Separation and Analysis of Aspirin and Metformin HCl Using Green Subcritical Water Chromatography
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Preparation of Internal Standard and Working Standard Solutions
2.3. Preparation of Sample Solutions
2.4. Subcritical Water Chromatography and Traditional HPLC
3. Results and Discussion
3.1. Separation and Analysis of Aspirin
3.2. Separation and Analysis of Metformin HCl
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rebecca, K.; Denisa, F.; Elina, J. Single nonsteroidal anti-inflammatory drug induced serum sickness-like reaction to naproxen in a patient able to tolerate both aspirin and ibuprofen. J. Allergy Clin. Immunol. Pract. 2016, 4, 160–161. [Google Scholar]
- Boggara, M.; Krishnamoorti, R. Partitioning of Nonsteroidal Antiinflammatory Drugs in Lipid Membranes. A Molecular Dynamics Simulation Study. Biophys. J. 2010, 98, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Nonsteroidal anti-inflammatory drugs (NSAIDs) are a group of medications commonly used to treat pain and inflammation. JAMA 2015, 314, 1084. [Google Scholar] [CrossRef] [PubMed]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eikawa, S.; Nishida, M.; Mizukami, S.; Yamazaki, C.; Nakayama, E.; Udono, H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc. Natl. Acad. Sci. USA 2015, 112, 1809–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.; Frid, A.; Hermansen, K.; Shah, N.S.; Tankova, T.; Mitha, I.H. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: The LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care 2009, 32, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Hashem, H. Chromatographic application on calixarene bonded stationary phases: A stability indicating method for simultaneous determination of paracetamol, caffeine and acetylsalicylic acid in Excedrin tables. Chromatographia 2010, 71, 31–35. [Google Scholar] [CrossRef]
- Chhetri, H.; Thapa, P.; Schepdael, A. Simple HPLC-UV method for the quantification of metformin in human plasma with one step protein precipitation. Saudi Pharm. J. 2014, 22, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, H.; Thapa, P.; Schepdael, A. HPLC method for the quantification of metformin hydrochloride in bulk and dosage. Int. J. Pharm. Sci. Res. 2013, 4, 2600–2604. [Google Scholar]
- Yang, Y.; Kapalavavi, B. Subcritical water chromatography—An economical and Green separation technique. Encycl. Anal. Chem. 2011, a9217, 1–23. [Google Scholar] [CrossRef]
- Yang, Y. Subcritical water chromatography: A green approach to high temperature liquid chromatography. J. Sep. Sci. 2007, 30, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M. Superheated water chromatography—A green technology for the Future. J. Chromatogr. A 2008, 1184, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Sunamoto, T.; Matsushima, Y.; Kikuchi, A.; Okano, T. Temperature-responsive chromatographic separation of amino acid phenylthiohydantoins using aqueous media as the mobile phase. Anal. Chem. 2000, 72, 5961–5966. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jones, A.D.; Eaton, C.D. Retention behavior of phenols, anilines, and alkylbenzenes in liquid chromatographic separations using subcritical water as the mobile phase. Anal. Chem. 1999, 71, 3808–3813. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Rui-Juan, S.; Na, Y.; Yuan-De, L.; Tian-Bao, H. Separations of some alcohols, phenols, and caboxylic acids by coupling of subcritical water chromatography and flame ionization detection with post column splitting. Chin. J. Anal. Chem. 2007, 35, 1335–1338. [Google Scholar] [CrossRef]
- Kondo, T.; Yang, Y. Comparison of elution strength, column efficiency, and peak symmetry in subcritical water chromatography and traditional reversed-phase liquid chromatography. Anal. Chim. Acta 2003, 494, 157–166. [Google Scholar] [CrossRef]
- Yang, Y.; Belghazi, M.; Lagadec, A.; Miller, D.J.; Hawthorne, S.B. Elution of organic solutes from different polarity sorbents using subcritical water. J. Chromatogr. A 1998, 810, 149–159. [Google Scholar] [CrossRef]
- Akay, S.; Odabası, M.; Yang, Y.; Kayan, B. Synthesis and evaluation of NAPHEMAH polymer for use as a new stationary phase in high-temperature liquid chromatography. Sep. Purif. Technol. 2015, 152, 1–6. [Google Scholar] [CrossRef]
- Droux, S.; Roy, M.; Félix, G. Green chiral HPLC study of the stability of Chiralcel OD under high temperature liquid chromatography and subcritical water conditions. J. Chromatogr. B 2014, 968, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.F.; Thurbide, K.B.; Quickfall, D. A comparison of hydrocarbon and alkali metal response in the flame ionization detector used in subcritical water chromatography. Can. J. Chem. 2015, 93, 784–789. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, X.; Mao, Y.; Zhang, Y.; Liu, J.; Rong, L.; Xu, Z. Retention mechanism of phenolic compounds in subcritical water chromatography. Chem. Res. Chin. Univ. 2015, 31, 103–106. [Google Scholar] [CrossRef]
- Kondo, T.; Yang, Y.; Lamm, L. Separation of polar and non-polar analytes using dimethyl sulfoxide-modified subcritical water. Anal. Chim. Acta 2002, 460, 185–191. [Google Scholar] [CrossRef]
- Tiihonen, J.; Peuha, E.L.; Latva-Kokko, M.; Silander, S.; Paatero, E. Subcritical water as eluent for chromatographic separation of carbohydrates using cation-exchange resins. Sep. Purif. Technol. 2005, 44, 166–174. [Google Scholar] [CrossRef]
- Yang, Y.; Jones, A.D.; Mathis, J.A.; Francis, M.A. Flame ionization detection after spltting the water effluent in subcritical water chromatography. J. Chromatogr. A 2002, 942, 231–236. [Google Scholar] [CrossRef]
- Yarita, T.; Nakajima, R.; Shibukawa, M. Superheated water chromatography of phenols using poly (styrene-divinylbenzene) packings as a stationary phase. Anal. Sci. 2003, 19, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.D. Investigation of a range of stationary phases for the separation of model drugs by HPLC using superheated water as the mobile phase. Chromatographia 2000, 52, S28–S34. [Google Scholar] [CrossRef]
- Yarita, T.; Nakajima, R.; Otsuka, S.; Ihara, T.; Takatsu, A.; Shibukawa, M. Determination of ethanol in alcoholic beverages by high-performance liquid chromatography-flame ionization detection using pure water as mobile phase. J. Chromatogr. A 2002, 976, 387–391. [Google Scholar] [CrossRef]
- Dugo, P.; Buonasera, K.; Crupi, M.L.; Cacciola, F.; Dugo, G.; Mondello, L. Superheated water as chromatographic eluent for parabens separation on octadecyl coated zirconia stationary phase. J. Sep. Sci. 2007, 30, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Strickland, Z.; Kapalavavi, B.; Marple, R.; Gamsky, C. Industrial application of green chromatography—I. Separation and analysis of niacinamide in skincare creams using pure water as the mobile phase. Talanta 2011, 84, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kapalavavi, B.; Marple, R.; Gamsky, C.; Yang, Y. Separation of sunscreens in skincare creams using greener high-temperature liquid chromatography and subcritical water chromatography. Int. J. Cosmet. Sci. 2012, 34, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kapalavavi, B.; Gujjar, L.; Hadrous, S.; Marple, R.; Gamsky, C. Industrial application of green chromatography—II. Separation and analysis of preservatives in skincare products using subcritical water chromatography. Int. J. Cosmet. Sci. 2012, 34, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Hawthorne, S.B. Subcritical water chromatography with flame ionization detection. Anal. Chem. 1997, 69, 623–627. [Google Scholar] [CrossRef]
- Kapalavavi, B.; Yang, Y.; Marple, R.; Gamsky, C. Separation and analysis of pharmaceuticals in cold drugs using green chromatography. Sep. Purif. Technol. 2016, 158, 308–331. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds studied in this work are not available from the authors. |
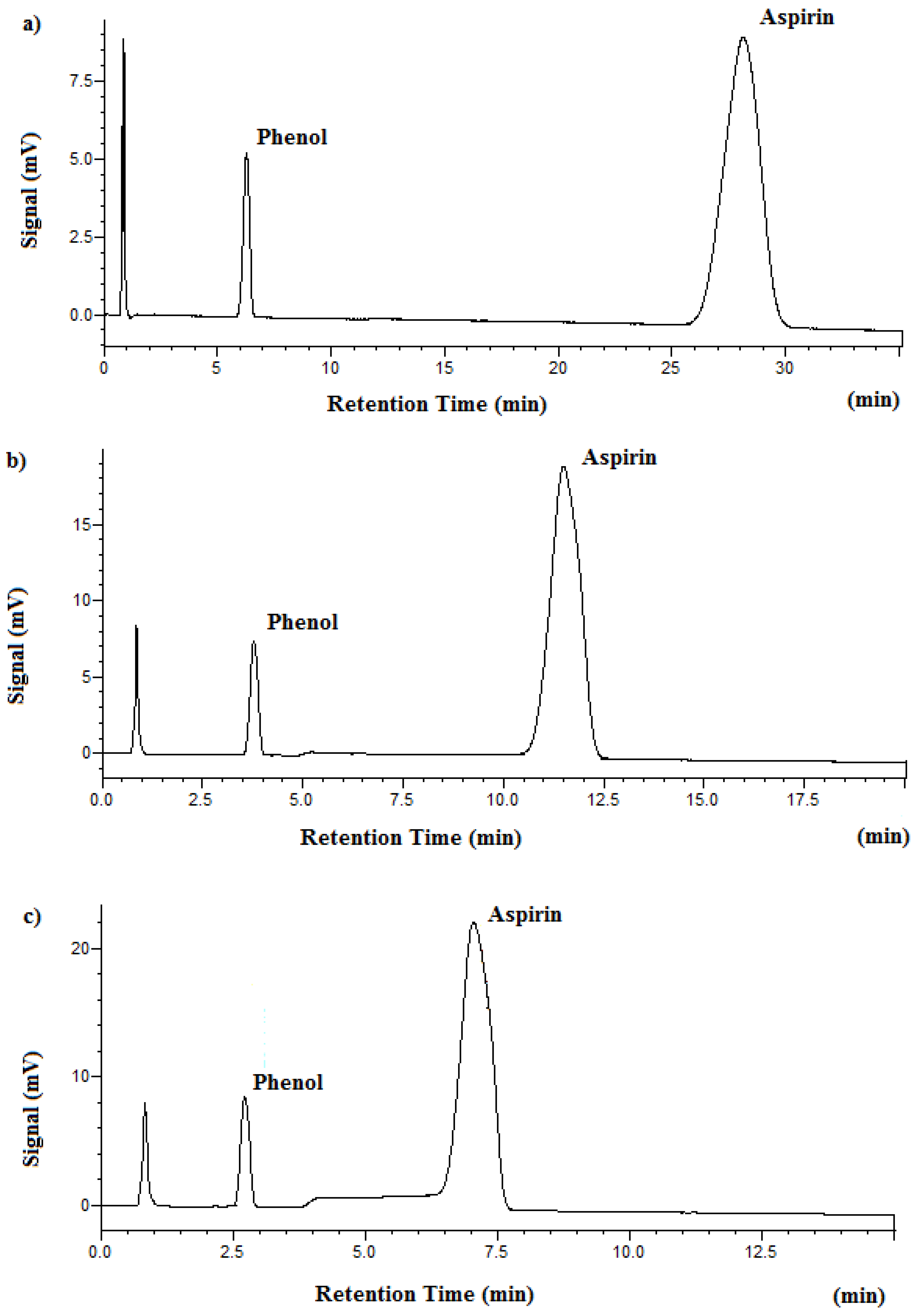
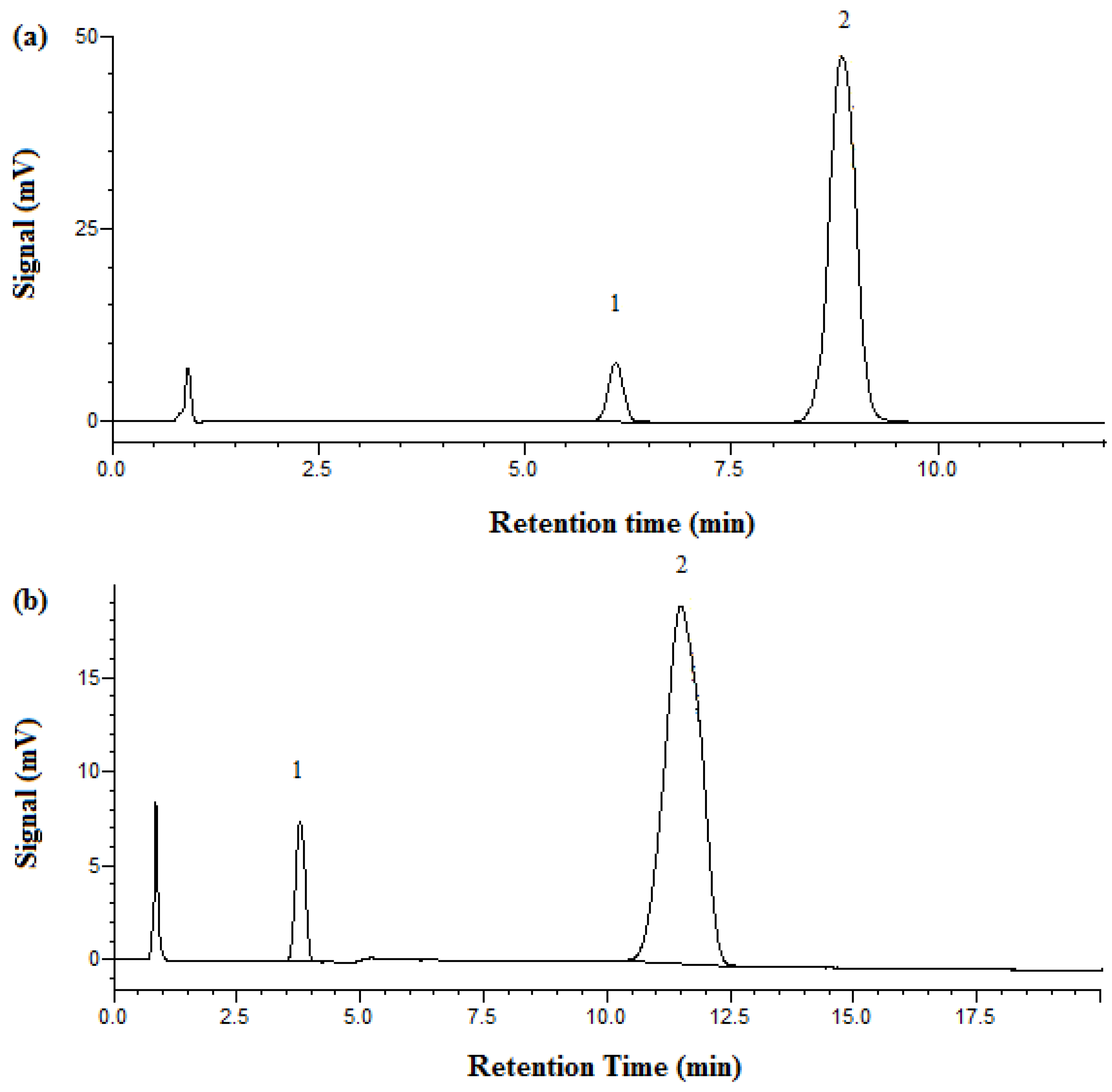
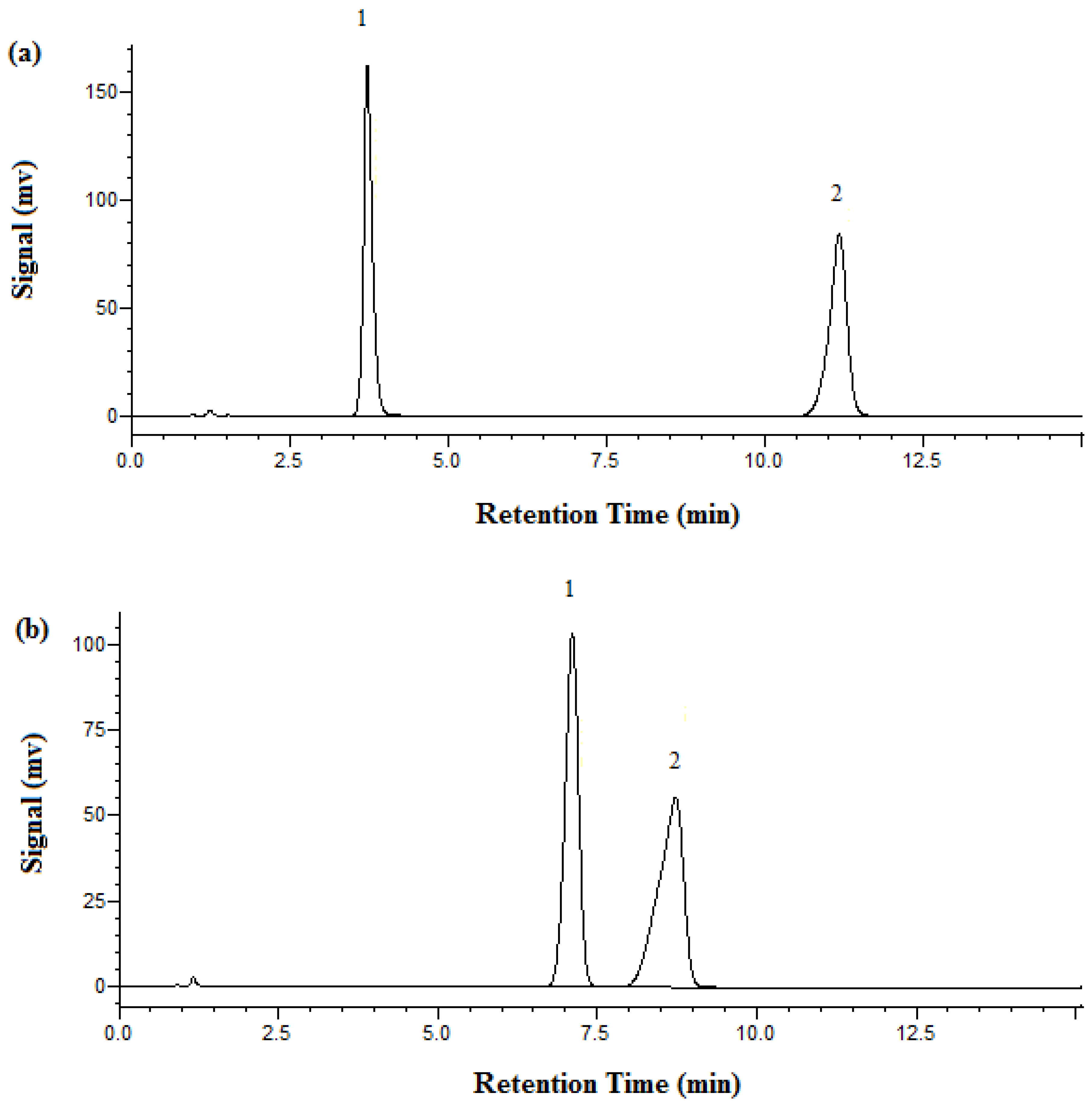
| Temperature (°C) | Retention Time (min) | Plate Number | |
|---|---|---|---|
| Aspirin | 95 °C | 28.2 | 1368 |
| Phenol | 95 °C | 6.28 | 2278 |
| Metformin | 95 °C | 7.11 | 5086 |
| Aspirin | 125 °C | 11.5 | 1021 |
| Phenol | 125 °C | 3.78 | 1580 |
| Aspirin | 150 °C | 7.05 | 655 |
| Phenol | 150 °C | 2.73 | 1099 |
| Method | Amount of API Used (mg) | Amount of API Found (mg) | %Recovery | %RSD a |
|---|---|---|---|---|
| Traditional HPLC | 63.7 | 63.1 | 99.0 | 0.7 |
| SBWC (125 °C) | 63.5 | 63.1 | 99.3 | 0.6 |
| Method | Amount of API Used (mg) | Amount of API Found (mg) | %Recovery | %RSD a |
|---|---|---|---|---|
| Traditional HPLC | 57.4 | 57.1 | 99.6 | 0.3 |
| SBWC (95 °C) | 54.2 | 53.5 | 98.8 | 0.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doctor, N.; Yang, Y. Separation and Analysis of Aspirin and Metformin HCl Using Green Subcritical Water Chromatography. Molecules 2018, 23, 2258. https://doi.org/10.3390/molecules23092258
Doctor N, Yang Y. Separation and Analysis of Aspirin and Metformin HCl Using Green Subcritical Water Chromatography. Molecules. 2018; 23(9):2258. https://doi.org/10.3390/molecules23092258
Chicago/Turabian StyleDoctor, Ninad, and Yu Yang. 2018. "Separation and Analysis of Aspirin and Metformin HCl Using Green Subcritical Water Chromatography" Molecules 23, no. 9: 2258. https://doi.org/10.3390/molecules23092258




