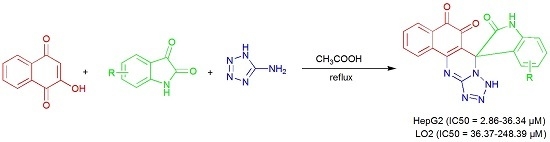
Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo[1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents
Abstract
:1. Introduction
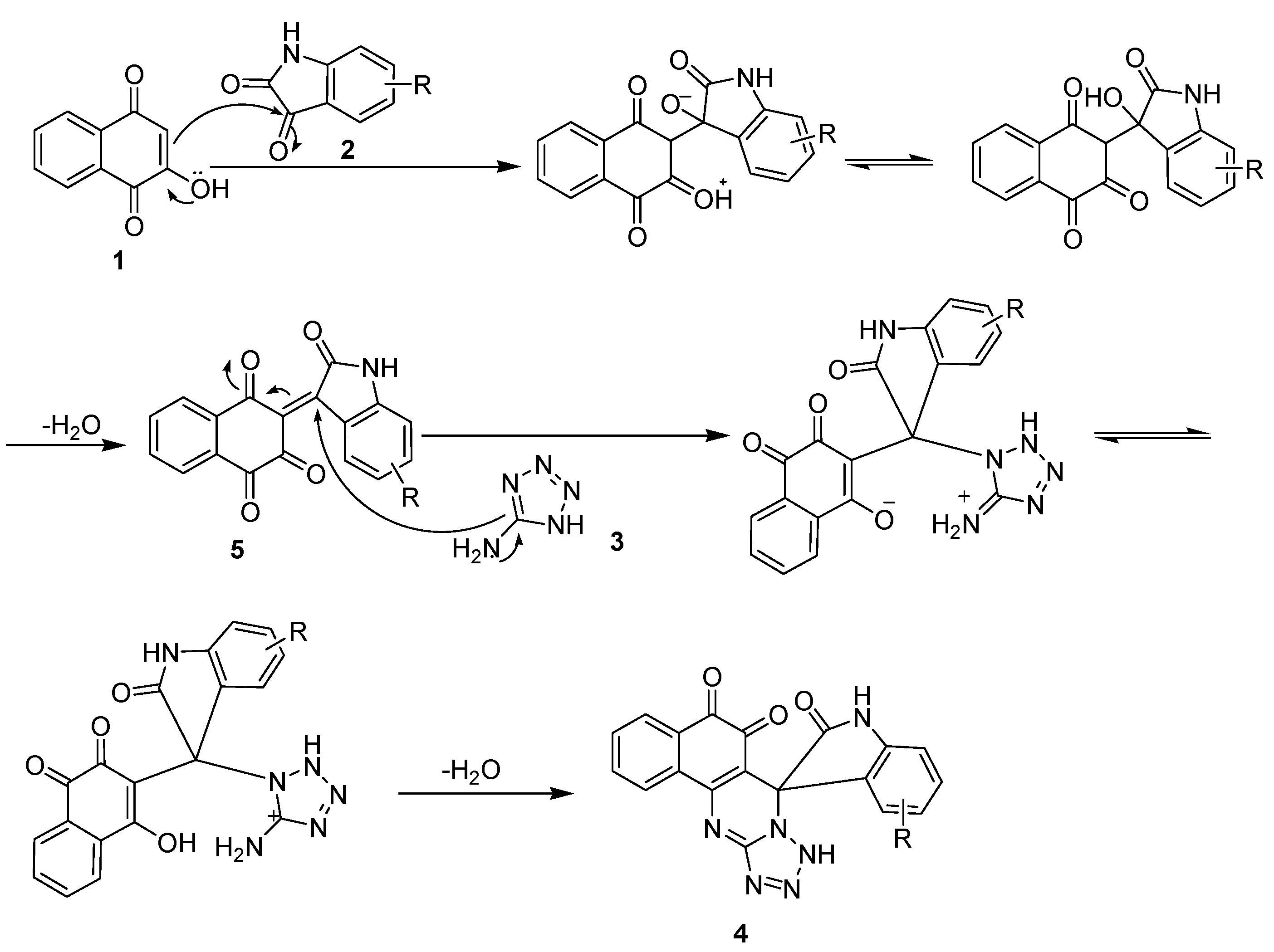
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Compound 4
3.3. Anti-Proliferative Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruijter, E.; Orru, R.V.A. Multicomponent reactions–opportunities for the pharmaceutical industry. Drug Discov. Today Technol. 2013, 10, e10–e15. [Google Scholar] [CrossRef] [PubMed]
- Ganem, B. Strategies for Innovation in Multicomponent Reaction Design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Yu, D.-Q.; Liu, H.-M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.P.; Kim, W.G.; Koshino, H.; Jung, J.; Yoo, I. Sesquiterpene O-naphthoquinones from the root bark of Ulmus. Phytochemistry 1996, 43, 425–430. [Google Scholar] [CrossRef]
- Errante, G.; La Motta, G.; Lagana, C.; Sarciron, M.E.; Barret, R. Synthesis and evaluation of antifungal activity of naphthoquinone derivatives. Eur. J. Med. Chem. 2006, 41, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Choi, Y.H.; Kim, N.D.; Park, Y.M.; Kim, G.Y. Anti-inflammatory effects of beta-lapachone in lipopolysaccharide-stimulated BV2 microglia. Int. Immunopharmacol. 2007, 7, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.K.; Singh, R.V.; Yadav, D.B. Synthesis and evaluation of novel 1,4-naphthoquinone derivatives as antiviral, antifungal and anticancer agents. Bioorg. Med. Chem. Lett. 2004, 14, 2901–2904. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Kaneko, M.; Iida, A.; Tokuda, H.; Nishimura, K. Stereoselective synthesis and cytotoxicity of a cancer chemopreventive naphthoquinone from Tabebuia avellanedae. Bioorg. Med. Chem. Lett. 2007, 17, 6417–6420. [Google Scholar] [CrossRef] [PubMed]
- Pink, J.J.; Planchon, S.; Tagliarino, M.C.; Varnes, M.E.; Siegel, D.; Boothman, D.A. NAD(P)H: Quinone qxidoreductase activity is the principal determinant of β-lapachone cytotoxicity. J. Biol. Chem. 2000, 275, 5416–5424. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hao, H.-P.; Wang, G.-J. NQO1-mediated biotransformation determines the cytotoxicity of tanshinone IIA. Chin. J. Nat. Med. 2012, 10, 353–357. [Google Scholar] [CrossRef]
- Bian, J.; Xu, L.; Deng, B.; Qian, X.; Fan, J.; Yang, X.; Liu, F.; Xu, X.; Guo, X.; Li, X.; et al. Synthesis and evaluation of (±)-dunnione and its ortho-quinone analogues as substrates for NAD(P)H:quinone oxidoreductase 1 (NQO1). Bioorg. Med. Chem. Lett. 2015, 25, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Q. Synthesis and biological evaluation of novel 1,2-naphthoquinones possessing tetrazolo[1,5-a]pyrimidine scaffolds as potent antitumor agents. RSC Adv. 2015, 5, 24960–24965. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 29, 500–536. [Google Scholar]
Sample Availability: Samples of the compounds 4 are available from the authors. |


| Entry | Solvent | Temperature/°C | Time/h | Yield/% 1 |
|---|---|---|---|---|
| 1 | EtOH | reflux | 10 | 20 |
| 2 | DMF | 120 | 6 | 29 |
| 3 | CH3COOH | reflux | 6 | 49 |
| 4 | CH3CN | reflux | 10 | 11 |
| 5 | CHCl3 | reflux | 10 | trace |
| 6 | H2O | reflux | 10 | trace |
| 7 | CH3COOH | 25 | 24 | - |
| 8 | CH3COOH | 50 | 24 | trace |
| 9 | CH3COOH | 100 | 10 | 22 |
| 10 | CH3COOH | 110 | 7 | 29 |
| Entry | R | Time/h | Product | m.p./°C | Yield/% |
|---|---|---|---|---|---|
| 1 | H | 6 | 4a | 275–277 | 49 |
| 2 | 5-Br | 8 | 4b | >300 | 31 |
| 3 | 5-Cl | 8 | 4c | >300 | 32 |
| 4 | 6-Br | 8 | 4d | 281–283 | 35 |
| 5 | 1-CH3-7-F | 5 | 4e | >300 | 52 |
| 6 | 7-Cl | 5 | 4f | >300 | 61 |
| 7 | 5-F | 5 | 4g | 297–299 | 69 |
| 8 | 7-Br | 5 | 4h | 286–289 | 57 |
| 9 | 1-C6H5 | 6 | 4i | >300 | 55 |
| 10 | 5-CH3 | 6 | 4j | 267–269 | 48 |
| 10 | 6-Cl | 7 | 4k | 291–293 | 45 |
| 12 | 6-OCH3 | 7 | 4l | 281–283 | 46 |
| 13 | 5-OCF3 | 8 | 4m | 273–276 | 36 |
| 14 | 7-CF3 | 7 | 4n | 288–290 | 47 |
| Comp. | IC50 (μM) | Selectivity Ratio 1 | |
|---|---|---|---|
| HepG2 | LO2 | ||
| 4a | 23.63 | 40.15 | 1.7 |
| 4b | 23.41 | 65.29 | 2.79 |
| 4c | 21.93 | 53.03 | 2.42 |
| 4d | 36.34 | 248.39 | 6.84 |
| 4e | 31.83 | 54 | 1.70 |
| 4f | 3.03 | 49.47 | 16.33 |
| 4g | 2.86 | 58.92 | 20.60 |
| 4h | 20.74 | 82.34 | 3.97 |
| 4i | 27.87 | 75.57 | 2.71 |
| 4j | 12.58 | 36.37 | 2.89 |
| 4k | 21.19 | 51.99 | 2.45 |
| 4l | 17.29 | 78.6 | 4.55 |
| 4m | 18.29 | 48.42 | 2.65 |
| 4n | 7.9 | 49.9 | 6.32 |
| TSA | 23.85 | 65.29 | 2.74 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Liu, Y.; Li, Y. Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo[1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents. Molecules 2018, 23, 2330. https://doi.org/10.3390/molecules23092330
Wu L, Liu Y, Li Y. Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo[1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents. Molecules. 2018; 23(9):2330. https://doi.org/10.3390/molecules23092330
Chicago/Turabian StyleWu, Liqiang, Yunxia Liu, and Yazhen Li. 2018. "Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo[1,5-a]Pyrimidine Hybrids as Potential Anticancer Agents" Molecules 23, no. 9: 2330. https://doi.org/10.3390/molecules23092330