Metal Chelating, Inhibitory DNA Damage, and Anti-Inflammatory Activities of Phenolics from Rambutan (Nephelium lappaceum) Peel and the Quantifications of Geraniin and Corilagin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quantities of Corilagin and Geraniin from RPP
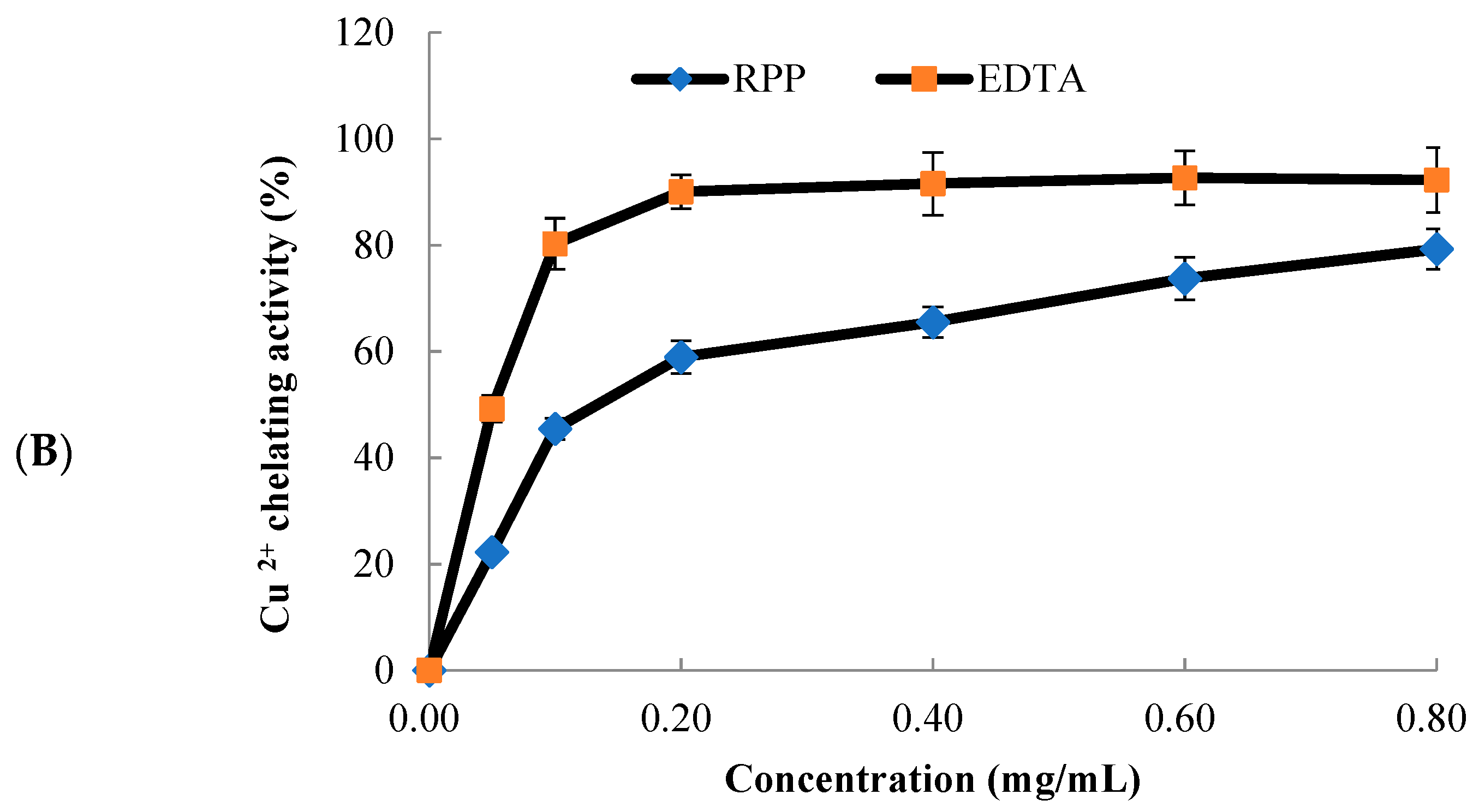
2.2. Metal Chelating Activity of RPP
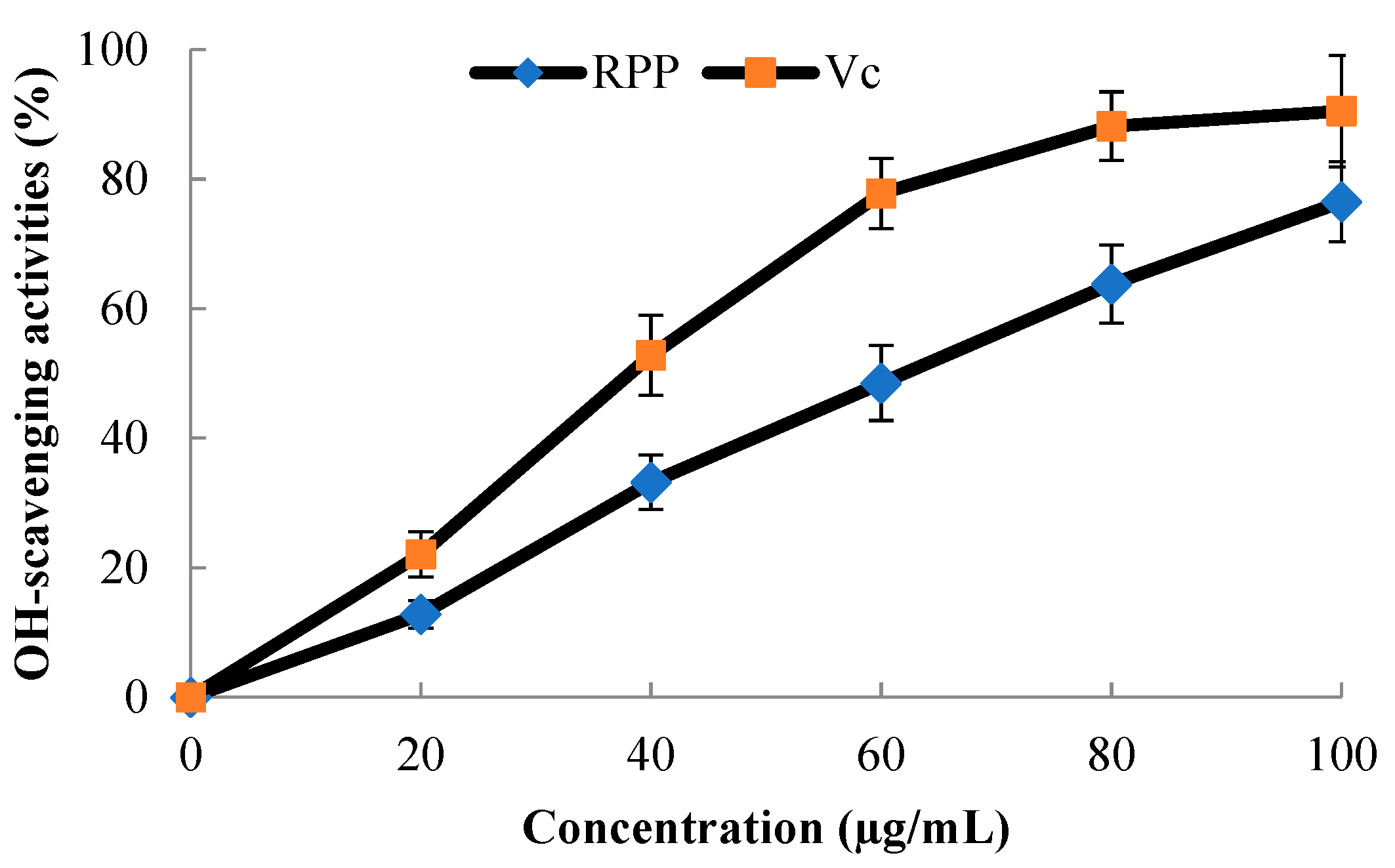
2.3. OH·-Scavenging Activity of RPP
2.4. Protective Effects of RPP on Supercolied Plasmid DNA Strain Breakage
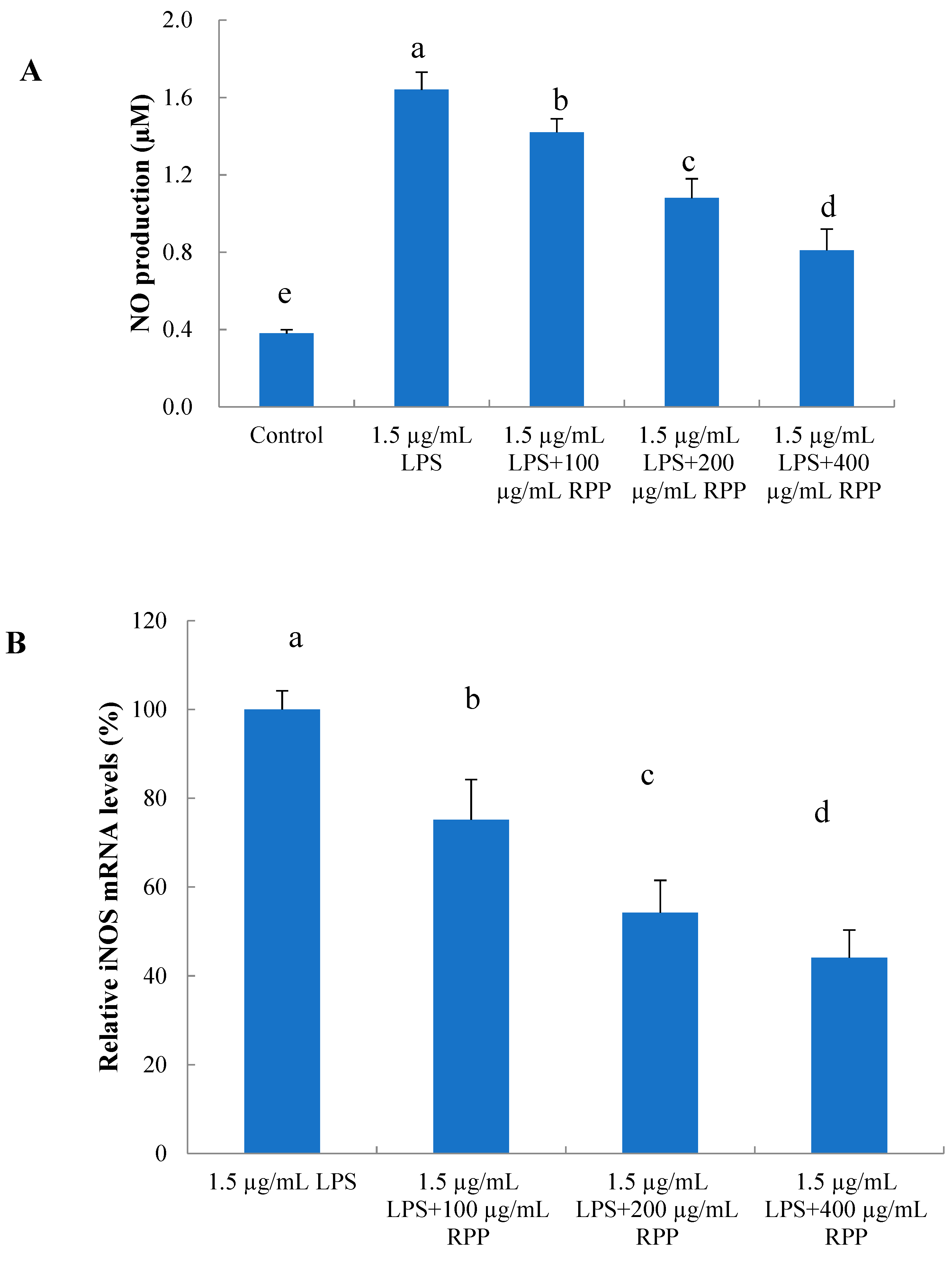
2.5. Protective Effects of RPP on Inflammationin Lipopolysaccharide (LPS)-Stimulated RAW 264.7 Cells
3. Materials and Methods
3.1. Materials and Reagents
3.2. Quantity Analysis of Geraniin and Corilagin by UPLC-QQQ-MS
3.3. Metal-Chelating Activity Assay
3.3.1. Iron-Chelating Activity Assay
3.3.2. Copper-Chelating Activity Assay
3.4. OH·-Scavenging Activity Assay
3.5. Protective Effect Analysis of RPP on AAPH-Induced DNA Damage
3.6. Anti-Inflammation Effect Analysis of RPP
3.6.1. RAW 264.7 Cell Viability Assay
3.6.2. Determination of NO Production
3.6.3. Analysis of iNOS Gene Expression
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pérez-Jiménez, J.; Saura-Calixto, F. Macromolecular antioxidant or non-extracpolyphenols in fruit and vegetables: Intake in four European countries. Food Res. Int. 2015, 74, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Gracio, D.; Teixeira, J.P.; Magro, F. Oxidative stress and DNA damage: Implications in inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant, anti-inflammatory and DNA scission inhibitory activities of phenolic compounds in selected onion and potato varieties. J. Func. Food 2013, 5, 930–939. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karki, R.; Park, C.H.; Kim, D.W. Extract of buckwheat sprouts scavenges oxidation and inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages (RAW264.7). J. Integr. Med. 2013, 11, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Huang, S.; Cai, S.; Cao, J.; Han, P. Digestion property and synergistic effect on biological activity of purple rice (Oryza sativa L.) anthocyanins subjected to a simulated gastrointestinal digestion in vitro. Food Res. Int. 2015, 78, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Q.; Miao, J.; Rui, X.; Li, T.; Dong, M. Antioxidant activity and DNA damage protection of mungbeansprocessed by solid state fermentation with Cordyceps militaris SN-18. Innov. Food Sci. Emerg. Technol. 2015, 31, 216–225. [Google Scholar] [CrossRef]
- Carvalhoa, A.R.; Costa, G.; Figueirinha, A.; Liberal, J.; Prior, J.A.V.; Lopes, M.C.; Cruz, M.T.; Maria Batista, T. Urtica spp.: Phenolic composition, safety, antioxidant and anti inflammatory activities. Food Res. Int. 2017, 99, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Zhang, H.L.; Zhuang, Y.L. Preparation of free, soluble conjugate, and insoluble-bound phenolic compounds from peels of rambutan (Nephelium lappaceum) and evaluation of antioxidant activities in vitro. J. Food Sci. 2012, 77, C198–C204. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ma, Q.; Guo, Y.; Sun, L. Protective effects of rambutan (Nephelium lappaceum) peel phenolics on H2O2-induced oxidantive damages in HepG2 cells and d-galactose-induced aging mice. Food Chem. Toxicol. 2017, 108, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ma, Q.; Guo, Y.; Sun, L. Purification and identification of rambutan (Nephelium lappaceum) peel phenolics with evaluation of antioxidant and antiglycation activities in vitro. Int. J. Food Sci. Technol. 2017, 52, 1810–1819. [Google Scholar]
- Ma, Q.; Guo, Y.; Sun, L.; Zhuang, Y. Anti-diabetic effects of phenolic extract from rambutan peels (Nephelium lappaceum) in high-fat diet and streptozotocin-induced diabetic mice. Nutrients 2017, 9, 801. [Google Scholar]
- Sánchez-Vioque, R.; Polissiou, M.; Astraka, K.; Mozos-Pascual, M.D.L.; Tarantilis, P.; Herraiz-Peñalvera, D.; Santana-Méridas, O. Polyphenol composition and antioxidant and metal chelating activities of the solid residues from the essential oil industry. Ind. Crop. Prod. 2013, 49, 150–159. [Google Scholar] [CrossRef]
- Dalvi, L.T.; Moreira, D.C.; Andrade, R., Jr.; Ginani, J.; Alonso, A.; Hermes-Lima, M. Ellagic acid inhibits iron-mediated free radical formation. Spectrochim. Acta Part A 2017, 173, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Jaén, J.A.; González, L.; Vargas, A.; Olave, G. Gallic acid, ellagic acid and pyro-gallol reaction with metallic iron. Hyperfine Interact. 2003, 148, 227–235. [Google Scholar] [CrossRef]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, J.; Karonen, M.; Tähtinen, P.; Jacquet, R.; Quideau, S.; Salminen, J.P. Biological activity of ellagitannins: Effects as anti-oxidants, pro-oxidantsand metal chelators. Phytochemistry 2016, 125, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Thitilertdecha, N.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Identification of major phenolic compounds from Nephelium lappaceum L. and their antioxidant activities. Molecules 2010, 15, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food Chem. 2002, 50, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Xu, L.; Wu, P.; Xie, H.; Jiang, Y.; Chen, F.; Wei, X. Polyphenols from longan seeds and their radical-scavenging activity. Food Chem. 2009, 116, 433–436. [Google Scholar] [CrossRef]
- Lin, S.; He, J.; Jiang, Y.; Wu, F.; Wang, H.; Wu, D.; Sun, J.; Zhang, D.; Qu, H.; Yang, B. Production of nigragillin and dihydrophaseic acid by biotransformation of litchi pericarp with Aspergillus awamori and their antioxidant activities. J. Func. Food 2014, 7, 278–286. [Google Scholar] [CrossRef]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Wallace Hayes, A.; Tsatsakis, A.M.; Kouretas, D. Assessment of polyphenolic content, antioxidant activity, protection against ROS induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ma, Y.; Sun, D.; Fan, J.; Cai, S. In vitro DNA damage protection and anti-inflammatory effects of Tartary buckwheats (Fagopyrum tataricum L. Gaertn) fermented by filamentous fungi. Int. J. Food Sci. Technol. 2017, 9, 2006–2017. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, Q.; Li, J.; Wang, W.; Yao, L.P.; Fu, Y.J. Inhibitory effects of geraniin on LPS-induced inflammation via regulating NF-kB and Nrf2 pathways in RAW 264.7 cells. Chem. Biol. Interact. 2016, 253, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Boakye, Y.D.; Agyare, C.; Abotsi, W.K.; Ayande, P.G.; Ossei, P.P. Anti-inflammatory activity of aqueous leaf extract of Phyllanthus muellerianus (Kuntze) Exell. and its major constituent, geraniin. J. Ethnopharmacol. 2016, 187, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Marín, M.; Giner, R.M.; Ríos, J.L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Corbett, S.; Daniel, J.; Drayton, R.; Field, M.; Steinhardt, R.; Garrett, N. Evaluation of the anti-inflammatory effects of ellagic acid. J. Perianesth. Nurs. 2010, 25, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, S.; Singh, A.; Chourasia, M.; Ahmeda, S. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1β signaling in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharm. 2017, 329, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Bao, C.J.; Chang, W.D.; Zhuang, Y.L. Preparation, characterisation, antioxidant and antiglycation activities of the novel polysaccharides from the pileus of Dictyophora rubrovolvata. Int. Food Sci. Technol. 2017, 52, 161–170. [Google Scholar] [CrossRef]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef] [PubMed]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.C.T.; Shahidi, F. Low molecular weight phenolics of grape juice and wine making by products: Antioxidant activities and inhibition of oxidation of human low-density lipoprotein cholesterol and DNA strand breakage. J. Agric. Food Chem. 2014, 62, 12159–12171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Shen, S.C.; Lee, W.R.; Hou, W.C.; Yang, L.L.; Lee, T.J. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expressions by rutin, quercetin, and quercetin pentaacetate in RAW 264.7 macrophages. J. Cell. Biochem. 2001, 82, 537–548. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds (rambutan peel phenolics) are available from the authors. |




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, Z.; Hou, H.; Zhuang, Y.; Sun, L. Metal Chelating, Inhibitory DNA Damage, and Anti-Inflammatory Activities of Phenolics from Rambutan (Nephelium lappaceum) Peel and the Quantifications of Geraniin and Corilagin. Molecules 2018, 23, 2263. https://doi.org/10.3390/molecules23092263
Li Y, Li Z, Hou H, Zhuang Y, Sun L. Metal Chelating, Inhibitory DNA Damage, and Anti-Inflammatory Activities of Phenolics from Rambutan (Nephelium lappaceum) Peel and the Quantifications of Geraniin and Corilagin. Molecules. 2018; 23(9):2263. https://doi.org/10.3390/molecules23092263
Chicago/Turabian StyleLi, Yujing, Zhaojie Li, Hu Hou, Yongliang Zhuang, and Liping Sun. 2018. "Metal Chelating, Inhibitory DNA Damage, and Anti-Inflammatory Activities of Phenolics from Rambutan (Nephelium lappaceum) Peel and the Quantifications of Geraniin and Corilagin" Molecules 23, no. 9: 2263. https://doi.org/10.3390/molecules23092263



