The Role of (H2O)1-2 in the CH2O + ClO Gas-Phase Reaction
Abstract
:1. Introduction
2. Computational Methods
3. Results and Discussion
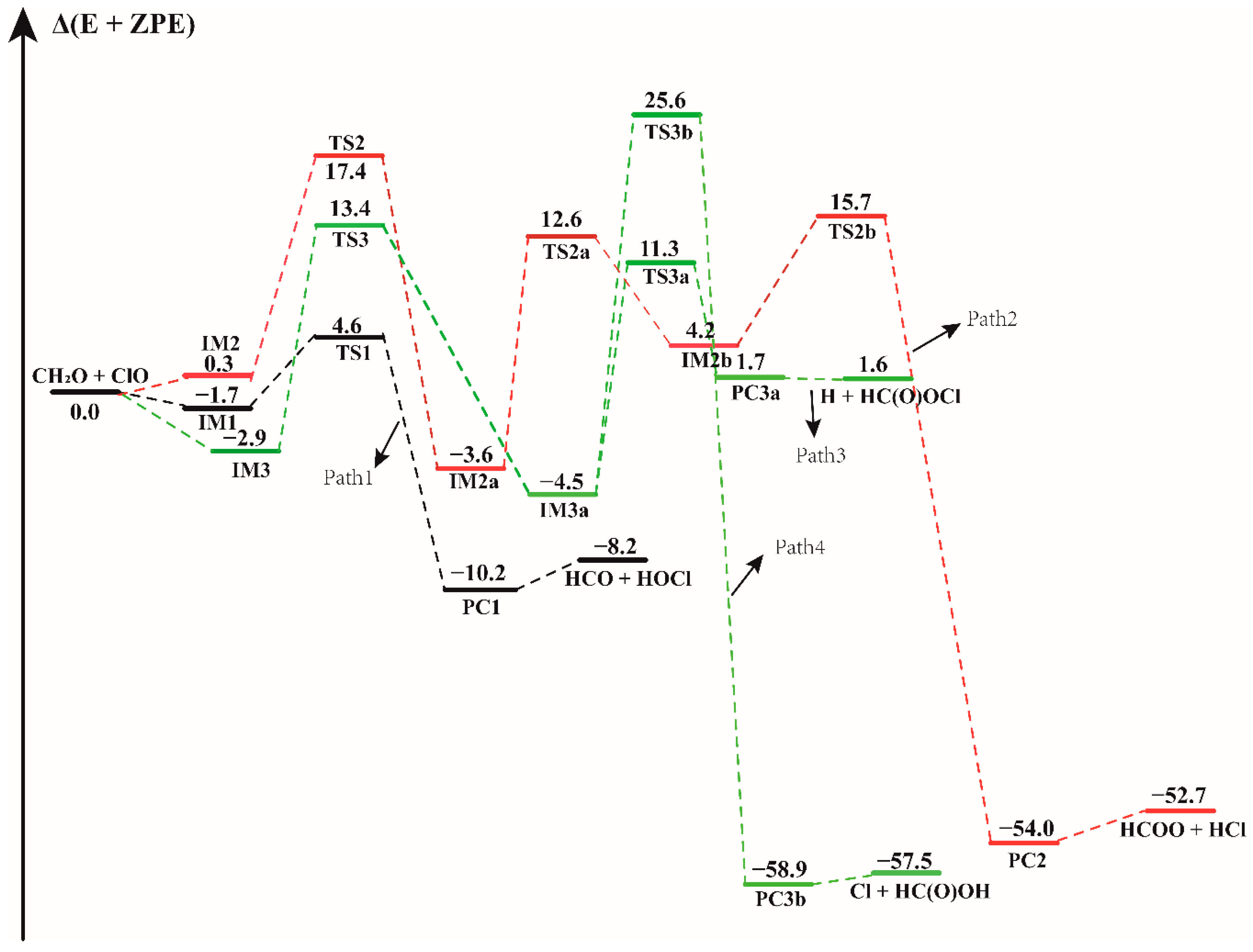
3.1. Mechanism of the CH2O + ClO Reaction in the Absence of Water Vapor
3.2. Mechanism of the CH2O + ClO Reaction in the Presence of Water Vapor
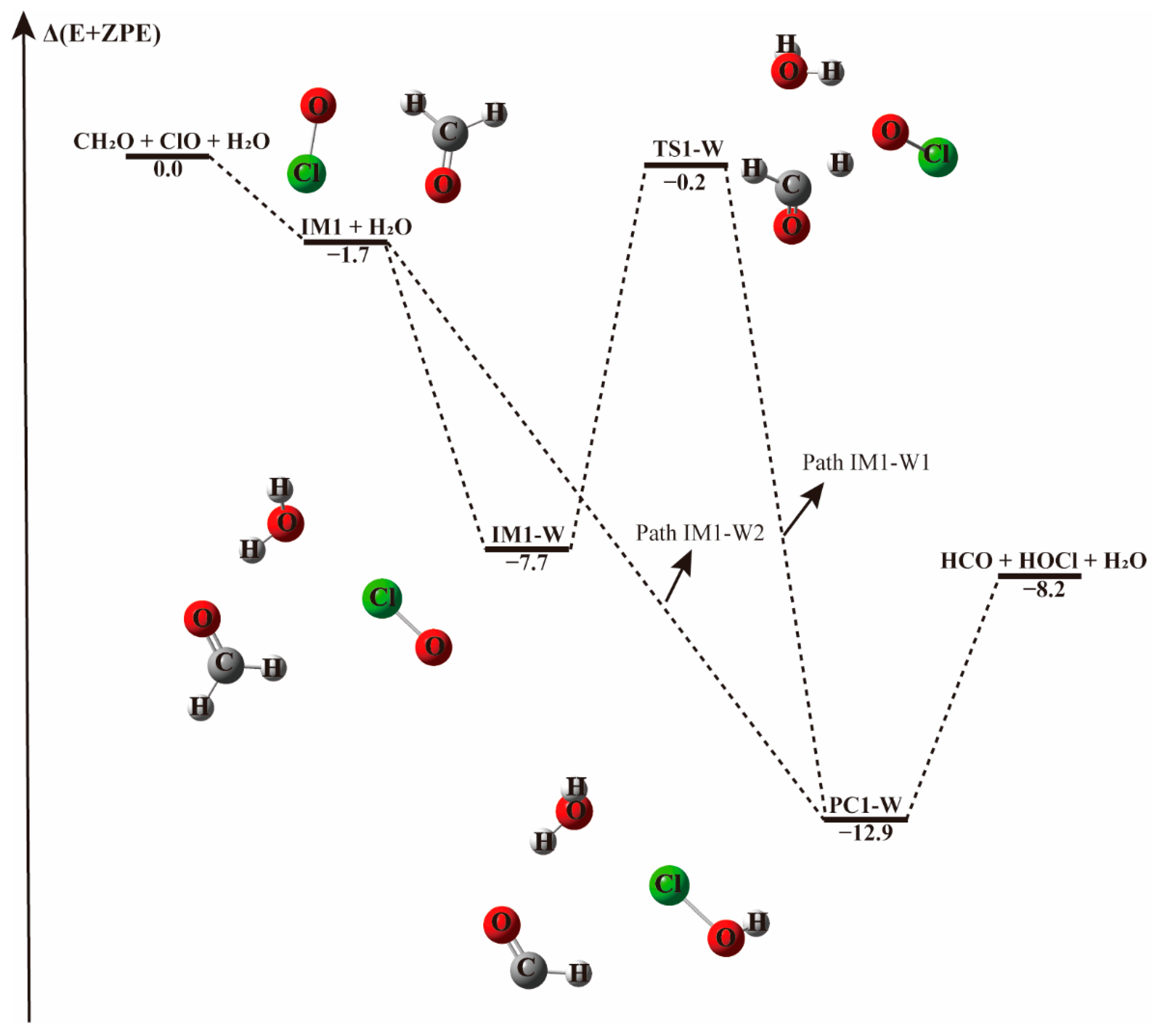
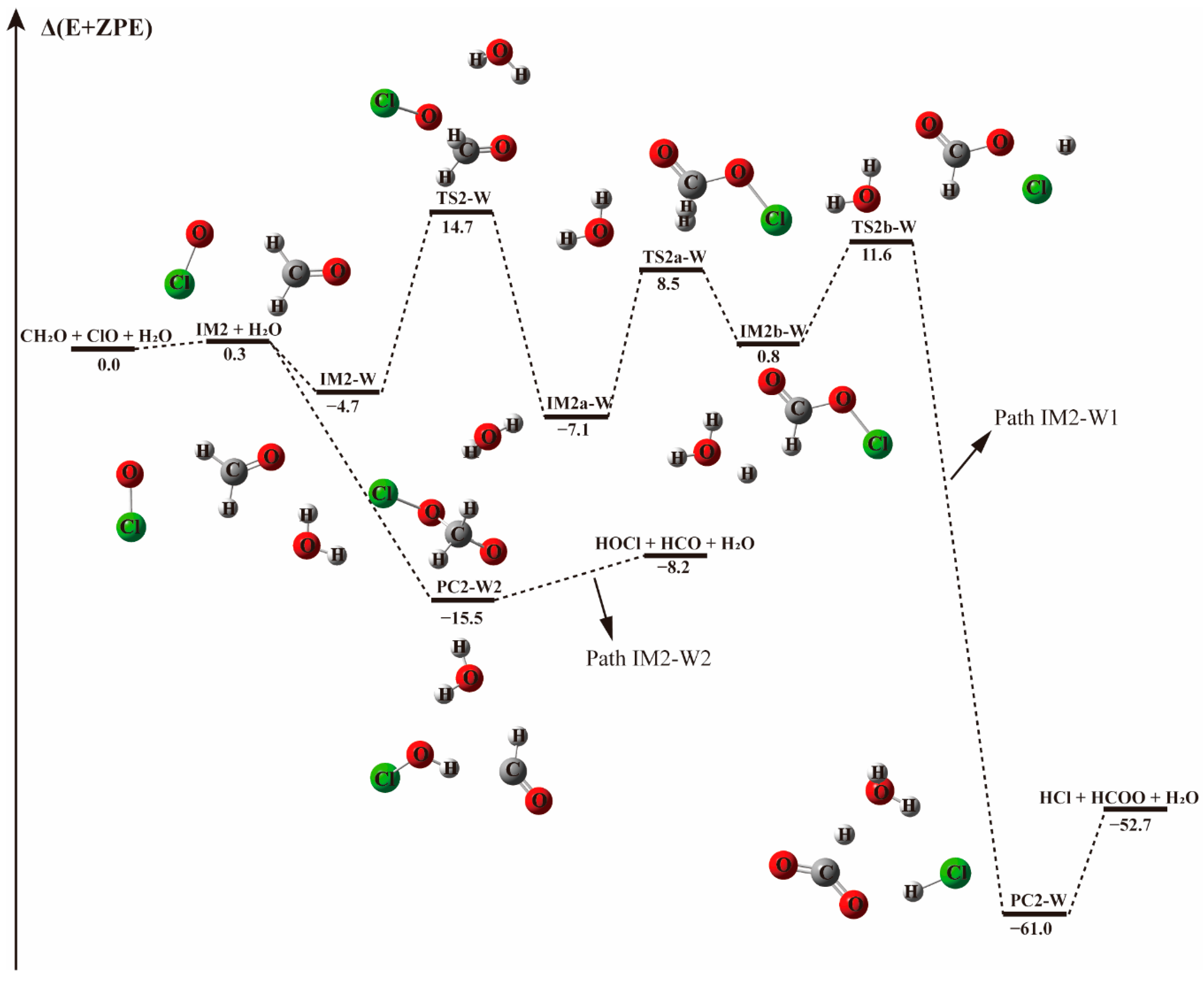
3.2.1. Reaction Between (H2O)1–2 and IM1/IM2/IM3 Complexes−
3.2.2. Mechanism of the CH2O···(H2O)1-2 + ClO and ClO···(H2O)1–2 + CH2O Reactions
3.3. Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, S.M.; Matross, D.M.; Andrews, A.E.; Millet, D.B.; Longo, M.; Gottlieb, E.W.; Hirsch, A.I.; Gerbig, C.; Lin, J.C.; Daube, B.C.; et al. Sources of carbon monoxide and formaldehyde in North America determined from high-resolution atmospheric data. Atmos. Chem. Phys. 2008, 8, 7673–7696. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Chen, Y.; Tabazadeh, A.; Fukui, Y.; Bey, I.; Yantosca, R.; Jacob, D.; Arnold, F.; Wohlfrom, K.; Atlas, E.; et al. Distribution and fate of selected oxygenated organic species in the troposphere and lower stratosphere over the Atlantic. J. Geophys. Res. Atmos. 2000, 105, 3795–3805. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Zhang, W. Theoretical Study on the Water-Assisted Reaction of NCO with HCHO. J. Phys. Chem. A 2013, 117, 6883–6892. [Google Scholar] [CrossRef] [PubMed]
- Possanzini, M.; Palo, V.D.; Cecinato, A. Sources and photodecomposition of formaldehyde and acetaldehyde in Rome ambient air. Atmos. Environ. 2002, 36, 3195–3201. [Google Scholar] [CrossRef]
- Grosjean, D. Formaldehyde and other carbonyls in Los Angeles ambient air. Environ. Sci. Technol. 1982, 16, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Schnell, M.; Mühlhäuser, M.; Peyerimhoff, S.D. Can the Methoxyradical CH3O Act as Sink for Cl and ClO in the Atmosphere? J. Phys. Chem. A 2004, 108, 1298–1304. [Google Scholar] [CrossRef]
- Bondy, A.L.; Wang, B.; Laskin, A.; Craig, R.L.; Nhliziyo, M.V.; Bertman, S.B.; Pratt, K.A.; Shepson, P.B.; Ault, A.P. Inland Sea Spray Aerosol Transport and Incomplete Chloride Depletion: Varying Degrees of Reactive Processing Observed during SOAS. Environ. Sci. Technol. 2017, 51, 9533–9542. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, S.; Yushina, S.; Uchida, T.; Shiraishi, N. Ignition and combustion of ammonium perchlorate in a hydrogen atmosphere. Proc. Combust. Inst. 2000, 28, 863–870. [Google Scholar] [CrossRef]
- Kosmas, A.M.; Drougas, E. A computational investigation of the atmospheric reaction CH3O2 + ClO. Chem. Phys. 2009, 358, 230–234. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, R.; Lin, M. Ab initio studies of ClOx reactions: vi. theoretical prediction of total rate constant and product branching probabilities for the HO2 + ClO reaction. J. Phys. Chem. A 2003, 107, 3841–3850. [Google Scholar] [CrossRef]
- Zhu, R.; Lin, M. Ab Initio Study of the ClO + NH2 Reaction: Prediction of the Total Rate Constant and Product Branching Ratios. J. Phys. Chem. A 2007, 111, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.L.; Pinto, J.P.; Watson, R.T.; Sander, S.P. Atmospheric Bromine and Ozone Perturbations in the Lower Stratosphere. J. Atmos. Sci. 1980, 37, 339–353. [Google Scholar] [CrossRef] [Green Version]
- Gilles, P.; Michel, P.; Françoise, M.; Radiela, R.; Georges, L.B. Role of the BrO + HO2 reaction in the stratospheric chemistry of bromine. Geophys. Res. Lett. 1992, 19, 2305–2308. [Google Scholar]
- Poulet, G.; Bras, G.L.; Combourieu, J.E.P.R. study of the reactivity of ClO with H2CO at 298 K. Geophys. Res. Lett. 1980, 7, 413–414. [Google Scholar] [CrossRef]
- DeMore, W.B.; Sander, S.P.; Golden, D.M.; Hampson, R.F.; Kurylo, M.J.; Howard, C.J.; Ravishankara, A.R.; Kolb, C.E.; Molina, M.J. Chemical Kinetics and Photochemical Data for Use in Stratospheric Modeling. Evaluation Number 12; Jet Propulsion Laboratory, California Institute of Technology: Pasadena, CA, USA, 1997. [Google Scholar]
- Tian, Y.; Wei, W.-M.; Tian, Z.-M.; Yang, H.-Y.; He, T.-J.; Liu, F.-C.; Chen, D.-M. Ab Initio/Density Functional Theory and Multichannel RRKM Study for the ClO + CH2O Reaction. J. Phys. Chem. A 2006, 110, 11145–11150. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, C.; Zhao, Y.; Bai, J.; Zhang, Q.; Wang, W. Atmospheric Chemical Reactions of 2,3,7,8-Tetrachlorinated Dibenzofuran Initiated by an OH Radical: Mechanism and Kinetics Study. Environ. Sci. Technol. 2012, 46, 8148–8155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gao, R.; Xu, F.; Zhou, Q.; Jiang, G.; Wang, T.; Chen, J.; Hu, J.; Jiang, W.; Wang, W. Role of Water Molecule in the Gas-Phase Formation Process of Nitrated Polycyclic Aromatic Hydrocarbons in the Atmosphere: A Computational Study. Environ. Sci. Technol. 2014, 48, 5051–5057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buszek, R.J.; Francisco, J.S.; Anglada, J.M. Water effects on atmospheric reactions. Int. Rev. Phys. Chem. 2011, 30, 335–369. [Google Scholar] [CrossRef]
- Leung, H.O.; Marshall, M.D.; Suenram, R.D.; Lovas, F.J. Microwave spectrum and molecular structure of the N2-H2O complex. J. Chem. Phys. 1989, 90, 700–712. [Google Scholar] [CrossRef]
- Sennikov, P.G.; Ignatov, S.K.; Schrems, O. Complexes and clusters of water relevant to atmospheric chemistry: H2O complexes with oxidants. Chem. Phys. Chem. 2005, 6, 392–412. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Narciso-Lopez, M.; Francisco-Marquez, M. Water Complexes of Important Air Pollutants: Geometries, Complexation Energies, Concentrations, Infrared Spectra, and Intrinsic Reactivity. J. Phys. Chem. A 2010, 114, 5796–5809. [Google Scholar] [CrossRef] [PubMed]
- Cirtog, M.; Asselin, P.; Soulard, P.; Tremblay, B.; Madebène, B.; Alikhani, M.E.; Georges, R.; Moudens, A.; Goubet, M.; Huet, T.R.; et al. The (CH2)2O−H2O Hydrogen Bonded Complex. Ab Initio Calculations and Fourier Transform Infrared Spectroscopy from Neon Matrix and a New Supersonic Jet Experiment Coupled to the Infrared AILES Beamline of Synchrotron SOLEIL. J. Phys. Chem. A 2011, 115, 2523–2532. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.; Gerber, R.B. Dynamics of vibrational overtone excitations of H2SO4, H2SO4-H2O: Hydrogen-hopping and photodissociation processes. J. Am. Chem. Soc. 2006, 128, 9594–9595. [Google Scholar] [CrossRef] [PubMed]
- Karpfen, A.; Kryachko, E.S. The dimers of glyoxal and acrolein with H2O and HF: Negative intramolecular coupling and blue-shifted C-H stretch. Chem. Phys. Lett. 2010, 489, 39–43. [Google Scholar] [CrossRef]
- Staikova, M.; Donaldson, D.J. Ab initio investigation of water complexes of some atmospherically important acids: HONO, HNO3 and HO2NO2. Phys. Chem. Chem. Phys. 2001, 3, 1999–2006. [Google Scholar] [CrossRef]
- Favero, L.B.; Caminati, W. Hydrated Complexes of Atmospheric Interest: Rotational Spectrum of Diacetyl-Water. J. Phys. Chem. A 2009, 113, 14308–14311. [Google Scholar] [CrossRef] [PubMed]
- Aloisio, S.; Francisco, J.S. Radical-water complexes in Earth’s atmosphere. Acc. Chem. Res. 2000, 33, 825–830. [Google Scholar] [CrossRef] [PubMed]
- McCabe, D.C.; Rajakumar, B.; Marshall, P.; Smith, I.W.M.; Ravishankara, A.R. The relaxation of OH (v = 1) and OD (v = 1) by H2O and D2O at temperatures from 251 to 390 K. Phys. Chem. Chem. Phys. 2006, 8, 4563–4574. [Google Scholar] [CrossRef] [PubMed]
- Alongi, K.S.; Dibble, T.S.; Shields, G.C.; Kirschner, K.N. Exploration of the Potential Energy Surfaces, Prediction of Atmospheric Concentrations, and Prediction of Vibrational Spectra for the HO2···(H2O)n (n = 1–2) Hydrogen Bonded Complexes. J. Phys. Chem. A 2006, 110, 3686–3691. [Google Scholar] [CrossRef] [PubMed]
- Brauer, C.S.; Sedo, G.; Grumstrup, E.M.; Leopold, K.R.; Marshall, M.D.; Leung, H.O. Effects of partially quenched orbital angular momentum on the microwave spectrum and magnetic hyperfine splitting in the OH-water complex. Chem. Phys. Lett. 2005, 401, 420–425. [Google Scholar] [CrossRef]
- McCoy, A.B.; Diken, E.G.; Johnson, M.A. Generating Spectra from Ground-State Wave Functions: Unraveling Anharmonic Effects in the OH−·H2O Vibrational Predissociation Spectrum. J. Phys. Chem. A 2009, 113, 7346–7352. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Francisco, J.S.; Schenter, G.K.; Iordanov, T.D.; Garrett, B.C.; Dupuis, M.; Li, J. The OH radical-H2O molecular interaction potential. J. Chem. Phys. 2006. [Google Scholar] [CrossRef] [PubMed]
- Kanno, N.; Tonokura, K.; Koshi, M. Equilibrium constant of the HO2-H2O complex formation and kinetics of HO2 + HO2 − H2O: Implications for tropospheric chemistry. J Geophys. Res. Atmos. 2006. [Google Scholar] [CrossRef]
- Du, B.; Zhang, W. The effect of (H2O)n (n= 1–2) or H2S on the hydrogen abstraction reaction of H2S by OH radicals in the atmosphere. Comput. Theor. Chem. 2015, 1069, 77–85. [Google Scholar] [CrossRef]
- English, A.M.; Hansen, J.C.; Szente, J.J.; Maricq, M.M. The effects of water vapor on the CH3O2 self-reaction and reaction with HO2. J. Phys. Chem. A 2008, 112, 9220–9228. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Tan, X.F.; Long, Z.W.; Wang, Y.B.; Ren, D.S.; Zhang, W.J. Theoretical Studies on Reactions of the Stabilized H2COO with HO2 and the HO2···H2O Complex. J. Phys. Chem. A 2011, 115, 6559–6567. [Google Scholar] [CrossRef] [PubMed]
- Buszek, R.J.; Barker, J.R.; Francisco, J.S. Water effect on the OH + HCl reaction. J. Phys. Chem. A 2012, 116, 4712–4719. [Google Scholar] [CrossRef] [PubMed]
- Vöhringer-Martinez, E.; Hansmann, B.; Hernandez, H.; Francisco, J.S.; Troe, J.; Abel, B. Water Catalysis of a Radical-Molecule Gas-Phase Reaction. Science 2007, 315, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.; Kjaergaard, H.G. Effect of Hydration on the Hydrogen Abstraction Reaction by HO in DMS and its Oxidation Products. J. Phys. Chem. A 2010, 114, 4857–4863. [Google Scholar] [CrossRef] [PubMed]
- Buszek, R.J.; Torrent-Sucarrat, M.; Anglada, J.M.; Francisco, J.S. Effects of a single water molecule on the OH + H2O2 reaction. J. Phys. Chem. A 2012, 116, 5821–5829. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Maeda, S.; Ohno, K. Water-catalyzed gas-phase reaction of formic acid with hydroxyl radical: A computational investigation. Chem. Phys. Lett. 2009, 469, 57–61. [Google Scholar] [CrossRef]
- Chen, H.-T.; Chang, J.-G.; Chen, H.-L. A Computational Study on the Decomposition of Formic Acid Catalyzed by (H2O)x, x = 0−3: Comparison of the Gas-Phase and Aqueous-Phase Results. J. Phys. Chem. A 2008, 112, 8093–8099. [Google Scholar] [CrossRef] [PubMed]
- Buszek, R.J.; Francisco, J.S. The Gas-Phase Decomposition of CF3OH with Water: A Radical-Catalyzed Mechanism. J. Phys. Chem. A 2009, 113, 5333–5337. [Google Scholar] [CrossRef] [PubMed]
- Smith, I. Single-Molecule Catalysis. Science 2007, 315, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, B.; Qin, Z. Catalytic Effect of Water, Formic Acid, or Sulfuric Acid on the Reaction of Formaldehyde with OH Radicals. J. Phys. Chem. A 2014, 118, 4797–4807. [Google Scholar] [CrossRef] [PubMed]
- Iuga, C.; Alvarez-Idaboy, J.R.; Vivier-Bunge, A. Single water-molecule catalysis in the glyoxal + OH reaction under tropospheric conditions: Fact or fiction? A quantum chemistry and pseudo-second order computational kinetic study. Chem. Phys. Lett. 2010, 501, 11–15. [Google Scholar] [CrossRef]
- Iuga, C.; Alvarez-Idaboy, J.R.; Vivier-Bunge, A. On the possible catalytic role of a single water molecule in the acetone + OH gas phase reaction: A theoretical pseudo-second-order kinetics study. Theor. Chem. Acc. 2011, 129, 209–217. [Google Scholar] [CrossRef]
- Iuga, C.; Alvarez-Idaboy, J.R.; Reyes, L.; Vivier-Bunge, A. Can a Single Water Molecule Really Catalyze the Acetaldehyde + OH Reaction in Tropospheric Conditions? J. Phys. Chem. Lett. 2010, 1, 3112–3115. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, Q.; Wang, W. Atmospheric oxidation of phenanthrene initiated by OH radicals in the presence of O2 and NOx—A theoretical study. Sci. Total Environ. 2016, 1, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhang, W. Catalytic effect of water, water dimer, or formic acid on the tautomerization of nitroguanidine. Comput. Theor. Chem. 2014, 1049, 90–96. [Google Scholar] [CrossRef]
- Viegas, L.P.; Varandas, A.J.C. Role of (H2O)n (n = 2–3) Clusters on the HO2 + O3 Reaction: A Theoretical Study. J. Phys. Chem. B 2016, 120, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.E.; Pokon, E.K.; Shields, G.C. Thermodynamics of Forming Water Clusters at Various Temperatures and Pressures by Gaussian-2, Gaussian-3, Complete Basis Set-QB3, and Complete Basis Set-APNO Model Chemistries; Implications for Atmospheric Chemistry. J. Am. Chem. Soc. 2004, 126, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Thom, H.; Dunning, J. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar]
- Kendall, R.A.; Thom, H.; Dunning, J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, D.L.; Kurtén, T.; Jørgensen, S.; Wallington, T.J.; Baggesen, S.B.; Aalling, C.; Kjaergaard, H.G. On the possible catalysis by single water molecules of gas-phase hydrogen abstraction reactions by OH radicals. Phys. Chem. Chem. Phys. 2012, 14, 12992–12999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, C.; Feng, X.; Kang, J.; Song, L.; Lu, Y.; Wang, Z.; Xu, Q.; Wang, W.; Wang, Z. The catalytic effect of water, water dimers and water trimers on H2S + 3O2 formation by the HO2 + HS reaction under tropospheric conditions. Phys. Chem. Chem. Phys. 2016, 18, 17414–17427. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. The path of chemical reactions—The IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kucharski, S.A.; Bartlett, R.J. A coupled cluster approach with triple excitations. J. Chem. Phys. 1984, 81, 5906–5912. [Google Scholar] [CrossRef]
- Purvis, G.D.; Bartlett, R.J. A full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bork, N.; Kurtén, T.; Vehkamäki, H. Exploring the atmospheric chemistry of O2SO3− and assessing the maximum turnover number of ion-catalysed H2SO4 formation. Atmos. Chem. Phys. 2013, 13, 3695–3703. [Google Scholar] [CrossRef]
- Billing, G.; Mikkelsen, K.; Truhlar, D.G. Introduction to Molecular Dynamics and Chemical Kinetics; Physics Today: Princeton, NJ, USA, 1996; Volume 49, p. 74. [Google Scholar]
- Miller, W.H. Unified statistical model for “complex” and “direct” reaction mechanisms. J. Chem. Phys. 1976, 65, 2216–2223. [Google Scholar] [CrossRef]
- Hu, W.-P.; Truhlar, D.G. Factors Affecting Competitive Ion−Molecule Reactions: ClO− + C2H5Cl and C2D5Cl via E2 and SN2 Channels. J. Am. Chem. Soc. 1996, 118, 860–869. [Google Scholar] [CrossRef]
- Gonzalez, J.; Anglada, J.M.; Buszek, R.J.; Francisco, J.S. Impact of water on the OH + HOCl reaction. J. Am. Chem. Soc. 2011, 133, 3345–3353. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kang, J.; Zhang, S.; Shao, X.; Jin, L.; Zhang, T.; Wang, Z. Catalytic effect of (H2O)n (n = 1–2) on the hydrogen abstraction reaction of H2O2+HS → H2S+HO2 under tropospheric conditions. Comput. Theor. Chem. 2017, 1110, 25–34. [Google Scholar] [CrossRef]
- Kumar, P.; Biswas, P.; Bandyopadhyay, B. Isomerization of the methoxy radical revisited: The impact of water dimers. Phys. Chem. Chem. Phys. 2016, 18, 27728–27732. [Google Scholar] [CrossRef] [PubMed]
- Anglada, J.M.; Hoffman, G.J.; Slipchenko, L.V.; Costa, M.M.; Ruiz-López, M.F.; Francisco, J.S. Atmospheric Significance of Water Clusters and Ozone–Water Complexes. J. Phys. Chem. A 2013, 117, 10381–10396. [Google Scholar] [CrossRef] [PubMed]
- Miro, P.; Cramer, C.J. Water clusters to nanodrops: A tight-binding density functional study. Phys. Chem. Chem. Phys. 2013, 15, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rojas, J.; Wales, D.J. The effect of dispersion damping functions on the structure of water clusters. Chem. Phys. 2014, 444, 23–29. [Google Scholar] [CrossRef]
- Cobar, E.A.; Horn, P.R.; Bergman, R.G.; Head-Gordon, M. Examination of the hydrogen-bonding networks in small water clusters (n = 2–5, 13, 17) using absolutely localized molecular orbital energy decomposition analysis. Phys. Chem. Chem. Phys. 2012, 14, 15328–15339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lan, X.; Qiao, Z.; Wang, R.; Yu, X.; Xu, Q.; Wang, Z.; Jin, L.; Wang, Z. Role of the (H2O)n (n = 1-3) cluster in the HO2 + HO → 3O2 + H2O reaction: Mechanistic and kinetic studies. Phys. Chem. Chem. Phys. 2018, 20, 8152–8165. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tsona, N.T.; Du, L. Effect of a single water molecule on the HO2 + ClO reaction. Phys. Chem. Chem. Phys. 2018, 20, 10650–10659. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Ma, C. Reactive Halogen Chemistry. Prog. Chem. 2009, 21, 307–334. [Google Scholar]
- Francisco, J.; Sander, S. Existence of a Chlorine Oxide and Water (ClO·H2O) Radical Complex. J. Am. Chem. Soc. 1995, 117, 9917–9918. [Google Scholar] [CrossRef]
- Li, Y.M.; Francisco, J.S. Complete active space self-consistent field and multireference configuration interaction studies of the low-lying excited states of the ClO–H2O radical complex. J. Chem. Phys. 2001, 115, 8381–8383. [Google Scholar] [CrossRef]
- Anglada, J.M.; Crehuet, R.; Martins-Costa, M.; Francisco, J.S.; Ruiz-Lopez, M. The atmospheric oxidation of CH3OOH by the OH radical: The effect of water vapor. Phys. Chem. Chem. Phys. 2017, 19, 12331–12342. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| System | ΔE | Δ(E + ZPE) | ΔH(298 K) | ΔG(298 K) |
|---|---|---|---|---|
| CH2O + ClO | 0 | 0 | 0 | 0 |
| IM1 | −2.5 | −1.7 | −0.7 | 6.0 |
| TS1 | 6.9 | 4.6 | 1.7 | 9.8 |
| PC1 | −10.0 | −10.2 | −10.0 | −5.3 |
| HCO + HOCl | −6.7 | −8.2 | −9.4 | −10.9 |
| IM2 | −0.2 | 0.3 | 1.1 | 6.9 |
| TS2 | 16.8 | 17.4 | 17.1 | 26.7 |
| IM2a | −5.7 | −3.6 | −2.6 | 7.5 |
| TS2a | 14.9 | 12.6 | 9.4 | 19.4 |
| IM2b | 7.0 | 4.2 | 1.3 | 8.8 |
| TS2b | 18.3 | 15.7 | 12.5 | 21.4 |
| PC2 | −52.6 | −54.0 | −55.6 | −48.2 |
| HCOO + HCl | −49.0 | −52.7 | −56.0 | −56.4 |
| IM3 | −3.7 | −2.9 | −1.9 | 4.6 |
| TS3 | 12.8 | 13.4 | 13.0 | 22.6 |
| IM3a | −6.8 | −4.5 | −3.2 | 6.9 |
| TS3a | 13.4 | 11.3 | 8.1 | 18.2 |
| PC3a | 4.4 | 1.7 | −1.0 | −1.0 |
| H + HC(O)OCl | 4.7 | 1.6 | −1.6 | 1.5 |
| TS3b | 27.1 | 25.6 | 23.2 | 32.9 |
| PC3b | −62.2 | −58.9 | −56.0 | −48.3 |
| Cl + HC(O)OH | −60.5 | −57.5 | −54.9 | −52.6 |
| System | ΔE | Δ(E + ZPE) | ΔH(298 K) | ΔG(298 K) |
|---|---|---|---|---|
| CH2O + ClO + H2O | 0 | 0 | 0 | 0 |
| IM1-W | −10.5 | −7.7 | −5.0 | 9.3 |
| TS1-W | 0 | −0.2 | −1.2 | 15.2 |
| PC1-W | −14.3 | −12.9 | −11.2 | 2.2 |
| IM2-W | −7.0 | −4.7 | −2.2 | 9.8 |
| TS2-W | 12.8 | 14.7 | 15.8 | 32.4 |
| IM2a-W | −10.5 | −7.1 | −4.6 | 12.4 |
| TS2a-W | 9.2 | 8.5 | 6.8 | 23.8 |
| IM2b-W | 2.0 | 0.8 | −0.5 | 14.4 |
| TS2b-W | 12.6 | 11.6 | 9.9 | 24.8 |
| PC2-W PC2-W2 | −62.3 −17.4 | −61.0 −15.5 | −61.1 −13.7 | −43.2 0.4 |
| HCl + HCOO + H2O | −49.0 | −52.7 | −56.0 | −56.4 |
| System | ΔE | Δ(E + ZPE) | ΔH(298 K) | ΔG(298 K) |
|---|---|---|---|---|
| CH2O + ClO + H2O | 0 | 0 | 0 | 0 |
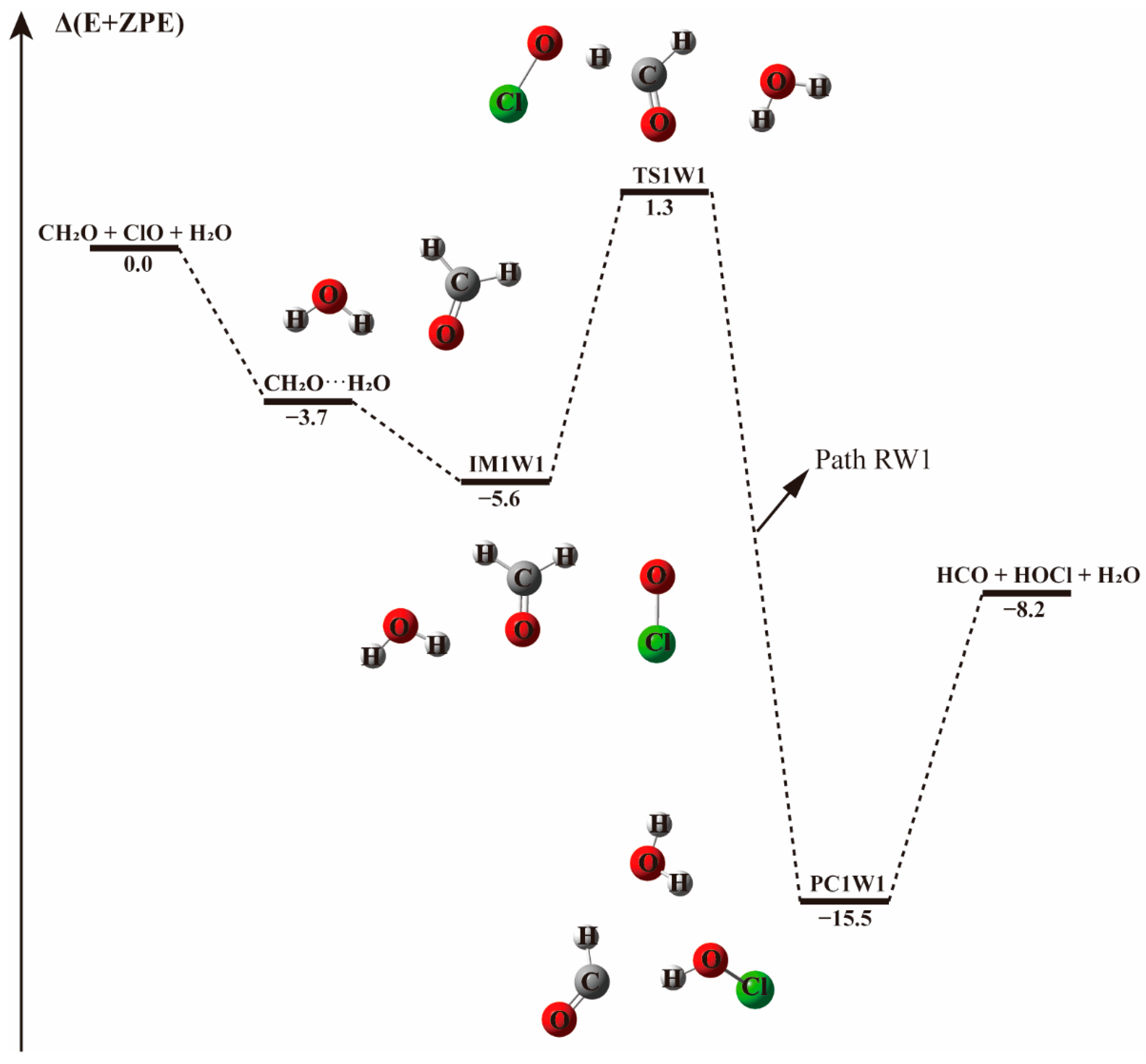
| CH2O···H2O + ClO | −5.6 | −3.7 | −2.0 | 4.5 |
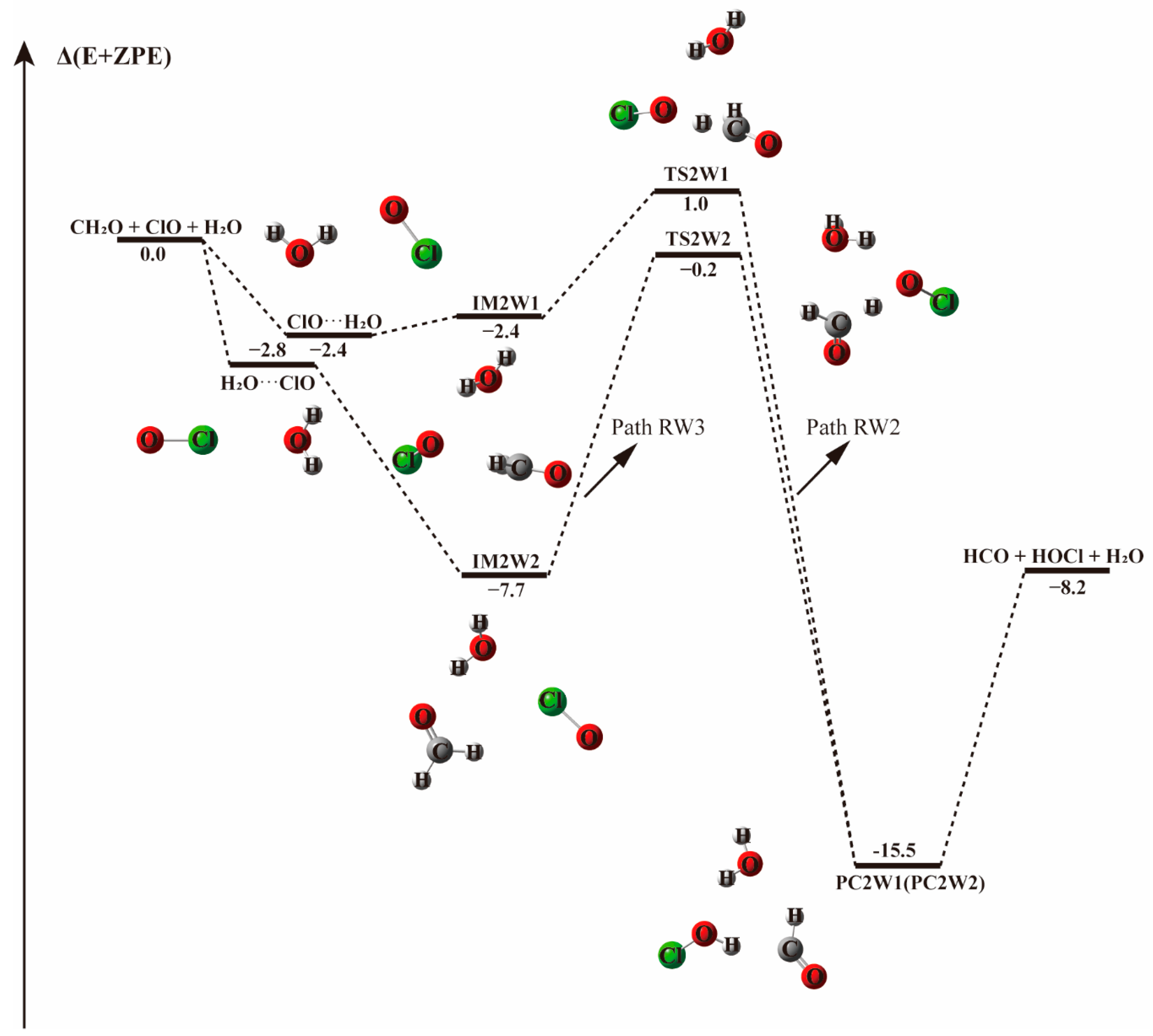
| ClO···H2O + CH2O | −3.5 | −2.4 | −1.4 | 4.0 |
| H2O···ClO + CH2O | −3.6 | −2.8 | −1.8 | 3.8 |
| IM1W1 | −8.1 | −5.6 | −3.0 | 10.2 |
| TS1W1 | 2.3 | 1.3 | −0.1 | 14.8 |
| PC1W1 | −17.4 | −15.5 | −13.7 | 0.4 |
| IM2W1 | −4.8 | −2.4 | −0.1 | 14.9 |
| TS2W1 | 1.1 | 1.0 | −0.1 | 16.5 |
| IM2W2 | −10.5 | −7.7 | −5.0 | 9.3 |
| TS2W2 | 0 | −0.2 | −1.2 | 15.2 |
| IM2W3 | −9.2 | −6.8 | −4.3 | 9.8 |
| TS2W3 | 41.8 | 39.4 | 35.7 | 53.7 |
| PC2W1(PC2W2) | −17.4 | −15.5 | −13.7 | 0.4 |
| PC2W3 | −19.7 | −17.3 | −15.3 | −0.1 |
| HCO + HOCl + H2O | −6.7 | −8.2 | −9.4 | −10.9 |
| System | ΔE | Δ(E + ZPE) | ΔH(298 K) | ΔG(298 K) |
|---|---|---|---|---|
| CH2O + ClO + (H2O)2 | 0 | 0 | 0 | 0 |
| CH2O···(H2O)2 + ClO | −14.5 | −9.9 | −6.4 | 9.5 |
| ClO···(H2O)2 + CH2O | −10.3 | −7.1 | −4.4 | 10.1 |
| IMWW1 | −16.9 | −11.8 | −7.5 | 15.4 |
| TSWW1 | −6.8 | −5.3 | −4.9 | 19.4 |
| PCWW1 | −22.6 | −18.8 | −15.3 | 6.1 |
| IMWW2 | −15.9 | −11.2 | −6.9 | 10.1 |
| TSWW2 PCWW2 | −5.4 −22.6 | −4.2 −18.8 | −4.0 −15.3 | 15.2 21.2 |
| HCO + HOCl + 2H2O | −6.7 | −8.2 | −9.4 | −10.9 |
| h (km) | T (K) | k1 | kIM1-W1 | kRW1 | kRW2 | kRW3 | |
|---|---|---|---|---|---|---|---|
| 0 | 298.15 | 2.6 × 10−16 | 4.2 × 10−20 | 5.7 × 10−20 | 6.7 × 10−21 | 4.2 × 10−20 | |
| 0 | 288.19 | 1.8 × 10−16 | 3.5 × 10−20 | 4.4 × 10−20 | 5.4 × 10−21 | 3.5 × 10−20 | |
| 2 | 275.21 | 1.1 × 10−16 | 2.7 × 10−20 | 3.0 × 10−20 | 3.9 × 10−21 | 2.7 × 10−20 | |
| 4 | 262.23 | 6.5 × 10−17 | 2.1 × 10−20 | 2.0 × 10−20 | 2.8 × 10−21 | 2.1 × 10−20 | |
| 6 | 249.25 | 3. 6 × 10−17 | 1.5 × 10−20 | 1.3 × 10−20 | 2.0 × 10−21 | 1.5 × 10−20 | |
| 8 | 236.27 | 1.8 × 10−17 | 1.1 × 10−20 | 8.0 × 10−21 | 1.3 × 10−21 | 1.1 × 10−20 | |
| 10 | 223.29 | 8.8 × 10−18 | 7.6 × 10−21 | 4.7 × 10−21 | 8.3 × 10−22 | 7.6 × 10−21 | |
| 12 | 216.69 | 5.9 × 10−18 | 6.2 × 10−21 | 3.5 × 10−21 | 6.5 × 10−22 | 6.2 × 10−21 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Tsona, N.T.; Du, L. The Role of (H2O)1-2 in the CH2O + ClO Gas-Phase Reaction. Molecules 2018, 23, 2240. https://doi.org/10.3390/molecules23092240
Li J, Tsona NT, Du L. The Role of (H2O)1-2 in the CH2O + ClO Gas-Phase Reaction. Molecules. 2018; 23(9):2240. https://doi.org/10.3390/molecules23092240
Chicago/Turabian StyleLi, Junyao, Narcisse T. Tsona, and Lin Du. 2018. "The Role of (H2O)1-2 in the CH2O + ClO Gas-Phase Reaction" Molecules 23, no. 9: 2240. https://doi.org/10.3390/molecules23092240