Design of Novel Pyrene-Bodipy Dyads: Synthesis, Characterization, Optical Properties, and FRET Studies
Abstract
:1. Introduction
2. Results and Discussion
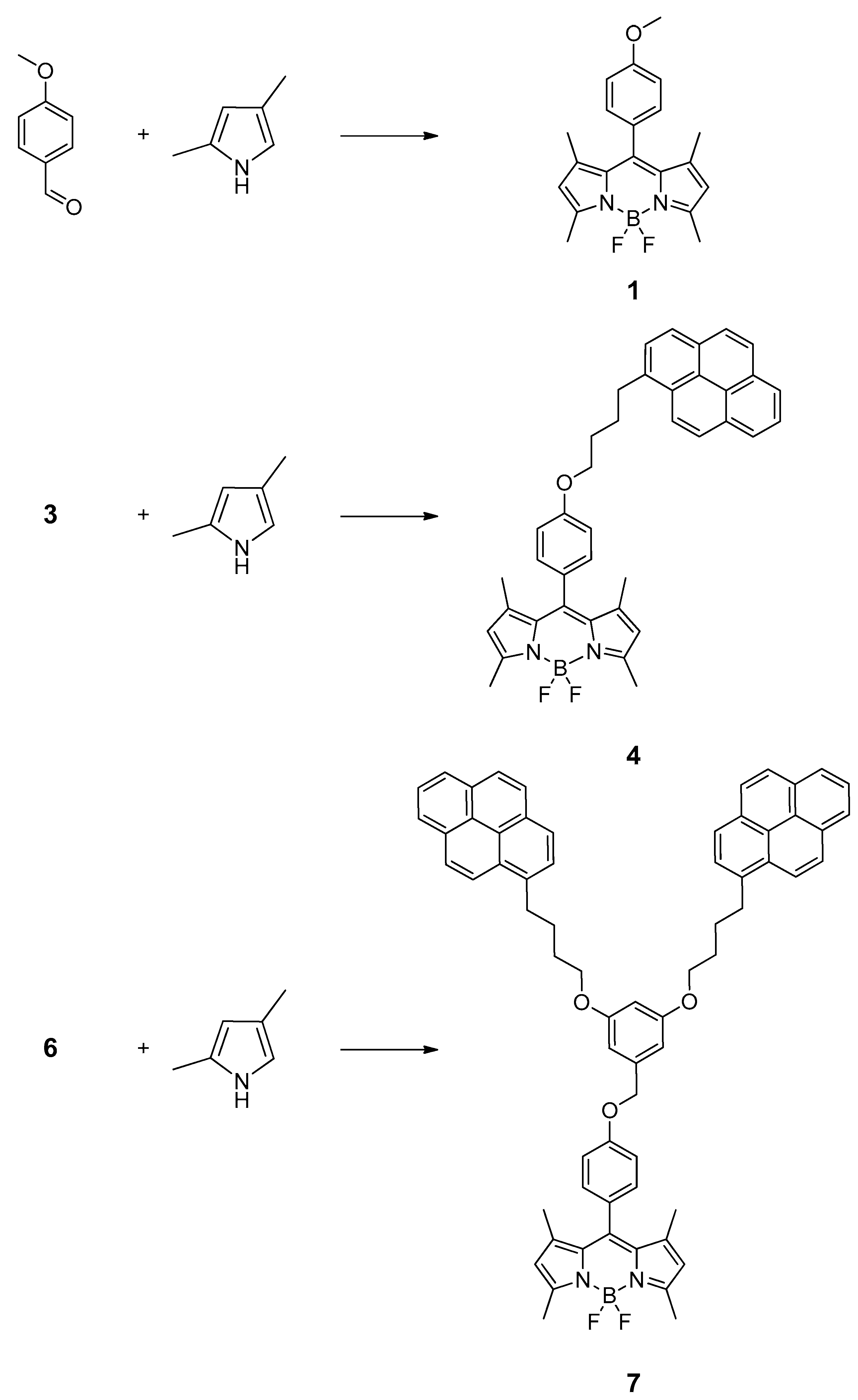
2.1. Synthesis of the Pyrene-Bodipy Compounds
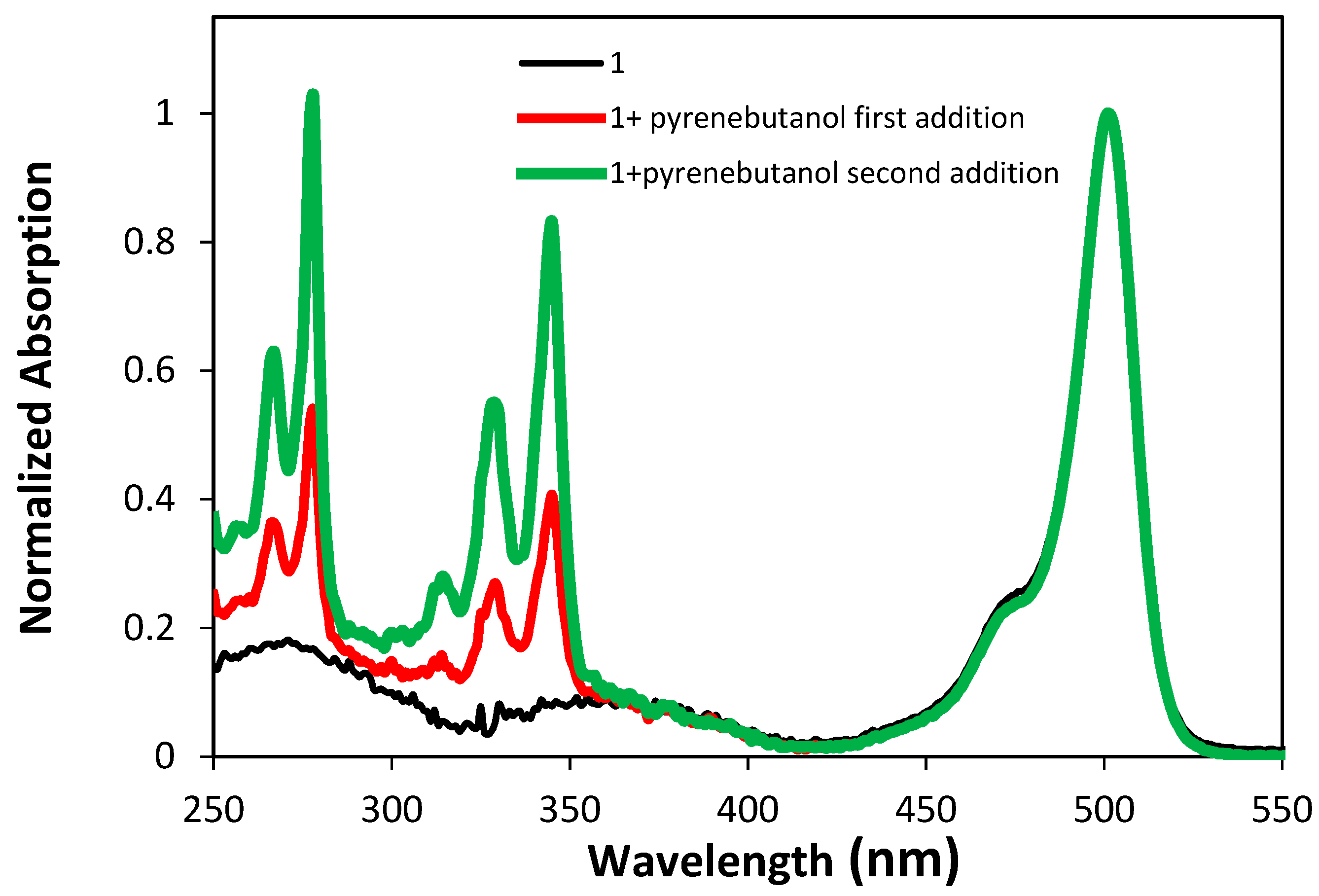
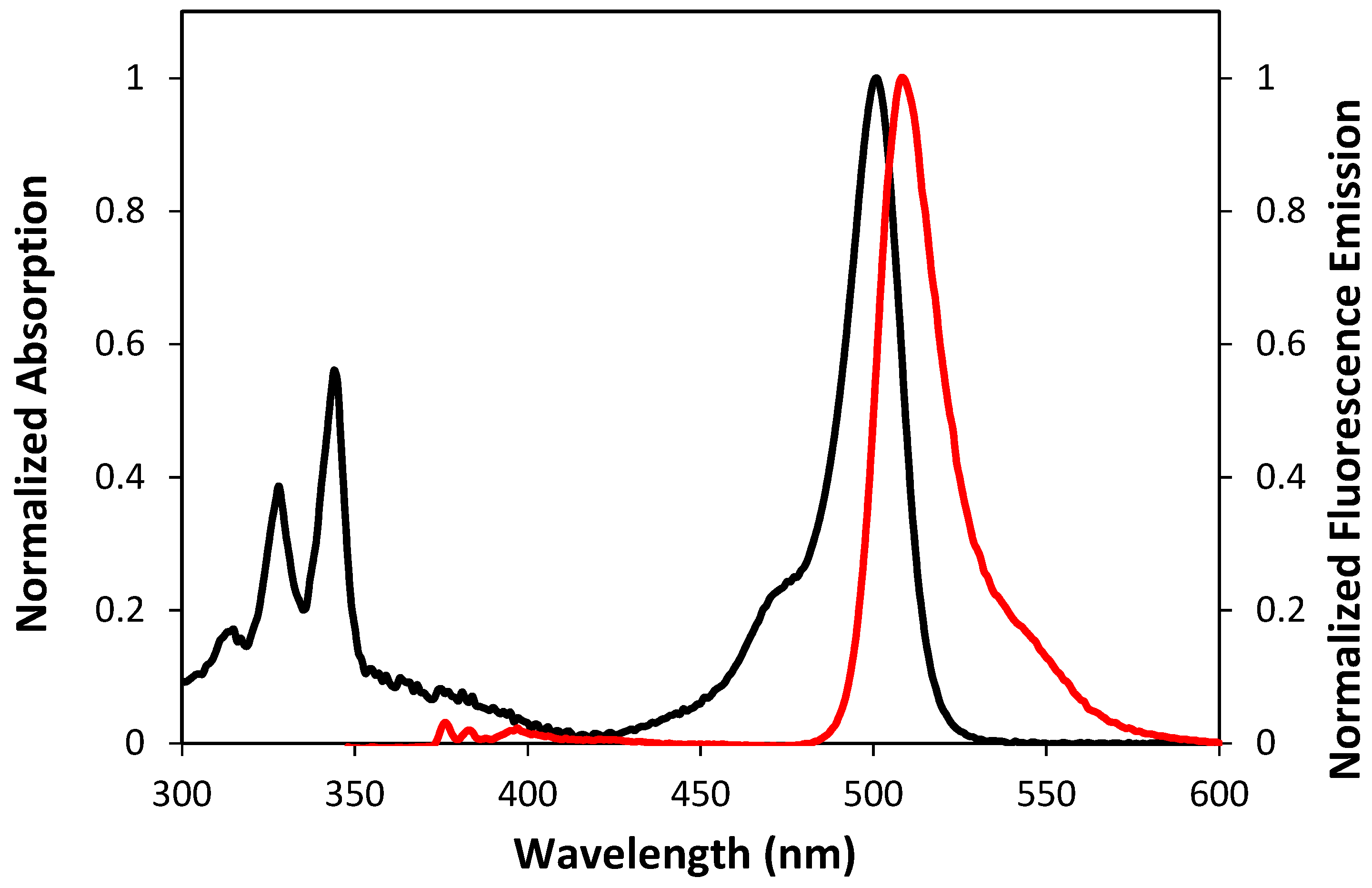
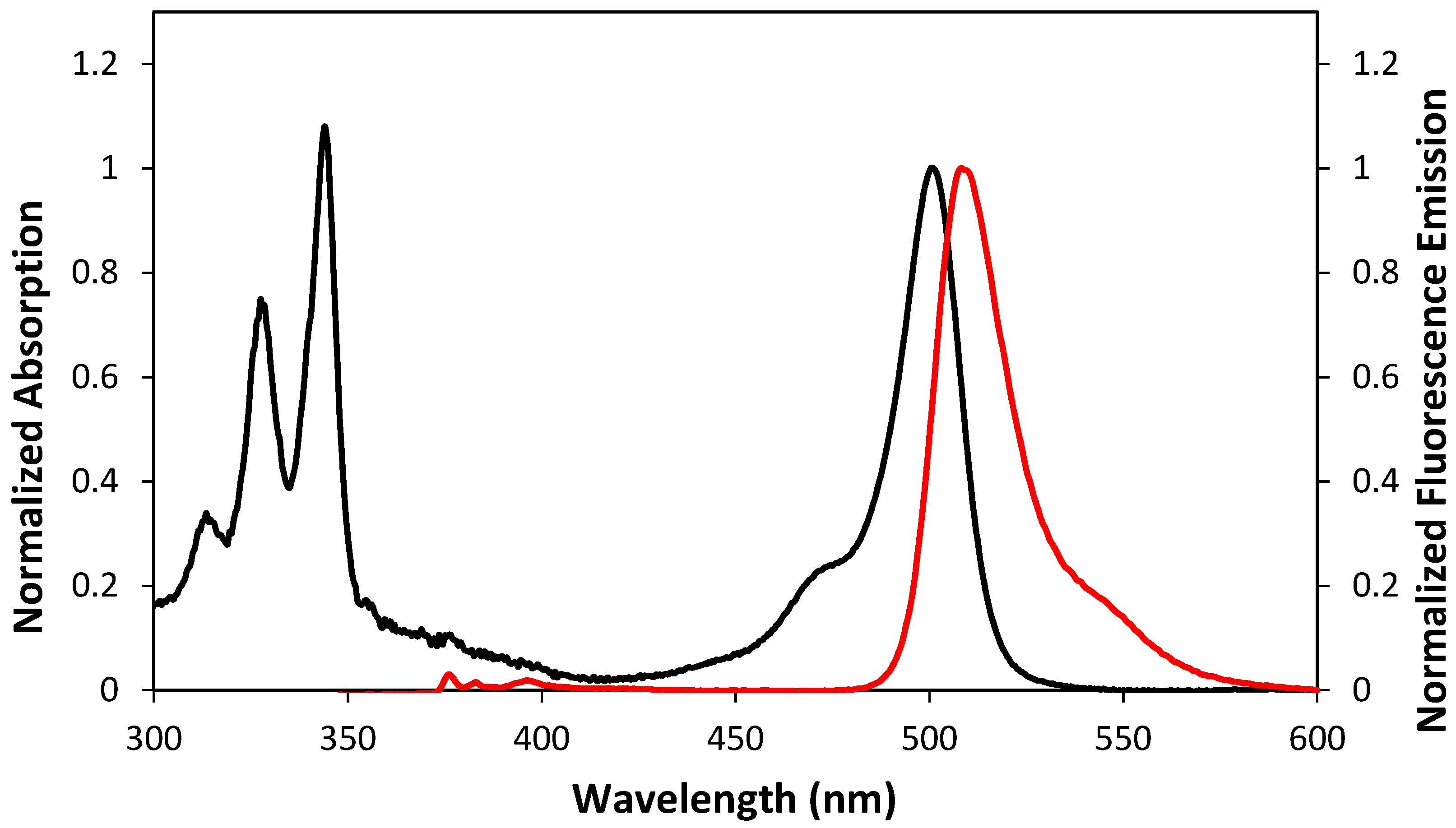
2.2. Optical and Photophysical Properties of the Pyrene-Labeled Bodipy Compounds
3. Materials and Methods
3.1. General Notes
3.2. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sapsford, K.E.; Wildt, B.; Mariani, A.; Yeatts, A.B.; Medintz, I. FRET—Förster Resonance Energy Transfer: From Theory to Applications; Medintz, I., Hildebrandt, N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 9783527656028. [Google Scholar]
- Bruns, N.; Pustelny, K.; Bergeron, L.M.; Whitehead, T.A.; Clark, D.S. Mechanical nanosensor based on fret within a thermosome: Damage-reporting polymeric materials. Angew. Chem. Int. Ed. 2009, 48, 5666–5669. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Lee, J.; Mazor, B.A.; Forrest, S.R. Transforming the cost of solar-to-electrical energy conversion: Integrating thin-film GaAs solar cells with non-tracking mini-concentrators. Light Sci. Appl. 2015, 4, e288. [Google Scholar] [CrossRef]
- Jing, D.; Liu, H.; Zhang, X.; Zhao, L.; Guo, L. Photocatalytic hydrogen production under direct solar light in a CPC based solar reactor: Reactor design and preliminary results. Energy Convers. Manag. 2009, 50, 2919–2926. [Google Scholar] [CrossRef]
- Balzani, V.; Ceroni, P.; Juris, A. Photochemistry and Photophysics; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 9783527334797. [Google Scholar]
- Berney, C.; Danuser, G. FRET or no FRET: A quantitative comparison. Biophys. J. 2003, 84, 3992–4010. [Google Scholar] [CrossRef]
- Hoi, H.; Ding, Y.; Campbell, R.E.; Algar, W.R.; Massey, M.; Krull, U.J.; van der Meer, B.W.; Claussen, J.C.; Hildebrandt, N.; Medintz, I.; et al. FRET—Förster Resonance Energy Transfer; Medintz, I., Hildebrandt, N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 9783527656028. [Google Scholar]
- Vogel, S.S.; Blank, P.S.; Koushik, S.V.; Thaler, C. Chapter 8 Spectral imaging and its use in the measurement of Förster resonance energy transfer in living cells. Lab. Tech. Biochem. Mol. Biol. 2009, 33, 351–394. [Google Scholar]
- Balzani, V.; Bergamini, G.; Ceroni, P.; Marchi, E. Designing light harvesting antennas by luminescent dendrimers. New J. Chem. 2011, 35, 1944–1954. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Balzani, V.; Ceroni, P.; Maestri, M.; Vicinelli, V. Light-harvesting dendrimers. Curr. Opin. Chem. Biol. 2003, 7, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Ziessel, R.; Ulrich, G.; Harriman, A. The chemistry of Bodipy: A new El Dorado for fluorescence tools. New J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- Jameson, L.P.; Dzyuba, S.V. Expeditious, mechanochemical synthesis of BODIPY dyes. Beilstein J. Org. Chem. 2013, 9, 786–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, I.J.; Hu, R.; Merino, G.; Tang, B.Z.; Peña-Cabrera, E. The smallest and one of the brightest. Efficient preparation and optical description of the parent borondipyrromethene system. J. Org. Chem. 2009, 74, 5719–5722. [Google Scholar] [CrossRef] [PubMed]
- Suhina, T.; Amirjalayer, S.; Woutersen, S.; Bonn, D.; Brouwer, A.M. Ultrafast dynamics and solvent-dependent deactivation kinetics of BODIPY molecular rotors. Phys. Chem. Chem. Phys. 2017, 19, 19998–20007. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, J.; Arroyo-Córdoba, I.J.; Valois-Escamilla, I.; Alvarez-Hernández, A.; Peña-Cabrera, E.; Hu, R.; Tang, B.Z.; Esnal, I.; Martínez, V.; Arbeloa, I.L. Modulation of the photophysical properties of BODIPY dyes by substitution at their meso position. RSC Adv. 2011, 1, 677–684. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, J. Internal dynamics of dendritic molecules probed by pyrene excimer formation. Polymers 2012, 4, 211–239. [Google Scholar] [CrossRef]
- Zaragoza-Galán, G.; Fowler, M.A.; Duhamel, J.; Rein, R.; Solladié, N.; Rivera, E. Synthesis and characterization of novel pyrene-dendronized porphyrins exhibiting efficient fluorescence resonance energy transfer: Optical and photophysical properties. Langmuir 2012, 28, 11195–11205. [Google Scholar] [CrossRef] [PubMed]
- Topp, A.; Bauer, B.J.; Klimash, J.W.; Spindler, R.; Tomalia, D.A.; Amis, E.J. Probing the location of the terminal groups of dendrimers in dilute solution. Macromolecules 1999, 32, 7226–7231. [Google Scholar] [CrossRef]
- Ingratta, M.; Duhamel, J. Correlating pyrene excimer formation with polymer chain dynamics in solution. Possibilities and limitations. Macromolecules 2007, 40, 6647–6657. [Google Scholar] [CrossRef]
- Duhamel, J. Pyrene florescence to study polymeric systems. In Molecular Interfacial Phenomena of Polymers and Biopolymers; Elsevier: New York, NY, USA, 2005; pp. 214–248. ISBN 9781855739284. [Google Scholar]
- Rodríguez-Alba, E.; Ortíz-Palacios, J.; Morales-Espinoza, E.G.; Vonlanthen, M.; Valderrama, B.X.; Rivera, E. Synthesis, characterization and optical properties of novel oligothiophenes bearing pyrene units attached via well defined oligo(ethylene glycol) spacers. Synth. Met. 2015, 206, 92–105. [Google Scholar] [CrossRef]
- Vonlanthen, M.; Cevallos-Vallejo, A.; Aguilar-Ortíz, E.; Ruiu, A.; Porcu, P.; Rivera, E. Synthesis, characterization and photophysical studies of novel pyrene labeled ruthenium (II) trisbipyridine complex cored dendrimers. Polymer 2016, 99, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Mahapatra, A.K.; Maji, R.; Maiti, K.; Manna, S.K.; Mondal, S.; Ali, S.S.; Manna, S.; Sahoo, P.; Mandal, S.; Uddin, M.R.; et al. A BODIPY/pyrene-based chemodosimetric fluorescent chemosensor for selective sensing of hydrazine in the gas and aqueous solution state and its imaging in living cells. RSC Adv. 2015, 5, 58228–58236. [Google Scholar] [CrossRef]
- Bai, D.; Benniston, A.C.; Hagon, J.; Lemmetyinen, H.; Tkachenko, N.V.; Harrington, R.W. Tuning the Förster overlap integral: Energy transfer over 20 Ångstroms from a pyrene-based donor to borondipyrromethene (Bodipy). Phys. Chem. Chem. Phys. 2013, 15, 9854–9861. [Google Scholar] [CrossRef] [PubMed]
- Goze, C.; Ulrich, G.; Mallon, L.J.; Allen, B.D.; Harriman, A.; Ziessel, R. Synthesis and photophysical properties of borondipyrromethene dyes bearing aryl substituents at the boron center. J. Am. Chem. Soc. 2006, 128, 10231–10239. [Google Scholar] [CrossRef] [PubMed]
- Ziessel, R.; Goze, C.; Ulrich, G.; Césario, M.; Retailleau, P.; Harriman, A.; Rostron, J.P. Intramolecular energy transfer in pyrene-bodipy molecular dyads and triads. Chem. A Eur. J. 2005, 11, 7366–7378. [Google Scholar] [CrossRef] [PubMed]
- Stryer, L.; Haugland, R.P. Energy Transfer: A Specroscopic Ruler. Proc. Natl. Acad. Sci. USA 1967, 58, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Burghart, A.; Kim, H.; Welch, M.B.; Thoresen, L.H.; Reibenspies, J.; Burgess, K.; Bergström, F.; Johansson, L.B.-Å. 3,5-Diaryl-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) Dyes: Synthesis, Spectroscopic, Electrochemical, and Structural Properties. J. Org. Chem. 1999, 64, 7813–7819. [Google Scholar] [CrossRef]
- Lager, E.; Liu, J.; Aguilar-Aguilar, A.; Tang, B.Z.; Peña-Cabrera, E. Novel meso-Polyarylamine-BODIPY hybrids: Synthesis and study of their optical properties. J. Org. Chem. 2009, 74, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Donuru, V.R.; Zhu, S.; Green, S.; Liu, H. Near-infrared emissive BODIPY polymeric and copolymeric dyes. Polymer 2010, 51, 5359–5368. [Google Scholar] [CrossRef]
- Jana, S.; Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Saikia, G. Thermal, Spectroscopic and Chemical Characterization of Biofield Energy Treated Anisole. Org. Chem. Curr. Res. 2015, 4, 6. [Google Scholar] [CrossRef]
- Birks, J.B.; Christophorou, L.G. Excimer fluorescence spectra of pyrene derivatives. Spectrochim. Acta 1963, 19, 401–410. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Instrumentation for Fluorescence Spectroscopy 2.1. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 1999; pp. 25–61. ISBN 0387312781. [Google Scholar]
- Olmsted, J. Calorimetric determinations of absolute fluorescence quantum yields. J. Phys. Chem. 1979, 83, 2581–2584. [Google Scholar] [CrossRef]
- Magde, D.; Wong, R.; Seybold, P.G. Fluorescence Quantum Yields and Their Relation to Lifetimes of Rhodamine 6G and Fluorescein in Nine Solvents: Improved Absolute Standards for Quantum Yields. Photochem. Photobiol. 2002, 75, 327–334. [Google Scholar] [CrossRef]
- Cevallos-Vallejo, A.; Vonlanthen, M.; Porcu, P.; Ruiu, A.; Rivera, E. New cyclen-cored dendrimers functionalized with pyrene: Synthesis characterization, optical and photophysical properties. Tetrahedron Lett. 2017, 58, 1319–1323. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Compound | λmax Absorption (nm)/ε(M−1cm−1) a | λmax Emission (nm) | Φpyrene b λex = 344 | Φbodipy c λex = 344 | Φbodipy c λex = 475 | EFRET d |
|---|---|---|---|---|---|---|
| 3 | 344/54,000 | 375 | 0.30 ± 0.03 | - | - | - |
| 6 | 344/101,000 | 375, 480 | 0.39 ± 0.02 | - | - | - |
| 1 | 501/87,000 | 509 | - | 0.32 ± 0.01 | 0.71 ± 0.02 | - |
| 4 | 344/47,000 501/79,000 | 509 | 0.0085 ± 0.0004 | 0.48 ± 0.02 | 0.65 ± 0.01 | 0.98 |
| 7 | 344/87,000 501/70,000 | 509 | 0.0097 ± 0.0003 | 0.49 ± 0.01 | 0.71 ± 0.01 | 0.99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcu, P.; Vonlanthen, M.; González-Méndez, I.; Ruiu, A.; Rivera, E. Design of Novel Pyrene-Bodipy Dyads: Synthesis, Characterization, Optical Properties, and FRET Studies. Molecules 2018, 23, 2289. https://doi.org/10.3390/molecules23092289
Porcu P, Vonlanthen M, González-Méndez I, Ruiu A, Rivera E. Design of Novel Pyrene-Bodipy Dyads: Synthesis, Characterization, Optical Properties, and FRET Studies. Molecules. 2018; 23(9):2289. https://doi.org/10.3390/molecules23092289
Chicago/Turabian StylePorcu, Pasquale, Mireille Vonlanthen, Israel González-Méndez, Andrea Ruiu, and Ernesto Rivera. 2018. "Design of Novel Pyrene-Bodipy Dyads: Synthesis, Characterization, Optical Properties, and FRET Studies" Molecules 23, no. 9: 2289. https://doi.org/10.3390/molecules23092289






