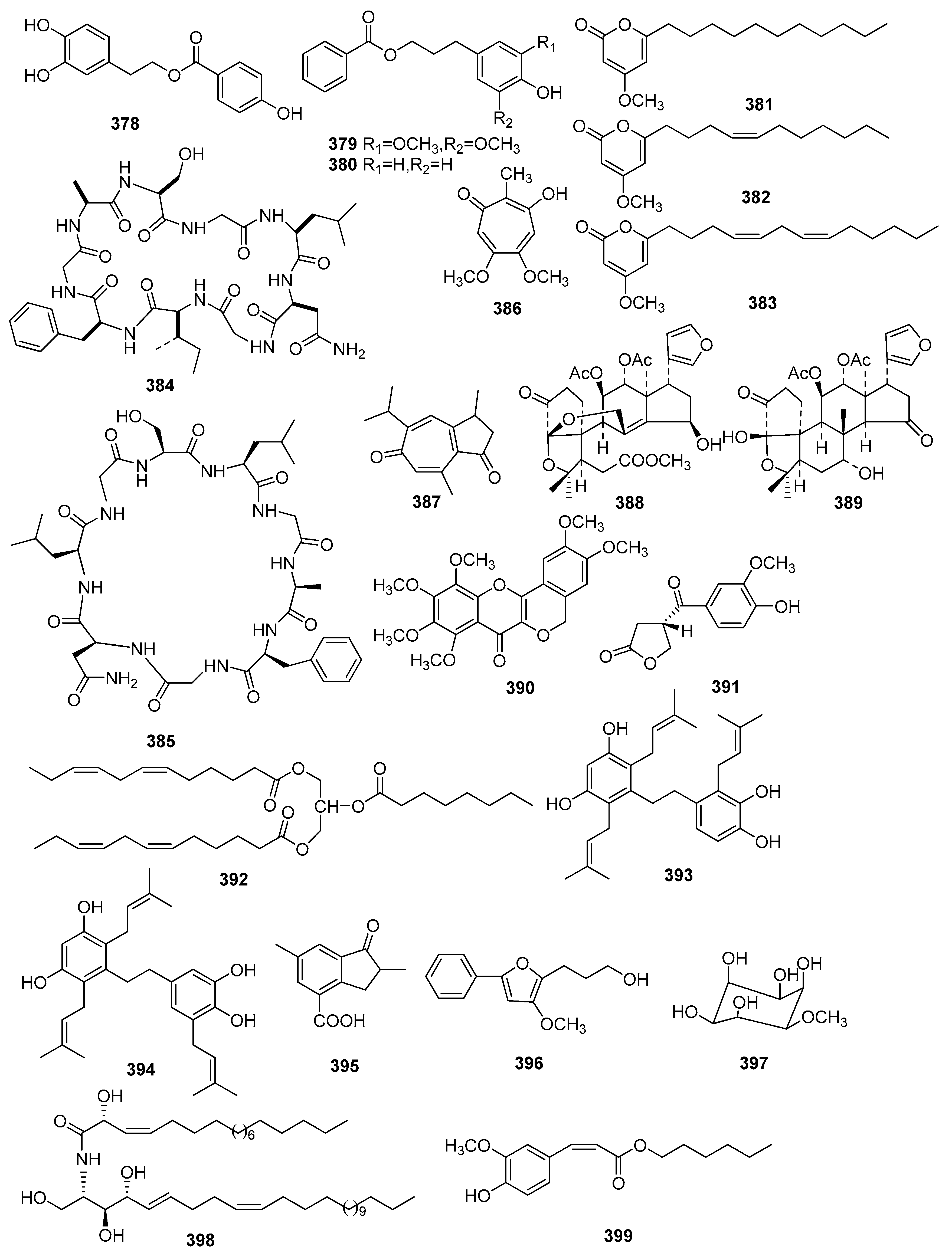
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 1 | ent-3,13E-clerodadiene-15-formate | C21H34O2 | C. sylvaticus | [12] |
| 2 | 9-[2-(2(5H)-furanone-4-yl)ethyl]-4,8,9-trimethyl-1,2,3,4,5,6,7,8-octahydronaphthalene-4-carboxylic acid | C20H28O4 | C. crassifolius | [14] |
| 3 | 9-[2-(2(5H)-furanone-4-yl)ethyl]-4,8,9-trimethyl-1,2,3,4,5,6,7,8-octahydronaphthalene-4-carboxylic ester | C21H30O4 | C. crassifolius | [14] |
| 4 | Centrafricine I | C21H24O6 | C. mayumbensis | [19] |
| 5 | Marrubiagenin | C20H28O4 | C. glabellus | [15] |
| 6 | Methyl 15,16-epoxy-3,13(16),14-ent-clerodatrien-18,19-olide-17-carboxylate | C21H26O5 | C. oblongifolius | [29] |
| 7 | Dimethyl 15,16-epoxy-12-oxo-3,13(16),14-ent-clerodatriene-17,18-dicarboxylate | C22H28O6 | C. oblongifolius | [29] |
| 8 | Isoteucvin | C19H20O5 | C. jatrophoides | [30] |
| 9 | Jatrophoidin | C21H22O7 | C. jatrophoides | [30] |
| 10 | 8-Epicordatin | C21H26O6 | C. palanostigma | [31] |
| 11 | laevigatbenzoate | C27H31O5 | C. laevigatus | [13] |
| 12 | 3,4,15,16-diepoxy-cleroda-13(16),14-diene-12,17-olide | C20H26O4 | C. oblongifolius | [22] |
| 13 | Crassifolin A | C21H30O4 | C. crassifolius | [16] |
| 14 | Crassifolin B | C20H29O4 | C. crassifolius | [16] |
| 15 | Crassifolin C | C21H24O5 | C. crassifolius | [16] |
| 16 | Crassifolin D | C21H24O6 | C. crassifolius | [16] |
| 17 | Crassifolin E | C20H23O6 | C. crassifolius | [16] |
| 18 | Crassifolin F | C23H29O7 | C. crassifolius | [16] |
| 19 | Crassifolin G | C19H20O6 | C. crassifolius | [16] |
| 20 | Methyl 3-oxo-12-epibarbascoate | C21H26O6 | C. urucurana | [32] |
| 21 | Laevinoids A | C20H22O5 | C. laevigatus | [20] |
| 22 | Laevinoids B | C20H23O5Cl | C. laevigatus | [20] |
| 23 | Crotonolide A | C20H18O6 | C. laui | [21] |
| 24 | Crotonolide B | C21H24O6 | C. laui | [21] |
| 25 | Isocrotonolide B | C21H24O6 | C. laui | [21] |
| 26 | Crotonolide C | C23H26O8 | C. laui | [21] |
| 27 | Isocrotonolide C | C23H26O8 | C. laui | [21] |
| 28 | Crotonolide D | C21H26O6 | C. laui | [21] |
| 29 | Isocrotonolide D | C21H26O6 | C. laui | [21] |
| 30 | Crotonolide E | C20H26O4 | C. laui | [21] |
| 31 | Crotonolide F | C20H26O4 | C. laui | [21] |
| 32 | Crotonolide G | C20H32O | C. laui | [21] |
| 33 | Crotonolide H | C20H32O4 | C. laui | [21] |
| 34 | 12-Deoxycrotonolide H | C20H32O3 | C. laui | [21] |
| 35 | Crotonoligaketone | C23H26O8 | C. oligandrum | [33] |
| 36 | Crotonpene A | C20H26O3 | C. yanhuii | [23] |
| 37 | Crotonpene B | C21H28O5 | C. yanhuii | [23] |
| 38 | Crassifolin I | C20H22O6 | C. crassifolius | [34] |
| 39 | Crassifolin H | C19H20O5 | C. crassifolius | [34] |
| 40 | Crotoeurin A | C38H36O1 | C. euryphyllus | [25] |
| 41 | Crotoeurin B | C20H24O6 | C. euryphyllus | [25] |
| 42 | Crotoeurin C | C20H22O6 | C. euryphyllus | [25] |
| 43 | 3-Oxo-15,16-epoxy-4α,12-dihydroxy-ent-neo-clerodan-13(16),14-diene | C20H30O4 | C. limae | [35] |
| 44 | 15,16-Epoxy-3α,4α,12-trihydroxy-ent-neo-clerodan- 13(16),14-diene | C20H32O4 | C. limae | [35] |
| 45 | 3α,4α,15,16-Tetrahydroxy-ent-neo-cleroda-13E-ene | C20H36O4 | C. limae | [35] |
| 46 | Cracroson A | C19H21O6 | C. crassifolius | [26] |
| 47 | Cracroson B | C20H22O6 | C. crassifolius | [26] |
| 48 | Cracroson C | C19H19O4N | C. crassifolius | [26] |
| 49 | Crassifolin J | C20H20O5 | C. crassifolius | [36] |
| 60 | Crotocorylifuran-2-one | C22H24O8 | C.megalocarpoides | [27] |
| 61 | Megalocarpoidolide D | C22H22O8 | C.megalocarpoides | [27] |
| 62 | 7,8-Dehydrocrotocorylifuran | C22H24O7 | C.megalocarpoides | [27] |
| 63 | Megalocarpoidolide E | C22H24O8 | C.megalocarpoides | [27] |
| 64 | Megalocarpoidolide F | C22H24O8 | C.megalocarpoides | [27] |
| 65 | Megalocarpoidolide G | C22H24O9 | C.megalocarpoides | [27] |
| 66 | Megalocarpoidolide H | C24H28O10 | C.megalocarpoides | [27] |
| 67 | Launine K | C27H36O3 | C. laui | [37] |
| 68 | Crassin A | C17H20O4 | C. crassifolius | [17] |
| 69 | Crassin B | C17H20O4 | C. crassifolius | [17] |
| 70 | Crassin C | C21H24O6 | C. crassifolius | [17] |
| 71 | Crassin D | C20H20O5 | C. crassifolius | [17] |
| 72 | Crassin E | C19H20O3 | C. crassifolius | [17] |
| 73 | Crassin F | C19H18O7 | C. crassifolius | [17] |
| 74 | Crassin G | C20H26O5 | C. crassifolius | [17] |
| 75 | Crassin H | C21H30O5 | C. crassifolius | [17] |
| 76 | Crassifolius A | C20H22O5 | C. crassifolius | [38] |
| 77 | Crassifolius B | C21H24O6 | C. crassifolius | [38] |
| 78 | Crassifolius C | C21H26O5 | C. crassifolius | [38] |
| 79 | Crolaevinoid C | C27H28O6 | C. laevigatus | [39] |
| 80 | Crolaevinoid D | C27H32O8 | C. laevigatus | [39] |
| 81 | Crolaevinoid E | C20H28O6 | C. laevigatus | [39] |
| 82 | Crolaevinoid F | C21H30O5 | C. laevigatus | [39] |
| 83 | Norcrassifolin | C19H18O4 | C. crassifolius | [28] |
| 84 | Hypolein A | C20H26O4 | C. hypoleucus | [24] |
| 85 | Hypolein B | C20H28O3 | C. hypoleucus | [24] |
| 86 | Hypolein C | C20H28O3 | C. hypoleucus | [24] |
| 87 | Cracroson E | C19H20O6 | C. crassifolius | [40] |
| 88 | Cracroson F | C19H20O6 | C. crassifolius | [40] |
| 89 | Cracroson G | C21H26O7 | C. crassifolius | [40] |
| 90 | 12-Epi-megalocarpoidolide D | C22H22O8 | C. oligandrus | [18] |
| 91 | Crotonolins A | C22H22O10 | C. oligandrus | [18] |
| 92 | Crotonolins B | C22H22O10 | C. oligandrus | [18] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 93 | 12-O-isobutyrylphorbol-13-decanoate | C34H52O8 | C. tiglium | [45] |
| 94 | 12-O-(2-methyl)butyrylphorbol-13-octanoate | C33H50O8 | C. tiglium | [45] |
| 95 | 12-O-[(2R)-N,N-dimethyl-3-methylbutanoyl]-4-deoxyphorbol 13-acetate | C29H43NO7 | C. ciliatoglandulifer | [41] |
| 96 | 12-O-[(2S)-N,N-dimethyl-3-methylbutanoyl]-4-deoxyphorbol 13-acetate | C29H43NO7 | C. ciliatoglandulifer | [41] |
| 97 | 12-O-[(2R)-N,N-Dimethyl-3-methylbutanoyl]phorbol 13-acetate | C29H43NO8 | C. ciliatoglandulifer | [41] |
| 98 | 12-O-[3-Methyl-2-butenoyl]-4-deoxyphorbol 13-acetate | C27H36NO7 | C. ciliatoglandulifer | [41] |
| 99 | 12-O-(2-methyl)butyrylphorbol-13-tiglate | C30H42O8 | C. tiglium | [46] |
| 100 | 12-O-tiglylphorbol-13-propionate | C28H38O8 | C. tiglium | [46] |
| 101 | 13-O-acetylphorbol-20-oleate | C40H62O8 | C. tiglium | [46] |
| 106 | 12-O-tiglyl-4-deoxy-4α-phorbol-13-(2-methyl)butyrate | C30H42O7 | C. tiglium | [46] |
| 107 | Alienusolin | C42H66O8 | C. alienus | [42] |
| 108 | 12-O-acetyl-5,6-didehydro-7-oxophorbol-13-yl 2-methylbutanoate | C27H36O9 | C. tiglium | [47] |
| 109 | 12-O-acetyl-5,6-didehydro-7-oxophorbol-13-yl2-methylpropanoate | C26H34O9 | C. tiglium | [47] |
| 110 | 12-Oacetyl-5,6-didehydro-6,7-dihydro-7-hydroxyphorbol-13-yl 2-methylbutanoate | C27H38O9 | C. tiglium | [47] |
| 111 | 12-O-decanoyl-7-hydroperoxy-phorbol-5-ene-13-acetate | C32H42O10 | C. mauritianus | [43] |
| 112 | 20-deoxy-20-oxophorbol12-tiglate 13-(2-methyl)butyrate | C30H40O8 | C. tiglium | [48] |
| 113 | 12-O-acetylphorbol-13-isobutyrate | C26H36O8 | C. tiglium | [48] |
| 114 | 12-O-benzoylphorbol-13-(2-methyl)butyrate | C32H40O8 | C. tiglium | [48] |
| 115 | 12-O-tiglyl-7-oxo-5-ene-phorbol-13-(2-methyl)butyrate | C30H40O9 | C. tiglium | [48] |
| 116 | 13-O-(2-metyl)butyryl-4-deoxy-4a-phorbol | C25H36O6 | C. tiglium | [48] |
| 117 | Crotignoid A | C30H42O10 | C. tiglium | [49] |
| 118 | Crotignoid B | C29H40O10 | C. tiglium | [49] |
| 119 | Crotignoid C | C30H42O9 | C. tiglium | [49] |
| 120 | Crotignoid D | C29H40O9 | C. tiglium | [49] |
| 121 | Crotignoid E | C29H38O9 | C. tiglium | [49] |
| 122 | Crotignoid F | C28H36O9 | C. tiglium | [49] |
| 123 | Crotignoid G | C30H44O8 | C. tiglium | [49] |
| 124 | Crotignoid H | C29H38O8 | C. tiglium | [49] |
| 125 | Crotignoid I | C30H44O8 | C. tiglium | [49] |
| 126 | Crotignoid J | C31H38O8 | C. tiglium | [49] |
| 127 | Crotignoid K | C29H34O7 | C. tiglium | [49] |
| 128 | Crotusin A | C36H54O10 | C. caudatus | [44] |
| 129 | Crotusin B | C46H72O11 | C. caudatus | [44] |
| 130 | Crotusin C | C36H52O11 | C. caudatus | [44] |
| 131 | 12-O-tiglylphorbol-4-deoxy- 4β-phorbol-13-acetate | C27H36O7 | C. tiglium | [50] |
| 132 | 12-O-tiglylphorbol-4-deoxy-4β-phorbol-13-hexadecanoate | C41H64O7 | C. tiglium | [50] |
| 133 | 13-O-acetylphorbol-4-deoxy-4β-phorbol-20-oleate | C40H62O7 | C. tiglium | [50] |
| 134 | 13-O-acetylphorbol-4-deoxy-4β-phorbol-20-linoleate | C40H60O7 | C. tiglium | [50] |
| 135 | 4-deoxy-20-oxophorbol 12-tiglyl 13-acetate | C27H34O7 | C. tiglium | [51] |
| 136 | 7-oxo-5-ene-phorbol-13-(2-methylbutyrate) | C25H34O8 | C. tiglium | [51] |
| 137 | 7-hydroxyl-phorbol-5-ene-13-(2-methyl)butyrate | C25H36O8 | C. tiglium | [51] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 149 | Caracasine | C21H30O3 | C. caracasana | [53] |
| 150 | Caracasine acid | C20H28O3 | C. caracasana | [53] |
| 151 | Kongensin A | C22H30O5 | C. kongensis | [56] |
| 152 | Kongensin B | C22H30O6 | C. kongensis | [56] |
| 153 | Kongensin C | C20H28O5 | C. kongensis | [56] |
| 154 | Kongensin D | C20H28O4 | C. kongensis | [57] |
| 155 | Kongensin E | C26H36O7 | C. kongensis | [57] |
| 156 | Kongensin F | C24H34O5 | C. kongensis | [58] |
| 157 | Crotonkinin A | C20H30O2 | C. tonkinensis | [62] |
| 158 | Crotonkinin B | C22H32O4 | C. tonkinensis | [62] |
| 159 | 14-epi-hyalic acid | C20H28O4 | C. argyrophylloides | [63] |
| 160 | 14-[(2-methylbutanoyl)oxy]-3,4-seco-ent-kaura-4(19),16-dien-3-oic acid | C25H39O4 | C. megistocarpus | [54] |
| 161 | 14-{[(2Z)-2-methylbut-2-enoyl]oxy}-3,4-seco-ent-kaura-4(19),16-dien-3-oic acid | C25H37O4 | C. megistocarpus | [54] |
| 162 | ent-11β-acetoxykaur-16-en-18-ol | C22H34O3 | C. tonkinensis | [64] |
| 163 | ent-11α-hydroxy-18-acetoxykaur-16-ene | C22H34O3 | C. tonkinensis | [64] |
| 164 | ent-14β-hydroxy-18-acetoxykaur-16-ene | C22H34O3 | C. tonkinensis | [64] |
| 165 | ent-7α-hydroxy-18-acetoxykaur-16-ene | C22H34O3 | C. tonkinensis | [64] |
| 166 | ent-14S*-hydroxykaur-16-en-19-oic acid | C20H30O3 | C. pseudopulchellus | [65] |
| 167 | ent-14S*,17-dihydroxykaur-15-en-19-oic acid | C20H30O4 | C. pseudopulchellus | [65] |
| 168 | ent-3,4-seco-17-oxo-kaur-4(19),15(16)-dien-3-oic acid | C20H28O3 | C. oblongifolius | [55] |
| 169 | Crotonkinin C | C22H30O5 | C. tonkinensis | [66] |
| 170 | Crotonkinin D | C24H34O6 | C. tonkinensis | [66] |
| 171 | Crotonkinin E | C24H34O5 | C. tonkinensis | [66] |
| 172 | Crotonkinin F | C24H34O5 | C. tonkinensis | [66] |
| 173 | Crotonkinin G | C23H36O5 | C. tonkinensis | [66] |
| 174 | Crotonkinin H | C22H36O4 | C. tonkinensis | [66] |
| 175 | Crotonkinin I | C24H36O5 | C. tonkinensis | [66] |
| 176 | Crotonkinin J | C23H34O5 | C. tonkinensis | [66] |
| 177 | 14β-hydroxy-3-oxo-ent-kaur-16-ene | C20H30O2 | C. kongensis | [67] |
| 178 | Kongeniod A | C21H30O3 | C. kongensis | [59] |
| 179 | Kongeniod B | C21H30O4 | C. kongensis | [59] |
| 180 | Kongeniod C | C23H32O5 | C. kongensis | [59] |
| 181 | 15-oxo-17(10′-α-pinenyl)-kauran-18-oic acid | C30H44O3 | C. limae | [35] |
| 182 | Micansinoic acid | C40H58O7 | C. micans | [60] |
| 183 | Isomicansinoic acid | C40H58O7 | C. micans | [60] |
| 184 | Dimethylester of micansinoic | C42H62O7 | C. micans | [60] |
| 185 | Methyl-micansinoic acid | C41H60O7 | C. micans | [60] |
| 186 | Ethyl-micansinoic acid | C42H62O7 | C. micans | [60] |
| 187 | Crotonkinensin C | C40H62O8 | C. tonkinensis | [61] |
| 188 | Crotonkinensin D | C44H66O10 | C. tonkinensis | [61] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 189 | Crotocascarin A | C25H32O7 | C. cascarilloides | [68] |
| 190 | Crotocascarin B | C25H32O7 | C. cascarilloides | [68] |
| 191 | Crotocascarin C | C25H32O8 | C. cascarilloides | [68] |
| 192 | Crotocascarin D | C25H32O6 | C. cascarilloides | [68] |
| 193 | Crotocascarin E | C26H34O8 | C. cascarilloides | [68] |
| 194 | Crotocascarin F | C24H30O7 | C. cascarilloides | [68] |
| 195 | Crotocascarin G | C24H30O7 | C. cascarilloides | [68] |
| 196 | Crotocascarin H | C24H30O8 | C. cascarilloides | [68] |
| 197 | Crotocascarin α | C24H32O8 | C. cascarilloides | [68] |
| 198 | Crotocascarin β | C24H32O7 | C. cascarilloides | [68] |
| 199 | (5β,6β)-5,6: 13,16-diepoxycrotofola-4(9),10(18),13,15-tetraen-1-one | C20H22O3 | C. argyrophyllus | [72] |
| 200 | (5β,6β)-5,6: 13,16-diepoxy-2-epicrotofola-4(9),10(18),13,15-tetraen-1-one | C20H22O3 | C. argyrophyllus | [72] |
| 201 | (5β,6β)-5,6: 13,16-diepoxy-16-hydroxycrotofola-4(9),10(18),13,15-tetraen-1-one | C20H22O4 | C. argyrophyllus | [72] |
| 202 | (5β,6β)-5,6: 13,16-diepoxy-16-hydroxy-2-epi-crotofola-4(9),10(18),13,15-tetraen-1-one | C20H22O4 | C. argyrophyllus | [72] |
| 203 | Crotocarasin A | C20H22O4 | C. caracasanus | [73] |
| 204 | Crotocarasin B | C20H22O4 | C. caracasanus | [73] |
| 205 | Crotocarasin C | C22H26O5 | C. caracasanus | [73] |
| 206 | Crotocarasin D | C22H26O5 | C. caracasanus | [73] |
| 207 | EBC-162 | C20H24O2 | C. insularis | [74] |
| 208 | EBC-233 | C20H24O4 | C. insularis | [74] |
| 209 | EBC-300 | C20H24O4 | C. insularis | [74] |
| 210 | EBC-240 | C20H26O5 | C. insularis | [74] |
| 211 | EBC-241 | C20H26O5 | C. insularis | [74] |
| 212 | Crotocascarin I | C20H24O5 | C. cascarilloides | [69] |
| 213 | Crotocascarin J | C20H24O6 | C. cascarilloides | [69] |
| 214 | Crotocascarin K | C20H24O5 | C. cascarilloides | [69] |
| 215 | Crotocascarin γ | C19H24O6 | C. cascarilloides | [69] |
| 216 | Crotocascarin L | C22H26O7 | C. cascarilloides | [70] |
| 217 | Crotocascarin M | C21H26O6 | C. cascarilloides | [70] |
| 218 | Crotocascarin N | C20H22O6 | C. cascarilloides | [70] |
| 219 | Crotocascarin O | C25H34O9 | C. cascarilloides | [70] |
| 220 | Crotocascarin P | C25H34O8 | C. cascarilloides | [70] |
| 221 | Crotocascarin Q | C25H32O7 | C. cascarilloides | [70] |
| 222 | Neocrotocascarin | C25H32O8 | C. cascarilloides | [70] |
| 223 | Crotodichogamoin A | C20H22O4 | C. dichogamus | [75] |
| 224 | Crotodichogamoin B | C20H22O2 | C. dichogamus | [75] |
| 225 | Cascarinoid A | C28H31NO5 | C. cascarilloides | [71] |
| 226 | Cascarinoid B | C28H31NO5 | C. cascarilloides | [71] |
| 227 | Cascarinoid C | C28H31NO6 | C. cascarilloides | [71] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 228 | Labdinine N | C20H34O3 | C. laui | [76] |
| 229 | ent-12,15-dioxo-3,4-seco-4,8,13-labdatrien-3-oic acid | C20H28O4 | C. stipuliformis | [78] |
| 230 | ent-12,15-epoxy-3,4-seco-4,8,12,14-labdatetraen-3-oic acid | C20H28O3 | C. stipuliformis | [78] |
| 231 | ent-15-nor-14-oxo-3,4-seco-4,8,12(E)-labdatrien-3-oic acid | C19H28O3 | C. stipuliformis | [78] |
| 232 | ent-12,15-dioxo-8,13-labdadien-3a-ol | C20H28O3 | C. stipuliformis | [78] |
| 233 | Crotonlaevin A | C18H30O4 | C. laevigatus | [79] |
| 234 | Crotonlaevin B | C20H32O5 | C. laevigatus | [79] |
| 235 | Crotonlaevin C | C21H34O5 | C. laevigatus | [79] |
| 236 | Crotonlaevin D | C18H30O3 | C. laevigatus | [79] |
| 237 | Crotonlaevin E | C20H32O5 | C. laevigatus | [79] |
| 238 | Crotonlaevin F | C22H34O6 | C. laevigatus | [79] |
| 239 | Crotonlaevin G | C22H36O5 | C. laevigatus | [79] |
| 240 | Crotonlaevin H | C22H36O5 | C. laevigatus | [79] |
| 241 | Crotonlaevin I | C20H34O4 | C. laevigatus | [79] |
| 242 | Crotonlaevin J | C20H30O3 | C. laevigatus | [79] |
| 243 | Crotonlaevin K | C20H28O3 | C. laevigatus | [79] |
| 244 | Crotonlaevin L | C20H30O4 | C. laevigatus | [79] |
| 245 | Crotonlaevin M | C20H30O4 | C. laevigatus | [79] |
| 246 | Crotonlaevin N | C20H30O3 | C. laevigatus | [79] |
| 247 | Crotonlaevin O | C20H30O3 | C. laevigatus | [79] |
| 248 | Crotonlaevin P | C20H30O3 | C. laevigatus | [79] |
| 249 | Crotonolide I | C20H34O3 | C. laui | [21] |
| 250 | Crotonolide J | C19H30O3 | C. laui | [21] |
| 251 | Launine A | C19H32O3 | C. laui | [82] |
| 252 | Launine B | C19H32O4 | C. laui | [82] |
| 253 | Launine C | C20H34O3 | C. laui | [82] |
| 254 | Launine D | C20H34O3 | C. laui | [82] |
| 255 | Launine E | C20H32O5 | C. laui | [82] |
| 256 | Launine F | C20H32O5 | C. laui | [82] |
| 257 | Launine G | C20H30O4 | C. laui | [82] |
| 258 | Launine H | C20H30O4 | C. laui | [82] |
| 259 | Launine I | C20H34O3 | C. laui | [82] |
| 260 | 15,16-epoxy-4-hydroxy-labda-13(16),14-dien-3,12-dione | C20H28O4 | C. jacobinensis | [77] |
| 261 | Crotondecalvatin A | C29H42O4 | C. decalvatus | [80] |
| 262 | Crotondecalvatin B | C30H42O6 | C. decalvatus | [80] |
| 263 | Bicrotonol A | C40H68O4 | C. crassifolius | [81] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 264 | Launine O | C20H34O2 | C. laui | [76] |
| 265 | Launine P | C21H36O2 | C. laui | [76] |
| 266 | Furanocembranoid 1 | C20H30O2 | C. oblongifolius | [83] |
| 267 | Furanocembranoid 2 | C20H30O3 | C. oblongifolius | [83] |
| 268 | Furanocembranoid 3 | C20H32O4 | C. oblongifolius | [83] |
| 269 | Furanocembranoid 4 | C20H32O5 | C. oblongifolius | [83] |
| 270 | Laevigatlactone A | C20H30O3 | C. laeVigatus | [84] |
| 271 | Laevigatlactone C | C20H30O3 | C. laeVigatus | [84] |
| 272 | Laevigatlactone B | C20H30O3 | C. laeVigatus | [84] |
| 273 | Laevigatlactone D | C20H30O3 | C. laeVigatus | [84] |
| 274 | Laevigatlactone E | C20H30O4 | C. laeVigatus | [84] |
| 275 | Laevigatlactone F | C20H30O5 | C. laeVigatus | [84] |
| 276 | (+)-[1R*,2S*,7S*,8S*,12R*]-7,8-Epoxy-2,12-cyclocembra-3E,10Zdien-20,10-olide | C20H28O3 | C. gratissimus | [85] |
| 277 | (+)-[1R*,10R*]-Cembra-2E,4E,7E,11Z-tetraen-20,10-olide | C20H28O2 | C. gratissimus | [85] |
| 278 | (+)-[1R*,4S*,10R*]-4-Hydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O3 | C. gratissimus | [85] |
| 279 | (−)-[1R*,4R*,10R*]-4-Hydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O3 | C. gratissimus | [85] |
| 280 | (−)-(1R*,4R*,10R*)-4-Methoxycembra-2E,7E,11Z-trien-20,10-olide | C21H32O3 | C. gratissimus | [86] |
| 281 | (−)-(1S*,4R*,10R*)-1-Hydroxy-4-methoxycembra-2E,7E,11Ztrien-20,10-olide | C21H32O4 | C. gratissimus | [86] |
| 282 | (−)-(1S*,4S*,10R*)-1,4-Dihydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O4 | C. gratissimus | [86] |
| 283 | (−)-(1S*,4S*,10R*)-1,4-Dihydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O4 | C. gratissimus | [86] |
| 284 | (+)-(10R*)-Cembra-1E,3E,7E,11Z,16-pentaen-20,10-olide | C20H26O | C. gratissimus | [86] |
| 285 | (+)-(10R*)-Cembra-1Z,3Z,7E,11Z,15-pentaen-20,10-olide | C20H26O | C. gratissimus | [86] |
| 286 | (+)-(5R*,10R*)-5-Methoxycembra-1E,3E,7E,11Z,15-pentaen-20,10-olide | C21H30O3 | C. gratissimus | [86] |
| 287 | (+)-(1S*,4S*,7R*,10R*)-1,4,7-Trihydroxycembra-2E,8(19),11Z-trien-20,10-olide | C20H30O5 | C. gratissimus | [86] |
| 288 | (−)-(1S*,4S*,7S*,10R*)-1,4,7-Trihydroxycembra-2E,8(19),11Z-trien-20,10-olide | C20H30O3 | C. gratissimus | [86] |
| 289 | (+)-(1S*,4R*,8S*,10R*)-1,4,8-Trihydroxycembra-2E,6E,11Z-trien-20,10-olide | C20H30O5 | C. gratissimus | [86] |
| 290 | Cembranoid 1 | C20H30O4 | C. longissimus | [87] |
| 291 | Cembranoid 2 | C20H30O3 | C. longissimus | [87] |
| 281 | (−)-(1S*,4R*,10R*)-1-Hydroxy-4-methoxycembra-2E,7E,11Ztrien-20,10-olide | C21H32O4 | C. gratissimus | [86] |
| 282 | (−)-(1S*,4S*,10R*)-1,4-Dihydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O4 | C. gratissimus | [86] |
| 283 | (−)-(1S*,4S*,10R*)-1,4-Dihydroxycembra-2E,7E,11Z-trien-20,10-olide | C20H30O4 | C. gratissimus | [86] |
| 284 | (+)-(10R*)-Cembra-1E,3E,7E,11Z,16-pentaen-20,10-olide | C20H26O | C. gratissimus | [86] |
| 285 | (+)-(10R*)-Cembra-1Z,3Z,7E,11Z,15-pentaen-20,10-olide | C20H26O | C. gratissimus | [86] |
| 286 | (+)-(5R*,10R*)-5-Methoxycembra-1E,3E,7E,11Z,15-pentaen-20,10-olide | C21H30O3 | C. gratissimus | [86] |
| 287 | (+)-(1S*,4S*,7R*,10R*)-1,4,7-Trihydroxycembra-2E,8(19),11Z-trien-20,10-olide | C20H30O5 | C. gratissimus | [86] |
| 288 | (−)-(1S*,4S*,7S*,10R*)-1,4,7-Trihydroxycembra-2E,8(19),11Z-trien-20,10-olide | C20H30O3 | C. gratissimus | [86] |
| 289 | (+)-(1S*,4R*,8S*,10R*)-1,4,8-Trihydroxycembra-2E,6E,11Z-trien-20,10-olide | C20H30O5 | C. gratissimus | [86] |
| 290 | Cembranoid 1 | C20H30O4 | C. longissimus | [87] |
| 291 | Cembranoid 2 | C20H30O3 | C. longissimus | [87] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 292 | Isolophanthin E | C20H30O3 | C. megalocarpoides | [27] |
| 293 | rel-(1R,4aR,5R,8R)-methyl-7-(1-(methoxycarbonyl)vinyl)-5,8-diacetoxy-1,2,3,4a,5,6,7,8,9,10,10a-dodecahydro-1,4a-dimethyl-2-oxophenanthrene-1-carboxylate | C26H34O9 | C. argyrophylloides | [63] |
| 294 | Crotontomentosin A | C20H26O2 | C. caudatus | [88] |
| 295 | Crotontomentosin B | C20H30O3 | C. caudatus | [88] |
| 296 | Crotontomentosin D | C20H24O2 | C. caudatus | [88] |
| 297 | Crotontomentosin C | C20H28O2 | C. caudatus | [88] |
| 298 | Crotontomentosin E | C22H32O3 | C. caudatus | [88] |
| 299 | Crotolaevigatone A | C20H24O3 | C. laevigatus | [89] |
| 300 | Crotolaevigatone B | C20H26O2 | C. laevigatus | [89] |
| 301 | Crotolaevigatone C | C20H26O3 | C. laevigatus | [89] |
| 302 | Crotolaevigatone D | C20H28O4 | C. laevigatus | [89] |
| 303 | Crotolaevigatone E | C19H24O2 | C. laevigatus | [89] |
| 304 | Crotolaevigatone F | C20H30O4 | C. laevigatus | [89] |
| 305 | Crotolaevigatone G | C20H30O4 | C. laevigatus | [89] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 306 | 1,4-dihydroxy-2E,6E,12E-trien-5-one-casbane | C20H30O3 | C.nepetaefolius | [90] |
| 307 | 4-hydroxy-2E,6E,12E-5-one-casbane | C20H29O3 | C.nepetaefolius | [90] |
| 308 | 1-hydroxy-(2E,6Z,12E)-casba-2,6,12-triene-4,5-dione | C20H28O3 | C.argyrophyllus | [91] |
| 309 | 6E,12E-casba-1,3,6,12-tetraen-1,4-epoxy-5-one | C20H26O2 | C.argyrophyllus | [91] |
| 310 | (2E,5β,6E,12E)-5-hydroxycasba-2,6,12-trien-4-one | C20H30O2 | C. argyrophyllus | [72] |
| 311 | EBC-324 | C20H28O5 | C. insularis | [92] |
| 312 | EBC-329 | C20H26O4 | C. insularis | [92] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 313 | Crassifoliusin A | C21H24O5 | C.crassifolius | [95] |
| 314 | Crotontomentosin F | C21H30O3 | C.caudatus | [88] |
| 315 | Crolaevinoid A | C27H30O7 | C.laevigatus | [39] |
| 316 | Crolaevinoid B | C20H26O4 | C.laevigatus | [39] |
| 317 | Crothalimene A | C20H26O4 | C.dichogamus | [75] |
| 318 | Crothalimene B | C20H30O2 | C.dichogamus | [75] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 319 | ent-3β-hydroxypimara-8(14),9,15-trien-12-one | C20H28O2 | C.insularis | [98] |
| 320 | EBC-316 | C20H26O2 | C.insularis | [99] |
| 321 | EBC-325 | C20H26O4 | C.insularis | [99] |
| 322 | EBC-326 | C20H26O4 | C.insularis | [99] |
| 323 | EBC-327 | C20H24O3 | C.insularis | [99] |
| 324 | EBC-345 | C20H30O4 | C.insularis | [99] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 325 | 3-hydroxycleistantha-13(17),15-diene | C20H32O | C.oblongifolius | [93] |
| 326 | 3,4-seco-cleistantha-4(18),13(17),15-trien-3-oic acid | C20H30O2 | C.oblongifolius | [93] |
| 327 | rel-(5β,8α,10α)-8-hydroxy-13-methylpodocarpa-9(11),13-diene-3,12-dione | C18H25O3 | C.regelianus | [94] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 328 | Crotonkinensin A | C20H24O4 | C.tonkinensis | [100] |
| 329 | Crotonkinensin B | C20H26O3 | C.tonkinensis | [100] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 330 | Crotobarin | C22H28O5 | C.barorum | [101] |
| 331 | Crotogoudin | C20H26O3 | C.goudotii | [101] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 332 | Launine L | C20H32O3 | C.laui | [37] |
| 333 | Launine M | C20H32O2 | C.laui | [37] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 334 | Crolaevinoid G | C20H24O6 | C.laevigatus | [39] |
| 335 | Crolaevinoid H | C21H26O6 | C.laevigatus | [39] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 336 | Steenkrotin A | C20H24O6 | C.steenkampianus | [102] |
| 337 | Steenkrotin B | C20H28O7 | C.steenkampianus | [102] |
| 338 | Norcrassin A | C17H22O7 | C.crassifolius | [81] |
| 339 | Cracroson D | C21H26O6 | C.crassifolius | [40] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 340 | 6α -methoxy-cyperene | C16H26O | C.muscicarpa | [103] |
| 341 | rel-(1R,4S,6R,7S,8αR)-decahydro-1-(hydroxymethyl)-4,9,9-trimethyl-4,7-(epoxymethano)azulen-6-ol | C15H26O3 | C. regelianus | [94] |
| 342 | Blumenol A | C13H20O3 | C. pedicellatus | [104] |
| 343 | Crocrassins A | C15H24O3 | C. crassifolius | [105] |
| 344 | Crocrassins B | C16H26O3 | C. crassifolius | [105] |
| 345 | 1,3,5-cadinatriene-(7R,10S)-diol | C15H25O2 | C. dichogamus | [75] |
| 346 | Cracroson H | C15H22O3 | C. crassifolius | [40] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 347 | Pseudopulchellol | C25H40O | C.pseudopulchellus | [106] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 348 | 3α-hydroxy-urs-12,15-dien | C30H48O | C.bonplandianum | [107] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 349 | Cyperenoic acid-9-O-β-d-glucopyranoside | C21H32O8 | C. crassifolius | [108] |
| 350 | 3-O-β-d-xylopyranosylspathodic acid | C35H56O9 | C. lachnocarpus | [109] |
| 351 | Helichrysoside-3′-methylether | C31H28O14 | C. zambesicus | [110] |
| 352 | Crotonionoside A | C29H42O11 | C. cascarilloides | [111] |
| 353 | Crotonionoside B | C30H44O12 | C. cascarilloides | [111] |
| 354 | Crotonionoside C | C24H42O12 | C. cascarilloides | [111] |
| 355 | Crotonionoside D | C31H46O14 | C. cascarilloides | [111] |
| 356 | Crotonionoside E | C35H52O16 | C. cascarilloides | [111] |
| 357 | Crotonionoside F | C24H42O11 | C. cascarilloides | [111] |
| 358 | Crotonionoside G | C29H40O11 | C. cascarilloides | [111] |
| 359 | Oblongionoside A | C24H42O12 | C. oblongifolius | [112] |
| 360 | Oblongionoside B | C24H42O12 | C. oblongifolius | [112] |
| 361 | Oblongionoside C | C24H44O11 | C. oblongifolius | [112] |
| 362 | Oblongionoside D | C24H44O11 | C. oblongifolius | [112] |
| 363 | Oblongionoside E | C19H36O8 | C. oblongifolius | [112] |
| 364 | Oblongionoside F | C19H36O8 | C. oblongifolius | [112] |
| 365 | Blumenol A glucoside | C19H30O8 | C. pedicellatus | [10] |
| 366 | Sparsioside | C53H102O10 | C. sparsiorus | [113] |
| 367 | 3,12-dioxo-15,16-epoxy-4α-hydroxy-6-(β-glucopyranosyl)-ent-neo-clerodan-13(16),14-diene | C26H38O10 | C. limae | [35] |
| 368 | Isocrotofolane glucoside | C26H38O9 | C. cascarilloides | [69] |
| 369 | 2-methoxyphenol-β-d-(6-O-β-d-apiofuranosyl) glucopyranoside | C18H26O11 | C. cascarilloides | [69] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 370 | Crotamide A | C36H65NO | C. sparsiflorus | [114] |
| 371 | Crotamide B | C38H69NO | C. sparsiflorus | [114] |
| 372 | Crotonine | C12H14N2O4 | C. tiglium | [97] |
| 373 | Crotonimide A | C16H20N2O3 | C. pullei | [115] |
| 374 | Crotsparsidine | C17H17O3N | C. sparsiflorus | [96] |
| 375 | Crotonimide C. | C20H20N2O3 | C. alienus | [42] |
| 376 | 6-Hydroxy-1-methyl-2-dimethyl-3,4-tetrahydro-b-carbo-line | C14H19N2O | C. heliotropiifolius | [116] |
| 377 | N-trans-feruloyl-3,5-dihydroxyindolin-2-one | C20H20N2O6 | C. echioides | [117] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 378 | 2′-(3′’,4′’-dihydroxyphenyl)-ethyl-4-hydroxybenzoate | C15H14O5 | C.sylvaticus | [118] |
| 379 | 3-(4-hydroxy-3,5-dimethoxyphenyl)-propyl benzoate | C18H20O5 | C.hutchinsonianus | [119] |
| 380 | 3-(4-hydroxyphenyl)-propyl benzoate | C16H16O3 | C.hutchinsonianus | [119] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 381 | Crotonpyrone A | C17H28O3 | C.crassifolius | [120] |
| 382 | Crotonpyrone B | C17H26O3 | C.crassifolius | [120] |
| 383 | Crotonpyrone C | C19H28O3 | C.crassifolius | [121] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 384 | Crotogossamide | C37H56N10O11 | C.gossypifolius | [122] |
| 385 | [1−9-NαC]-crourorb A1 | C37H56N10O11 | C.urucurana | [123] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 386 | Crototropone | C10H12O4 | C.zehntneri | [124] |
| 387 | Pernambucone | C15H18O2 | C.argyroglossum | [125] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 388 | Musidunin | C31H38O11 | C.jatrophoides | [126] |
| 389 | Musiduol | C30H38O10 | C.jatrophoides | [126] |
| No. | Compound Name | Molecular Formula | Sources | Ref |
|---|---|---|---|---|
| 390 | Crotoncaudatin | C22H22O9 | C.caudatus | [127] |
| 391 | 8S-(−)-8-(4-hydroxy-3-methoxybenzoyl)-dihydrofuran-8(8′H)-one | C20H30O2 | C.kongensis | [67] |
| 392 | Lobaceride | C35H58O6 | C.lobatus | [129] |
| 393 | Laevifolin A | C29H38O4 | C.laevifolius | [128] |
| 394 | Laevifolin B | C29H38O4 | C.laevifolius | [128] |
| 395 | 2,6-Dimethyl-1-oxo-4-indanecarboxylic acid | C12H12O3 | C. steenkampianus | [102] |
| 396 | 3(3′-Methoxy-5′-phenylfuran-2′-yl)propan-1-ol | C14H16O3 | C. oblongifolius | [22] |
| 397 | Sparsifol | C7H15O6 | C. sparsiflorus | [96] |
| 398 | Sparsioamide | C43H81NO5 | C. sparsiflorus | [113] |
| 399 | hexyl Z-ferulate | C16H22O4 | C. laevigatus | [89] |
| Compounds | Tumor Cell Line | Activity (IC50) | Ref |
|---|---|---|---|
| Methyl 15,16-epoxy-3,13(16),14-ent-clerodatrien-18,19-olide-17-carboxylate (6) | HuCCA-1 | 36.0 μg/mL | [29] |
| KB | 26.0 μg/mL | [29] | |
| HeLa | 30.0 μg/mL | [29] | |
| MDA-MB231 | 29.0 μg/mL | [29] | |
| T47D | 10.0 μg/mL | [29] | |
| Dimethyl-15,16-epoxy-12-oxo-3,13 (16)14-ent-clerodatriene-17,18-dicarboxylate (7) | HuCCA-1 | 39.0 μg/mL | [29] |
| KB | 27.0 μg/mL | [29] | |
| HeLa | 29.0 μg/mL | [29] | |
| MDA-MB231 | 27.0 μg/mL | [29] | |
| T47D | 25.0 μg/mL | [29] | |
| Laevigatbenzoate (8) | HeLa | 45.4 μM | [13] |
| Crotonolide A (23) | HL-60 | 9.42 μM | [21] |
| P-388 | 7.45 μM | [21] | |
| 15-oxo-17(10′-α-pinenyl)-kauran-18-oic acid (181) | HCT-116 | 7.14 μg/mL | [35] |
| OVCAR-8 | 8.19 μg/mL | [35] | |
| SF-295 | >10.0 μg/mL | [35] | |
| Launine K (67) | HeLa | 14.5 μM | [37] |
| MCF-7 | 62.5 μM | [37] | |
| Crassin H (75) | HL-60 | 11.8 ± 2.1 μM | [17] |
| A549 | 5.2 ± 0.4 μM | [17] | |
| Crassifolius A (76) | Hep3B | 17.91 μM | [38] |
| HepG2 | 42.04 μM | [38] | |
| Cracroson D (339) | T24 | 14.48 ± 0.65 μM | [40] |
| A549 | 25.64 ± 2.14 μM | [40] | |
| Cracroson E (87) | T24 | 22.99 ± 1.76 μM | [40] |
| A549 | 51.88 ± 14.07μM | [40] | |
| Hela | 3.9 μM | [48] | |
| DU145 | 7.2 μM | [48] | |
| A549 | 5.8 μM | [48] | |
| SGC-7091 | 13 μM | [48] | |
| H1975 | 10 μM | [48] | |
| HL60 | 12 μM | [48] | |
| 293T | 291.6 μM | [48] | |
| LX-2 | >500.0 μM | [48] | |
| 12-O-benzoylphorbol-13-(2-methyl)butyrate (114) | K562 | 15 μM | [48] |
| MOLT-4 | 12 μM | [48] | |
| U937 | 17 μM | [48] | |
| MCF-7 | 20 μM | [48] | |
| Hela | 4.6 μM | [48] | |
| DU145 | 4.3 μM | [48] | |
| A549 | 6.9 μM | [48] | |
| SGC-7091 | 10 μM | [48] | |
| H1975 | 3.3 μM | [48] | |
| HL60 | 6.8 μM | [48] | |
| 293T | 420.4 μM | [48] | |
| LX-2 | >500.0 μM | [48] | |
| 12-O-tiglyl-7-oxo-5-ene-phorbol-13-(2-methyl)butyrate (115) | K562 | 17 μM | [48] |
| MOLT-4 | 4.8 μM | [48] | |
| U937 | 21 μM | [48] | |
| MCF-7 | 20 μM | [48] | |
| Hela | 5.0 μM | [48] | |
| DU145 | 10 μM | [48] | |
| A549 | 19 μM | [48] | |
| SGC-7091 | 23 μM | [48] | |
| H1975 | 10 μM | [48] | |
| HL60 | 10 μM | [48] | |
| 293T | 455.3 μM | [48] | |
| LX-2 | >500.0 μM | [48] | |
| 13-O-(2-metyl)butyryl-4-deoxy-4a-phorbol (116) | K562 | 8.0 μM | [48] |
| MOLT-4 | 9.9 μM | [48] | |
| U937 | 18 μM | [48] | |
| MCF-7 | 24 μM | [48] | |
| H1975 | 10 μM | [48] | |
| HL60 | 10 μM | [48] | |
| 293T | 455.3 μM | [48] | |
| LX-2 | >500.0 μM | [48] | |
| Hela | 10 μM | [48] | |
| DU145 | 10 μM | [48] | |
| A549 | 4.5 μM | [48] | |
| SGC-7091 | 5.4 μM | [48] | |
| H1975 | 3.3 μM | [48] | |
| HL60 | 9.8 μM | [48] | |
| 293T | 191.0 μM | [48] | |
| LX-2 | >500.0 μM | [48] | |
| Crotignoid A (117) | HL-60 | 1.61 μM | [49] |
| A549 | 2.85 μM | [49] | |
| Crotignoid B (118) | HL-60 | 22.1 μM | [49] |
| A549 | 31.0 μM | [49] | |
| Crotignoid C (119) | HL-60 | 32.3 μM | [49] |
| A549 | 5.03 μM | [49] | |
| Crotignoid D (120) | HL-60 | 19.8 μM | [49] |
| A549 | 10.2 μM | [49] | |
| Crotignoid F (122) | HL-60 | 44.6 μM | [49] |
| A549 | 6.96 μM | [49] | |
| Crotignoid G (123) | HL-60 | 22.1 μM | [49] |
| A549 | 3.89 μM | [49] | |
| Crotignoid H (124) | HL-60 | 9.97 μM | [49] |
| A549 | 8.08 μM | [49] | |
| Crotignoid I (125) | HL-60 | 14.8 μM | [49] |
| A549 | 24.4 μM | [49] | |
| Crotignoid J (126) | HL-60 | 14.2 μM | [49] |
| A549 | 29.5 μM | [49] | |
| Crotusin A (128) | HL-60 | 12.53 ± 0.37 μM | [44] |
| SMMC-7721 | 7.06 ± 0.72 μM | [44] | |
| A549 | 9.69 ± 0.41 μM | [44] | |
| MCF-7 | 9.56 ± 0.76 μM | [44] | |
| SW480 | 14.88 ± 0.43 μM | [44] | |
| Crotusin B (129) | HL-60 | 19.39 ± 0.46 μM | [44] |
| SMMC-7721 | 21.13 ± 0.29 μM | [44] | |
| A549 | 14.66 ± 1.66 μM | [44] | |
| MCF-7 | 1.49 ± 0.23 μM | [44] | |
| SW480 | 31.21 ± 3.20 μM | [44] | |
| Crotusin C (130) | HL-60 | 4.19 ± 0.15 μM | [44] |
| SMMC-7721 | 3.87 ± 0.12 μM | [44] | |
| A549 | 2.44 ± 0.35 μM | [44] | |
| MCF-7 | 0.49 ± 0.04 μM | [44] | |
| SW480 | 2.89 ± 0.01 μM | [44] | |
| 12-O-tiglylphorbol-4-deoxy-4β-phorbol-13-acetate (131) | SNU387 | 59.5 ± 2.1 μM | [50] |
| SNU398 | 43.7 ± 1.5 μM | [50] | |
| 12-O-tiglylphorbol-4-deoxy-4β-phorbol-13-hexadecanoate (132) | SNU387 | 30.2 ± 1.4 μM | [50] |
| SNU398 | 91.2 ± 3.7 μM | [50] | |
| 13-O-acetylphorbol-4-deoxy-4β-phorbol-20-oleate (133) | SNU387 | 1.9 ± 0.2 μM | [50] |
| SNU398 | 13.5 ± 1.1 μM | [50] | |
| 13-O-acetylphorbol-4-deoxy-4β-phorbol-20-linoleate (134) | SNU387 | 0.71 ± 0.08 μM | [50] |
| SNU398 | 18.2 ± 1.7 μM | [50] | |
| 4-deoxy-20-oxophorbol 12-tiglyl 13-acetate (135) | K562 | 0.03 μM | [51] |
| A549 | 6.88 μM | [51] | |
| Huh-7 | 3.85 μM | [51] | |
| 7-oxo-5-ene-phorbol-13-(2-methylbutyrate) (136) | K562 | 0.03 μM | [51] |
| A549 | 6.33 μM | [51] | |
| Huh-7 | 20.9 μM | [51] | |
| 7-hydroxyl-phorbol-5-ene-13-(2-methyl)butyrate (137) | K562 | 0.07 μM | [51] |
| A549 | 8.86 μM | [51] | |
| Huh-7 | 11.6 μM | [51] | |
| 13-O-(2-metyl)butyryl-phorbol (139) | K562 | 0.05 μM | [51] |
| A549 | 43.5 μM | [51] | |
| Huh-7 | 34.2 μM | [51] | |
| 7-keto-12-O-tiglylphorbol-13-acetate (140) | HL-60 | 6.22 ± 3.24 μg/mL | [52] |
| A549 | 18.0 ± 9.48 μg/mL | [52] | |
| Phorbol-13-isobutyrate (148) | HL-60 | 0.22 ± 0.15 μg/mL | [52] |
| 14-epi-hyalic acid (159) | HL-60 | 8.2 μM | [63] |
| Kongeniod A (178) | HL-60 | 1.27 ± 0.24 μM | [59]] |
| A549 | 5.74 ± 0.25 μM | [59] | |
| Kongeniod B (179) | HL-60 | 0.47 ± 0.04 μM | [59] |
| A549 | 3.25 ± 0.91 μM | [59] | |
| Kongeniod C (180) | HL-60 | 0.58 ± 0.17 μM | [59] |
| Crotonkinensin D (188) | MCF-7 | 9.4 ± 1.7 μM | [61] |
| MCF-7/TAMR | 2.6 ± 0.9 μM | [61] | |
| MCF-7/ADR | 18.9 ± 0.6 μM | [61] | |
| MDA-MB-231 | 22.0 ± 0.9 μM | [61] | |
| EBC-162 (207) | HL-60 | 15 μg/mL | [74] |
| HT29 | 15 μg/mL | [74] | |
| MCF-7 | 30 μg/mL | [74] | |
| MM96 | 10 μg/mL | [74] | |
| NNF | 20 μg/mL | [74] | |
| K562 | 50 μg/mL | [74] | |
| EBC-233 (208) | HL-60 | 10 μg/mL | [74] |
| HT29 | 80 μg/mL | [74] | |
| MCF-7 | 20 μg/mL | [74] | |
| MM96 | 6 μg/mL | [74] | |
| NNF | 50 μg/mL | [74] | |
| K562 | 50 μg/mL | [74] | |
| EBC-300 (209) | HL-60 | 35 μg/mL | [74] |
| HT29 | 100 μg/mL | [74] | |
| MCF-7 | 100 μg/mL | [74] | |
| MM96 | 80 μg/mL | [74] | |
| NNF | 80 μg/mL | [74] | |
| K562 | 100 μg/mL | [74] | |
| EBC-240 (210) | HL-60 | 45 μg/mL | [74] |
| HT29 | 80 μg/mL | [74] | |
| MCF-7 | 50 μg/mL | [74] | |
| MM96 | 12 μg/mL | [74] | |
| NNF | 80 μg/mL | [74] | |
| K562 | 60 μg/mL | [74] | |
| EBC-241 (211) | HL-60 | 40 μg/mL | [74] |
| HT29 | 80 μg/mL | [74] | |
| MCF-7 | 40 μg/mL | [74] | |
| MM96 | 12 μg/mL | [74] | |
| NNF | 75 μg/mL | [74] | |
| K562 | 60 μg/mL | [74] | |
| Furanocembranoid 1 (266) | BT474 | 7.8 μg/mL | [83] |
| CHAGO | 7.0 μg/mL | [83] | |
| Hep-G2 | 5.6 μg/mL | [83] | |
| KATO-3 | 5.9 μg/mL | [83] | |
| SW-620 | 6.3 μg/mL | [83] | |
| Furanocembranoid 2 (267) | BT474 | 9.5 μg/mL | [83] |
| CHAGO | >10 μg/mL | [83] | |
| Hep-G2 | >10 μg/mL | [83] | |
| KATO-3 | 6.8 μg/mL | [83] | |
| SW-620 | 9.9 μg/mL | [83] | |
| Furanocembranoid 3 (268) | BT474 | 9.6 μg/mL | [83] |
| CHAGO | 7.1 μg/mL | [83] | |
| Hep-G2 | 5.7 μg/mL | [83] | |
| KATO-3 | 8.2 μg/mL | [83] | |
| SW-620 | 5.6 μg/mL | [83]] | |
| Furanocembranoid 4 (269) | BT474 | 9.6 μg/mL | [83] |
| CHAGO | 9.3 μg/mL | [83] | |
| Hep-G2 | 6.1 μg/mL | [83] | |
| KATO-3 | 8.1 μg/mL | [83] | |
| SW-620 | 6.0 μg/mL | [83] | |
| Laevigatlactone B (272) | Hela | 38.4 μM | [84] |
| (+)-[1R*,2S*,7S*,8S*,12R*]-7,8-Epoxy-2,12-cyclocembra-3E,10Zdien-20,10-olide (276) | PEO1 | 132 nM | [85] |
| PEO1TaxR | 200 nM | [85] | |
| (+)-[1R*,4S*,10R*]-4-Hydroxycembra-2E,7E,11Z-trien-20,10-olide (278) | PEO1 | 125 nM | [85] |
| PEO1TaxR | 135 nM | [85] | |
| Crotontomentosin A (294) | Hela | 24.0 ± 2.6 μM | [88] |
| Hep G2 | 87.9 ± 4.5 μM | [88] | |
| MDA-MB-231 | 54.1 ± 2.1 μM | [88] | |
| A549 | 40.6 ± 3.9 μM | [88] | |
| Crotontomentosin B (295) | Hela | >100 μM | [88] |
| Hep G2 | 28.1 ± 2.1 μM | [88] | |
| MDA-MB-231 | 28.7 ± 3.4 μM | [88] | |
| A549 | 29.1 ± 5.2 μM | [88] | |
| Crotontomentosin C (297) | Hela | 47.9 ± 3.3 μM | [88] |
| Hep G2 | 83.3 ± 5.3 μM | [88] | |
| MDA-MB-231 | >100 μM | [88] | |
| A549 | >100 μM | [88]] | |
| Crotontomentosin D (296) | Hela | 59.7 ± 4.5 μM | [88] |
| Hep G2 | >100 μM | [88] | |
| MDA-MB-231 | 49.3 ± 2.8 μM | [88] | |
| A549 | >100 μM | [88] | |
| Crotolaevigatone B (300) | A549 | 21.2 μM | [89] |
| MDA-MB-231 | 33.4 μM | [89] | |
| Crotolaevigatone G (305) | A549 | 25.6 μM | [89] |
| MDA-MB-231 | 32.7 μM | [89] | |
| EBC-324 (311) | MCF-7 | 40 μM | [92] |
| NFF | 50 μM | [92] | |
| K562 | 6 μM | [92] | |
| EBC-329 (312) | MCF-7 | 13 μM | [92] |
| NFF | 40 μM | [92] | |
| K562 | 0.6 μM | [92] | |
| ent-3β-hydroxypimara-8(14),9,15-trien-12-one (319) | NFF | 23 μg/mL | [98] |
| Hela | 13 μg/mL | [98] | |
| HT 29 | 13 μg/mL | [98] | |
| MCF-7 | 16 μg/mL | [98] | |
| MM96L | 2.8 μg/mL | [98] | |
| K562 | 17 μg/mL | [98] | |
| EBC-325 (321) | MCF-7 | 20 μM | [99] |
| NFF | 6 μM | [99] | |
| K562 | 3 μM | [99] | |
| EBC-326 (322) | MCF-7 | 14 μM | [99] |
| NFF | 6 μM | [99] | |
| K562 | 6 μM | [99] | |
| EBC-327 (323) | MCF-7 | 10 μM | [99] |
| NFF | 10 μM | [99] | |
| K562 | 10 μM | [99] | |
| 3-hydroxycleistantha-13(17),15-diene (325) | KATO-3 | 6.0 μg/mL | [93] |
| SW-620 | >10 μg/mL | [93] | |
| BT474 | 6.1 μg/mL | [93] | |
| Hep-G2 | 0.5 μg/mL | [93] | |
| CHAGO | 5.5 μg/mL | [93] | |
| 3,4-seco-cleistantha-4(18),13(17),15-trien-3-oic acid (326) | KATO-3 | 9.6 μg/mL | [93] |
| SW-620 | >10 μg/mL | [93] | |
| BT474 | 10 μg/mL | [93] | |
| Hep-G2 | 8.6 μg/mL | [93] | |
| CHAGO | >10 μg/mL | [93] | |
| Crotobarin (330) | KB | 2.5 ± 0.10 μM | [101] |
| HT29 | 2.1 ± 0.60 μM | [101] | |
| A549 | 0.79 ± 0.15 μM | [101] | |
| HL60 | 0.56 ± 0.02 μM | [101] | |
| Crotogoudin (331) | KB | 1.5 ± 0.03 μM | [101] |
| HT29 | 1.9 ± 0.25 μM | [101] | |
| A549 | 0.54 ± 0.02 μM | [101] | |
| HL60 | 0.49 ± 0.01 μM | [101] | |
| Crotonpyrone A (381) | Hela | 10.21 μg/mL | [120] |
| NCI-446 | 6.59 μg/mL | [120] | |
| Crotonpyrone B (382) | Hela | 9.54 μg/mL | [120] |
| [1−9-NαC]-crourorb A1 (385) | NCI-ADR/RES | 4.8 μM | [123] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.-H.; Liu, W.-Y.; Liang, Q. Chemical Constituents from Croton Species and Their Biological Activities. Molecules 2018, 23, 2333. https://doi.org/10.3390/molecules23092333
Xu W-H, Liu W-Y, Liang Q. Chemical Constituents from Croton Species and Their Biological Activities. Molecules. 2018; 23(9):2333. https://doi.org/10.3390/molecules23092333
Chicago/Turabian StyleXu, Wen-Hui, Wei-Yi Liu, and Qian Liang. 2018. "Chemical Constituents from Croton Species and Their Biological Activities" Molecules 23, no. 9: 2333. https://doi.org/10.3390/molecules23092333




