Dietary Natural N-Acetyl-d-Glucosamine Prevents Bone Loss in Ovariectomized Rat Model of Postmenopausal Osteoporosis
Abstract
:1. Introduction
2. Results
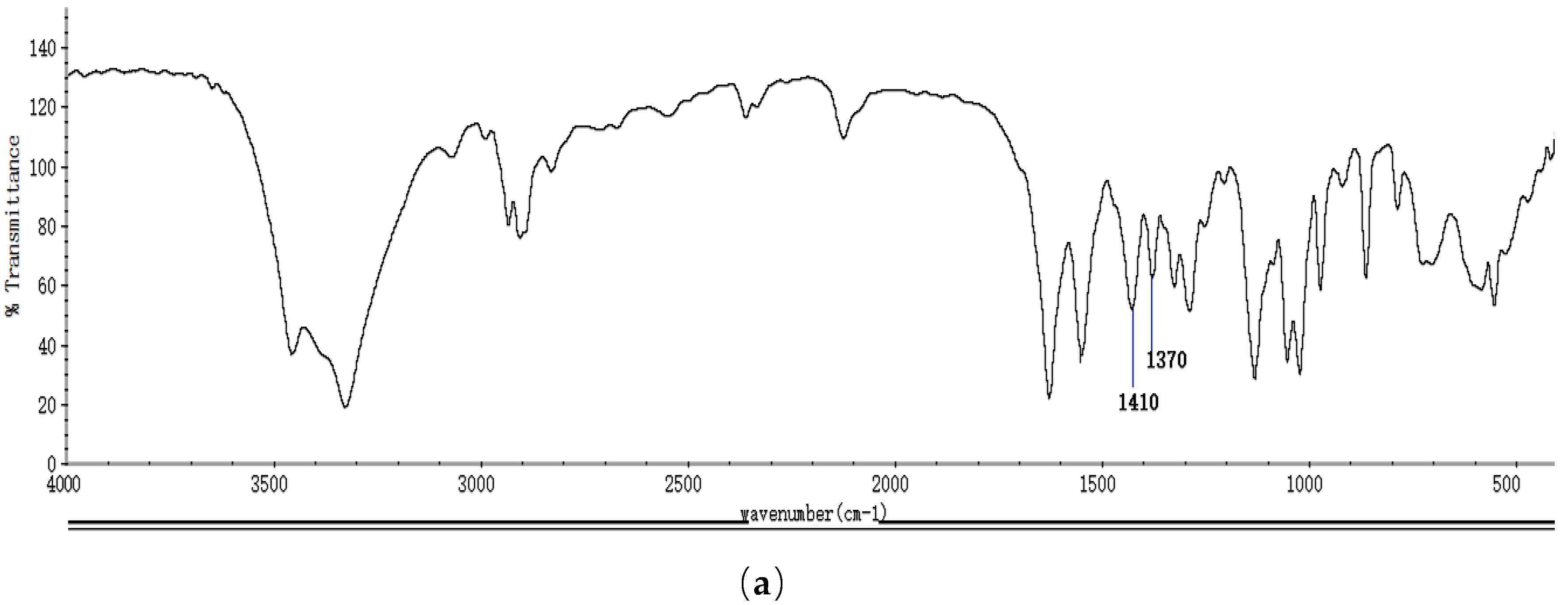
2.1. Identification and Properties Analysis of NAG
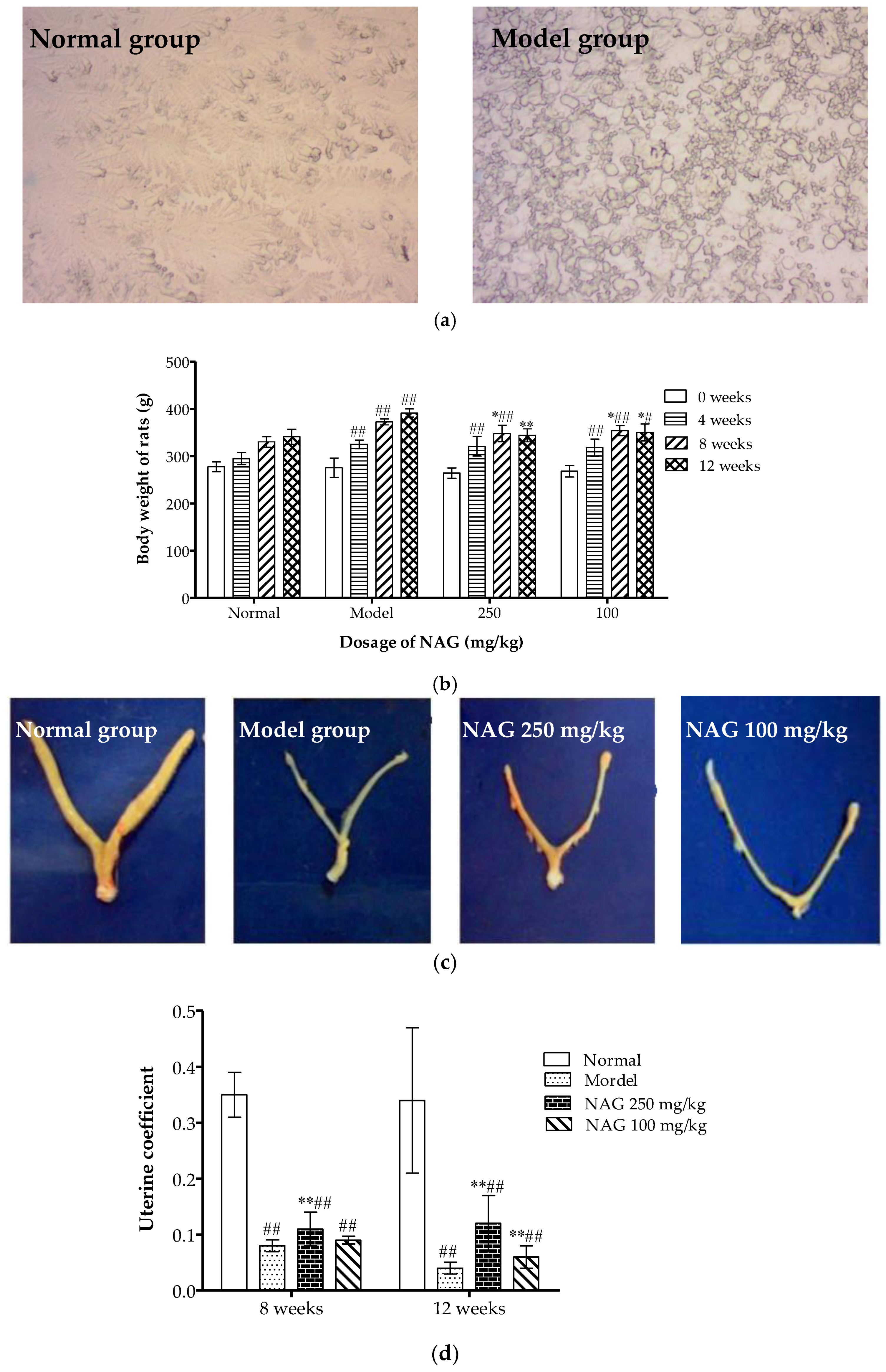
2.2. Dietary NAG Ameliorated Body and Uterine Weight of Ovariectomized Rats
2.3. NAG Consumption Changed Alkaline Phosphatase and Ca Contents in Serum of Ovariectomized Rats
2.4. NAG Administration Improved Bone Mechanical Properties of Ovariectomized Rats
2.5. Elevation of Bone Mineral Calcium Content in Femur and Tibia by NAG
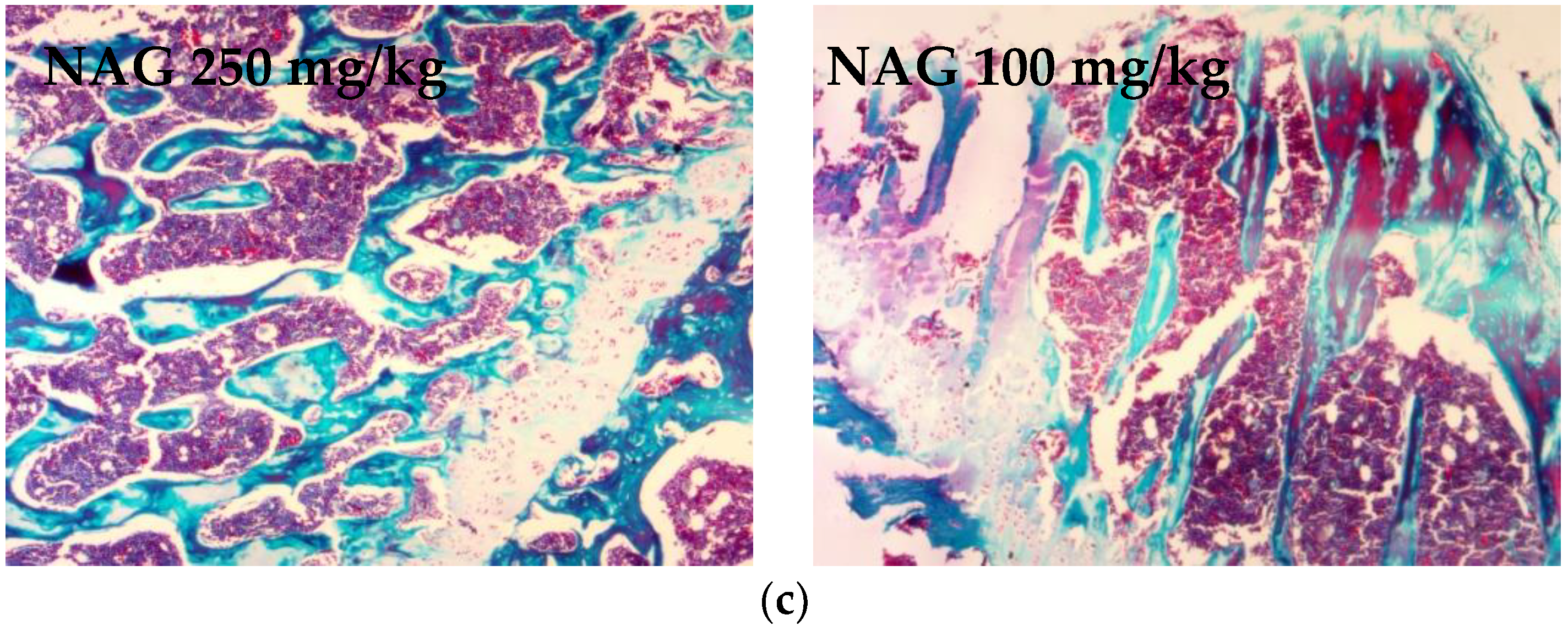
2.6. Antiosteoporotic Activity of NAG in Tibia Bones of Ovariectomized Rats
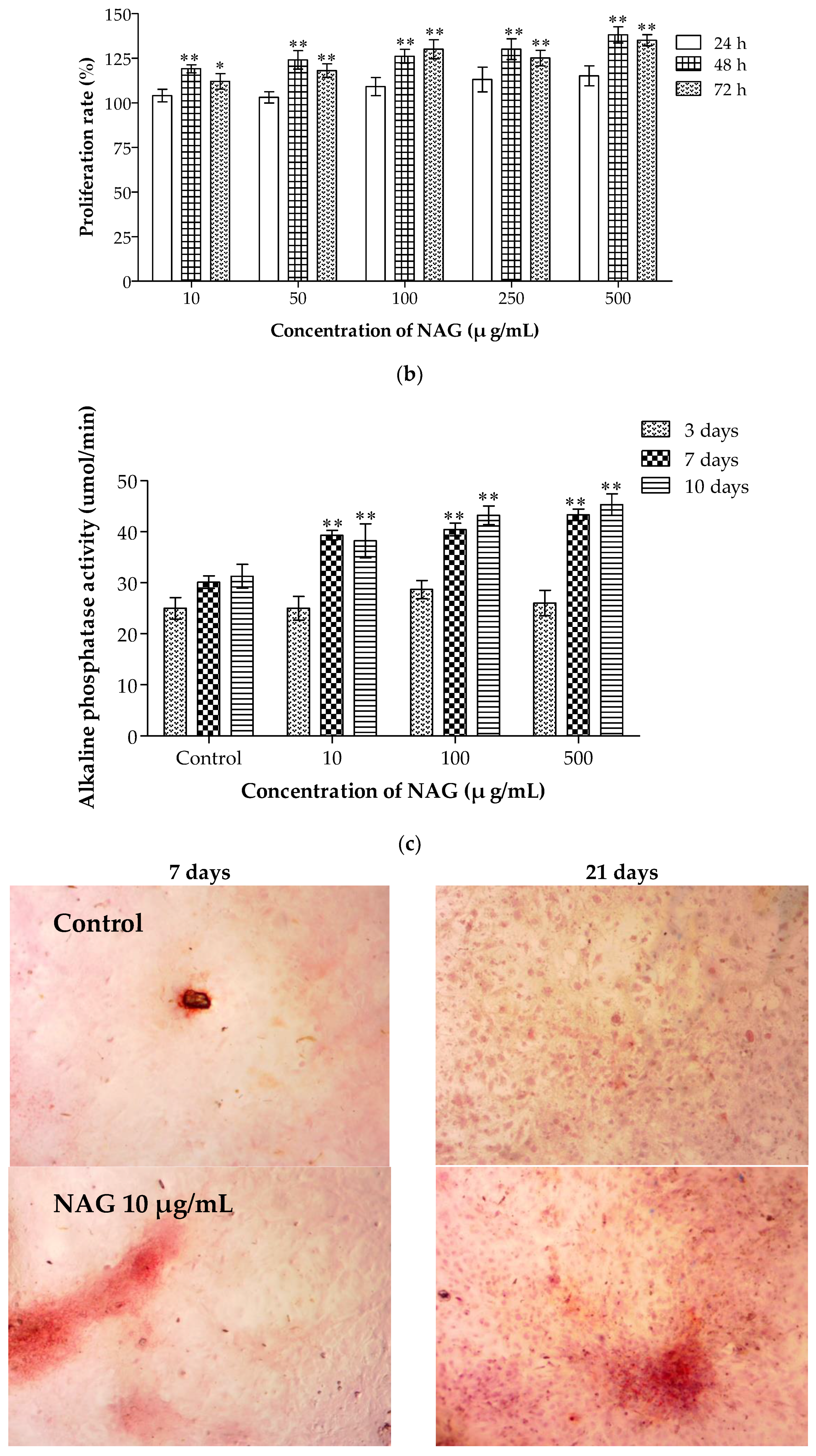
2.7. Elevation of Osteoblasts Proliferation and Differentiation by NAG
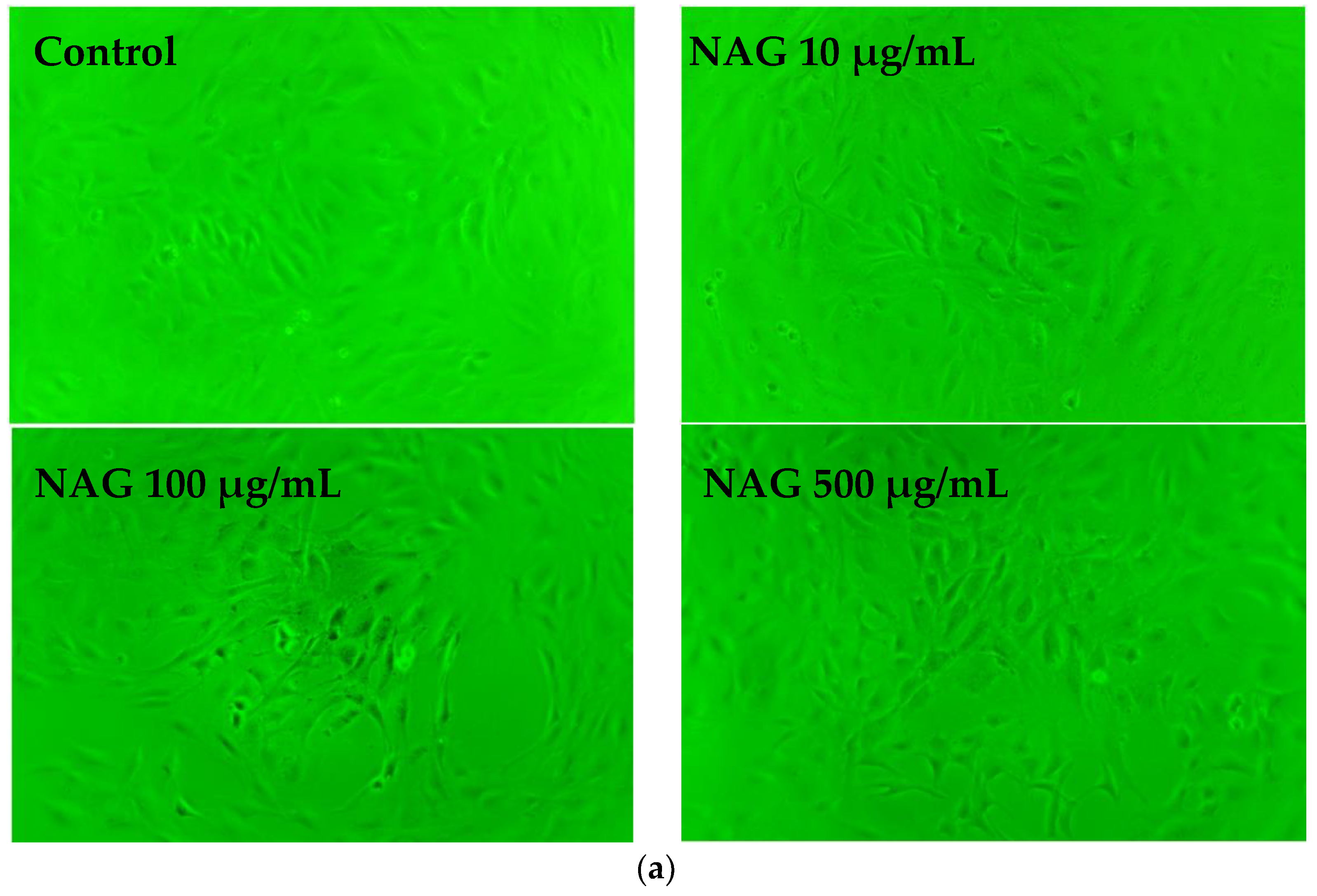
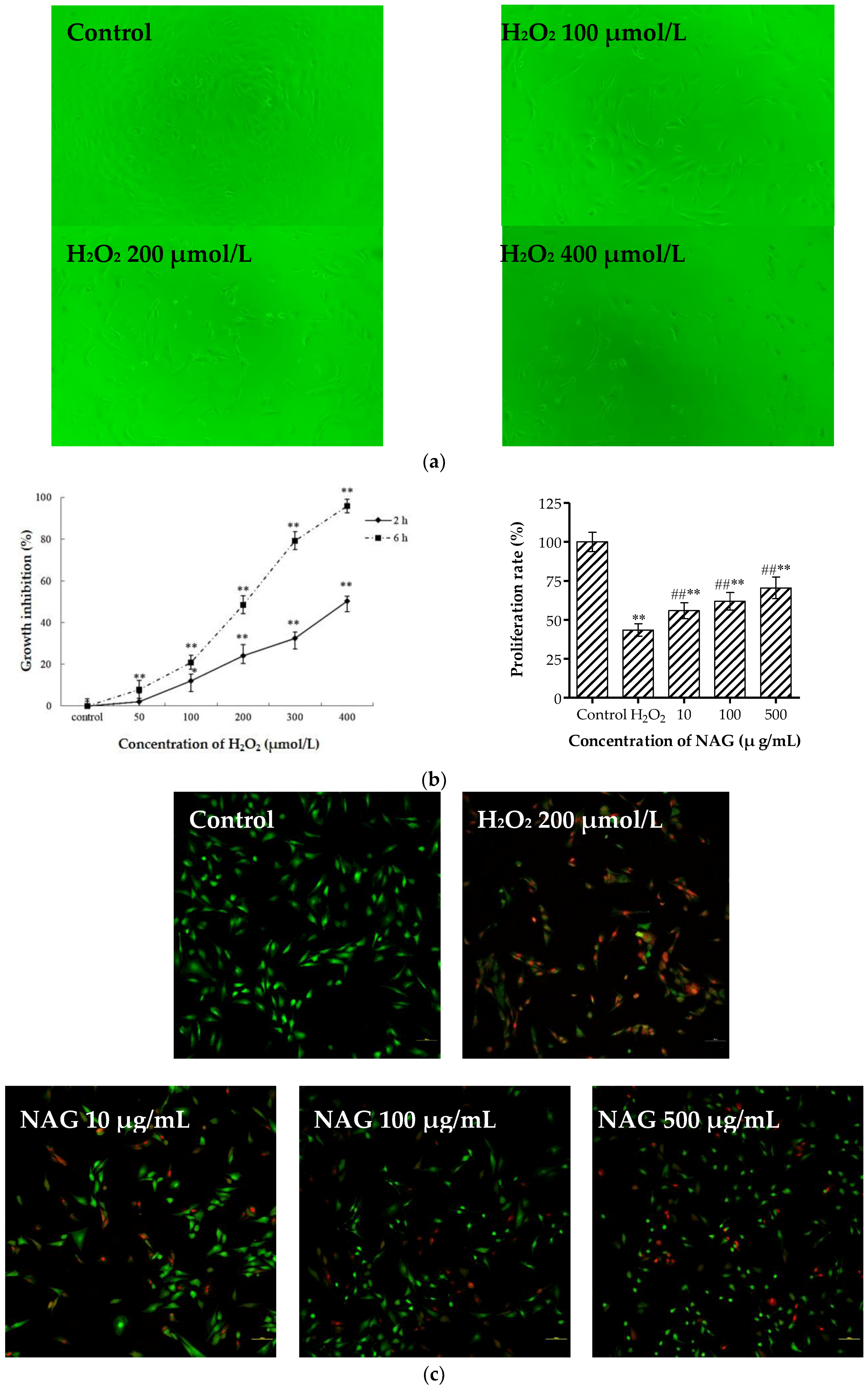
2.8. NAG Protects MC3T3-E1 Cells against H2O2 Oxidative Damage
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation and Physico-Chemical Characteristics of NAG
4.3. Animals and Feeding
4.4. Biochemical Analyses
4.5. Measurement of Femoral Mechanical Strength
4.6. Histological Examination of Tibias
4.7. MC3T3-E1 Cell Culture and Osteoblast Proliferation
4.8. Cellular ALP Activity Assay and Alizarin Red S Staining of Osteoblasts
4.9. Hydrogen Peroxide-Induced Oxidation Damage in Osteoblasts
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, H.; Shen, G.; Qiu, T.; Liang, D.; Yang, Z.; Yao, Z.; Tang, J.; Jiang, X.; Wei, Q. Animal models for glucocorticoid-induced postmenopausal osteoporosis: An updated review. Biomed. Pharmacother. 2016, 84, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Dick, I.M.; Devine, A.; Beilby, J.; Prince, R.L. Effects of endogenous estrogen on renal calcium and phosphate handling in elderly women. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E430. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, D.; Pan, N.; Sun, N.; Wang, Q.; Fan, J.; Zhou, P.; Zhu, W.; Jiang, L. Identification of mir-194-5p as a potential biomarker for postmenopausal osteoporosis. Peerj 2015, 3, e971. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, N.; Sánchez-Borrego, R.; Villero, J.; Baró, F.; Calaf, J.; Cancelo, M.J.; Coronado, P.; Estévez, A.; Fernández-Moya, J.M.; González, S.; et al. 2013 up-date of the consensus statement of the spanish menopause society on postmenopausal osteoporosis. Maturitas 2013, 76, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Nawata, H.; Soen, S.; Takayanagi, R.; Tanaka, I.; Takaoka, K.; Fukunaga, M.; Matsumoto, T.; Suzuki, Y.; Tanaka, H.; Fujiwara, S.; et al. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the japanese society for bone and mineral research (2004). J. Bone Miner. Metab. 2005, 23, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Felsenberg, D.; Cooper, C.; Stakkestad, J.A.; Miller, P.D.; Kendler, D.L.; Adami, S.; McClung, M.R.; Bolognese, M.A.; Civitelli, R.; et al. A new concept for bisphosphonate therapy: A rationale for the development of monthly oral dosing of ibandronate. Osteoporos. Int. 2006, 17, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B. Bone quality: Understanding what matters. J. Musculoskel. Neuron 2004, 4, 184–186. [Google Scholar]
- Cheung, R.K.; Leung, K.K.; Lee, K.C.; Chow, T.C. Sequential non-traumatic femoral shaft fractures in a patient on long-term alendronate. Hong Kong Med. J. 2007, 13, 485–489. [Google Scholar] [PubMed]
- Lenart, B.A.; Lorich, D.G.; Lane, J.M. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N. Engl. J. Med. 2008, 358, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Rahnama, M.; Jastrzębskajamrogiewicz, I.; Jamrogiewicz, R.; Trybek, G. Analysis of the influence of hormone replacement therapy on osteocalcin gene expression in postmenopausal women. BioMed Res. Int. 2015, 416929. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Dutta, J.; Chattopadhyaya, M.C.; Tripathi, V.S. Chitin and chitosan: Novel biomaterials waiting for future developments. J. Polym. Mater. 2004, 21, 321–333. [Google Scholar]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- Tamai, Y.; Miyatake, K.; Okamoto, Y.; Takamori, Y.; Sakamoto, K.; Minami, S. Enhanced healing of cartilaginous injuries by N-acetyl-d-glucosamine and glucuronic acid. Carbohydr. Polym. 2003, 54, 251–262. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Guzmán-Morales, J.; Tran-Khanh, N.; Lavallée, G.; Jolicoeur, M.; Lavertu, M. Chitosan rate of uptake in hek293 cells is influenced by soluble versus microparticle state and enhanced by serum-induced cell metabolism and lactate-based media acidification. Molecules 2013, 18, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Lippiello, L. Glucosamine and chondroitin sulfate: Biological response modifiers of chondrocytes under simulated conditions of joint stress. Osteoarthr. Cartilage 2003, 11, 335–342. [Google Scholar] [CrossRef]
- Richter, J.; Capková, K.; Hříbalová, V.; Vannucci, L.; Danyi, I.; Malý, M.; Fišerová, A. Collagen-induced arthritis: Severity and immune response attenuation using multivalent n-acetyl glucosamine. Clin. Exp. Immunol. 2014, 177, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Sashiwa, H.; Fujishima, S.; Yamano, N.; Kawasaki, N.; Nakayama, A.; Muraki, E.; Sukwattanasinitt, M.; Pichyangkura, R.; Aiba, S. Enzymatic production of N-acetyl-d-glucosamine from chitin. Degradation study of n-acetylchitooligosaccharide and the effect of mixing of crude enzymes. Carbohydr. Polym. 2003, 51, 391–395. [Google Scholar] [CrossRef]
- Terry, D.; Rees-Milton, K.; Pruss, C.; Hopwood, J.; Carran, J.; Anastassiades, T.P. Modulation of articular chondrocyte proliferation and anionic glycoconjugate synthesis by glucosamine (glcn), n-acetyl glcn (glcnac) glcn sulfate salt (glcn. s) and covalent glucosamine sulfates (glcn-so4). Osteoarthr. Cartilage 2007, 15, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, C.; Yeh, C.; Fang, B.; Huang, T.; Liu, C.L. N-acetyl glucosamine obtained from chitin by chitin degrading factors in chitinbacter tainanesis. Int. J. Mol. Sci. 2011, 12, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shen, C.; Liu, C.L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lim, D.; Baek, K.; Jang, S.; Park, B.; Mayakrishnan, V. Production of chitinase from escherichia fergusonii, chitosanase from chryseobacterium indologenes, comamonas koreensis and its application in n-acetylglucosamine production. Int. J. Biol. Macromol. 2018, 112, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radical Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Burdon, R.H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radical Biol. Med. 1995, 18, 775–794. [Google Scholar] [CrossRef]
- Demelash, A.; Karlsson, J.O.; Nilsson, M.; Björkman, U. Selenium has a protective role in caspase-3-dependent apoptosis induced by H2O2 in primary cultured pig thyrocytes. Eur. J. Endocrinol. 2004, 150, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Yoon, M. Swimming’s prevention of ovariectomy-induced obesity through activation of skeletal-muscle pparα. Int. J. Sport Nutr. Exerc. 2012, 22, 1–10. [Google Scholar] [CrossRef]
- Janicki, P.; Boeuf, S.; Steck, E.; Egermann, M.; Kasten, P.; Richter, W. Prediction of in vivo bone forming potency of bone marrow-derived human mesenchymal stem cells. Eur. Cells Mater. 2011, 21, 488–507. [Google Scholar] [CrossRef]
- Kourtis, L.C.; Carter, D.R.; Beaupre, G.S. Improving the estimate of the effective elastic modulus derived from three-point bending tests of long bones. Ann. Biomed. Eng. 2014, 42, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, Y.; Li, X.; Wang, Q.; Ouyang, Y.; Xia, Y.; Yu, B.; Lin, B.; Li, S.; Fan, Y.; et al. The use of injectable chitosan/nanohydroxyapatite/collagen composites with bone marrow mesenchymal stem cells to promote ectopic bone formation in vivo. J. Nanomater. 2013, 2013, 3805–3816. [Google Scholar] [CrossRef]
- Nakatoh, S. The importance of assessing the rate of bone turnover and the balance between bone formation and bone resorption during daily teriparatide administration for osteoporosis: A pilot study. J. Bone Miner. Metab. 2016, 34, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Woesz, A.; Rumpler, M.; Stampfl, J.; Varga, F.; Fratzl-Zelman, N.; Roschger, P.; Klaushofer, K.; Fratzl, P. Towards bone replacement materials from calcium phosphates via rapid prototyping and ceramic gelcasting. Mater. Sci. Eng. C 2005, 25, 181–186. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Ge, C.; Xiao, G.; Roca, H.; Jiang, D. Transcriptional regulation of osteoblasts. Cells Tissues Org. 2009, 189, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B.; Owen, T.A. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990, 4, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Burstone, M.S. Histochemical observation on enzymatic processes in bone and teeth. Ann. N.Y. Acad. Sci. 1960, 85, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, G.G.; Getz, B.; Pederson, L.; Sanders, E.S.; Subramaniam, M.; Ingle, J.N.; Spelsberg, T.C. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000, 60, 6001–6007. [Google Scholar] [PubMed]
- Fridovich, I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen. Ann. N.Y. Acad. Sci. 1999, 893, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Voronova, V.; Zhudenkov, K.; Helmlinger, G.; Peskov, K. Interpretation of metabolic memory phenomenon using a physiological systems model: What drives oxidative stress following glucose normalization. PLoS ONE 2017, 12, e0171781. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, P.; Böcker, W.; El, K.T.; Kampschulte, M.; Schlewitz, G.; Huerter, B.; Sommer, U.; Dürselen, L.; Ignatius, A.; Bauer, N.; et al. Bone matrix, cellularity, and structural changes in a rat model with high-turnover osteoporosis induced by combined ovariectomy and a multiple-deficient diet. Am. J. Pathol. 2014, 184, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.; Court, J.; Goodchild, R.; Griffiths, M.; Jaros, E.; Johnson, M.; Lloyd, S.; Piggott, M.; Spurden, D.; Ballard, C.; et al. Clinical neurochemistry: Developments in dementia research based on brain bank material. J. Neural Transm. 1998, 105, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, B.; Li, H.; Yang, Y.; Liu, W. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gullian, M.; Aguirremacedo, L. Seasonal variation of physiological parameters in the eastern oyster crassostrea virginica from a tropical region of the gulf of Mexico. J. Shellfish Res. 2017, 28, 439–446. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Hao, B.; Hao, L.; Zhu, S.; Wang, Q.; Li, R.; Xu, Y.; Zhang, X. Use of an osteoblast overload damage model to probe the effect of icariin on the proliferation, differentiation and mineralization of mc3t3-e1 cells through the wnt/β-catenin signalling pathway. Cell. Physiol. Biochem. 2017, 41, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Lee, S.Y.; Jeong, S.J.; Lim, D.S.; Cha, H.J.; Chung, W.G.; Jeong, M.J. Secretory leukocyte protease inhibitor promotes differentiation and mineralization of mc3t3-e1 preosteoblasts on a titanium surface. Mol. Med. Rep. 2016, 14, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |




| Group | Maximum Load (N) | Fracture Deflection (mm) | ||
|---|---|---|---|---|
| 8 Weeks | 12 Weeks | 8 Weeks | 12 Weeks | |
| Normal group | 138.32 ± 4.44 | 163.97 ± 7.16 | 1.39 ± 0.05 | 1.59 ± 0.11 |
| Model group | 111.81 ± 6.84 ## | 93.77 ± 9.4 ## | 1.09 ± 0.02 ## | 1.06 ± 0.03 ## |
| NAG 250 mg/kg | 123.63 ± 5.32 ##,* | 161.26 ± 8.33 ** | 1.22 ± 0.06 ##,** | 1.42 ± 0.07 ** |
| NAG 100 mg/kg | 117.08 ± 12.36 ## | 148.33 ± 6.38 #,** | 1.14 ± 0.10 ## | 1.29 ± 0.11 ##,** |
| Group | Bone Mineral Content | ||||
|---|---|---|---|---|---|
| Dry Weight (g) | Ash Weight (g) | Inorganic Content (%) | Femur Ca (mg/g) | Tibia Ca (mg/g) | |
| Normal group | 0.5271 ± 0.016 | 0.3427 ± 0.015 | 65.08% | 196.96 ± 1.41 | 180.79 ± 7.84 |
| Model group | 0.4682 ± 0.017 ## | 0.2612 ± 0.015 ## | 56.78% | 132.34 ± 1.35 ## | 104.16 ± 7.6 ## |
| NAG 250 mg/kg | 0.5226 ± 0.012 ** | 0.3445 ± 0.007 ** | 65.93% | 202.5 ± 8.73 ** | 163.88 ± 9.96 ** |
| NAG 100 mg/kg | 0.4943 ± 0.014 #,* | 0.3022 ± 0.021 #,* | 61.14% | 173.85 ± 13.56 #,** | 156.4 ± 8.36 ##,** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Li, Z.; Zhang, W.; Yang, Y.; Han, B.; Liu, W.; Peng, Y. Dietary Natural N-Acetyl-d-Glucosamine Prevents Bone Loss in Ovariectomized Rat Model of Postmenopausal Osteoporosis. Molecules 2018, 23, 2302. https://doi.org/10.3390/molecules23092302
Jiang Z, Li Z, Zhang W, Yang Y, Han B, Liu W, Peng Y. Dietary Natural N-Acetyl-d-Glucosamine Prevents Bone Loss in Ovariectomized Rat Model of Postmenopausal Osteoporosis. Molecules. 2018; 23(9):2302. https://doi.org/10.3390/molecules23092302
Chicago/Turabian StyleJiang, Zhiwen, Zhe Li, Wei Zhang, Yan Yang, Baoqin Han, Wanshun Liu, and Yanfei Peng. 2018. "Dietary Natural N-Acetyl-d-Glucosamine Prevents Bone Loss in Ovariectomized Rat Model of Postmenopausal Osteoporosis" Molecules 23, no. 9: 2302. https://doi.org/10.3390/molecules23092302




