Antioxidant and Antimutagenic Activities of Different Fractions from the Leaves of Rhododendron arboreum Sm. and Their GC-MS Profiling
Abstract
1. Introduction
2. Results
2.1. Antioxidant Activity
2.1.1. Nitric Oxide Quenching Assay
2.1.2. Lipid Peroxidation Assay
2.1.3. Site-Specific Deoxyribose Deprivation Assay
2.1.4. Non-Site Specific Deoxyribose Deprivation Assay
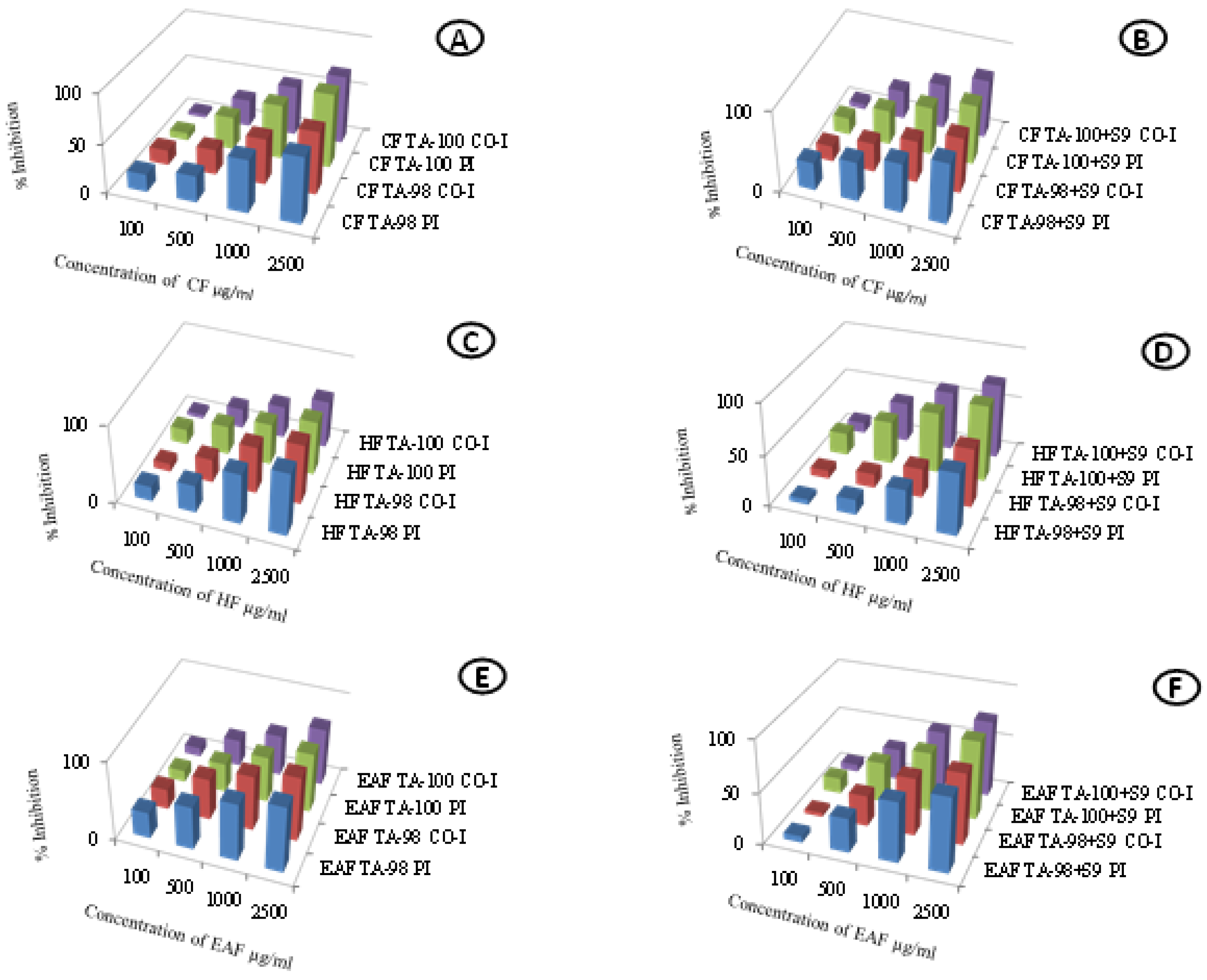
2.2. Antimutagenic Activity
2.3. Analysis Using GC-MS
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Antioxidant Activity
4.2.1. Lipid Peroxidation Assay
4.2.2. Nitric Oxide Quenching Assay
4.2.3. Deoxyribose Deprivation Assay
4.3. Antimutagenic Activity
4.4. Analysis Using GC-MS
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of interest
References
- Cocheme, H.M.; Murphy, M.P. Mitochondria as a source of reactive oxygen species. J. Biochem. 2009. [Google Scholar] [CrossRef]
- Galle, J. Oxidative stress in chronic renal failure. Nephrol. Dial. Transpl. 2001, 16, 2135–2137. [Google Scholar] [CrossRef]
- Stohs, S.J.; Bagghi, D. Review: Oxidative mechanisms in the toxicity of metal ions. Free Rad. Biol. Med. 1995, 18, 321–336. [Google Scholar] [CrossRef]
- Berlinera, J.A.; Heineckeb, W.J. Review: The role of oxidized lipoproteins in atherogenesis. Free Rad. Biol. Med. 1996, 20, 707–727. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncola, J.; Cronin, M.T.D.; Mazura, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- El-Hela, A.; Abdullah, A. Antioxidant and antimicrobial activities of methanol extracts of some Verbena species: In vitro evaluation of antioxidant and antimicrobial activity in relation to polyphenolic content. J. Appl. Sci. Res. 2010, 6, 683–689. [Google Scholar]
- Słoczyńska, K.; Powroźnik, B.; Pękala, E.; Waszkielewicz, A.M. Antimutagenic compounds and their possible mechanisms of action. J. Appl. Genet. 2014, 55, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Srivastava, P. Rhododendron arboreum: An overview. J. Appl. Pharm. Sci. 2012, 2, 158–162. [Google Scholar]
- The Retailers Code of Practice for Potentially Harmful Plants; Horticultural Trades Association; Guy’s & St Thomas’ Poisons Information Service and Royal Botanic Gardens: Kew, UK, 2008.
- Hajibabaei, K. Antioxidant properties of vitamin E. Ann. Res. Antioxid. 2016, 1, e22. [Google Scholar]
- Shibula, K.; Velavan, S. Determination of phytocomponents in methanolic extract of Annona muricata leaf using GC-MS technique. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 1251–1255. [Google Scholar]
- Salam, S.S.A.; Al Jaouni, S. Vitamin E (α-tocopherol) contents and antimutagenicity potentials Talbina (Hordeum vulgare L.). FEBS J. 2016, 283, 192. [Google Scholar]
- Ajith, T.A.; Ann, M.; Thomas, J. Evaluation of comparative and combined antimutagenic potential of vitamin C and vitamin E using histidine mutant Salmonella typhimurium strains. Ind. J. Clin. Biochem. 2008, 23, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003, 80, 393–397. [Google Scholar] [CrossRef]
- Dassprakash, M.V.; Arun, R.; Abraham, S.K.; Premkumar, K. In vitro and in vivo evaluation of antioxidant and antigenotoxic potential of leaf extract. Pharm. Biol. 2012, 50, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Bruni, R.; Righi, D.; Grandini, A.; Tognolini, M.; Pio Prencipe, F.; Poli, F.; Benvenuti, S.; Del Rio, D.; Rossi, D. Metabolite profiling of polyphenols in a Terminalia chebula Retzius ayurvedic decoction and evaluation of its chemopreventive activity. J. Ethnopharm. 2013, 147, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zahin, M.; Ahmad, I.; Gupta, R.C.; Aqil, F. Punicalagin and ellagic acid demonstrate antimutagenic activity and inhibition of benzo[a]pyrene induced DNA adducts. Biomed. Res. Int. 2014, 2014, 467465. [Google Scholar] [CrossRef] [PubMed]
- Okwu, D.E.; Ighodaro, B.U. GC-MS Evaluation of bioactive compounds and antibacterial activity of the oil fraction from the leaves of Alstonia boonei De Wild. Der Pharma Chemica 2010, 2, 261–272. [Google Scholar]
- Devi, K.V.; Shanmugasundaram, R.; Mohan, V.R. GC-MS analysis of ethanol extract of Entada pursaetha DC seed. Biosci. Discov. 2012, 3, 30–33. [Google Scholar]
- Rajeswari, G.; Murugan, M.; Mohan, V.R. GC-MS analysis of bioactive components of Hugonia mystax L. (Linaceae). Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 301–308. [Google Scholar]
- Lee, Y.S.; Kang, M.H.; Cho, Y.S.; Jeong, C.S. Effects of constituents of Amomum xanthioides on gastritis in rats and on growth of gastric cancer cells. Arch. Pharm. Res. 2007, 30, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.M.; Sree, A. Antibacterial activity and GCMS analysis of the extract of leaves of Finlaysonia obovata (a mangrove plant). Asian J. Plant. Sci. 2007, 6, 168–172. [Google Scholar]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Ng, T.B. Natural products with hypoglycemic, hypotensive, hypocholesterolemic, antiatherosclerotic and antithrombotic activities. Life Sci. 1999, 65, 2663–2677. [Google Scholar] [CrossRef]
- Moon, D.O.; Kim, M.O.; Choi, Y.H.; Kim, G.Y. Beta-Sitosterol induces G2/M arrest, endoreduplication, and apoptosis through the Bcl-2 and PI3K/Akt signaling pathways. Cancer Lett. 2008, 264, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, S.; Anand, S.; Sangeetha, K.N.; Shilpa, K.; Lakshmi, J.; Balakrishnan, A.; Lakshmi, B.S. Biological evaluation of (3β)-STIGMAST-5-EN-3-OL as potent anti-diabetic agent in regulating glucose transport using in vitro model. Int. J. Diabetes Mellitus 2010, 2, 101–109. [Google Scholar] [CrossRef]
- Nisar, M.; Ali, S.; Qaisar, M. Preliminary phytochemical screening of flowers, leaves, bark, stem and roots of Rhododendron arboreum. MEJSR 2011, 10, 472–476. [Google Scholar]
- Mary, S.J.; Indira, S. Phytochemical and FT-IR spectral analysis of Rhododendron arboreum Sm. ssp. nilagiricum (Zenker) Tagg. Biosci. Discov. 2017, 8, 9–13. [Google Scholar]
- Udgire, M.S.; Pathade, G.R. Evaluation of antimicrobial activities and phytochemical constituents of extracts of Valeriana wallichii. Asian J. Plant Sci. Res. 2013, 3, 55–59. [Google Scholar]
- Jubie, S.; Dhanabal, S.P. Gas chromatography-mass spectrometry analysis and antibacterial activity of fatty acid mixture of Spirulina platensis. J. Pharm. Sci. Res. 2012, 4, 1836–1838. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. The chemistry of free radicals and related reactive species. In Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2007; pp. 60–61. [Google Scholar]
- Marcocci, L.; Maguire, J.J.; Droylefaix, M.T.; Packer, L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O.I. The deoxyribose method: a simple ‘‘test-tube” assay for determination of rate constants for reaction of hydroxyl groups. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Grootveld, M.; Halliwell, B. The role of iron in ascorbate dependent deoxyribose degradation. Evidence consistent with a site specific hydroxyl radical generation caused by iron ions bound to the deoxyribose molecule. J. Inorg. Biochem. 1987, 29, 289–299. [Google Scholar] [CrossRef]
- Maron, D.H.; Ames, B.N. Revised methods for the Salmonella typhimurium mutagenicity test. Mut. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Bala, S.; Grover, I.S. Antimutagenicity of some citrus fruits. Mut. Res. 1989, 222, 141–148. [Google Scholar] [CrossRef]
- Garner, R.C.; Miller, E.C.; Miller, J.A. Liver microsomal metabolism ofaflatoxin B1 to a reactive derivative toxic to Salmonella typhimurium TA1530. Cancer Res. 1972, 32, 2058–2066. [Google Scholar] [PubMed]
Sample Availability: Not available. |
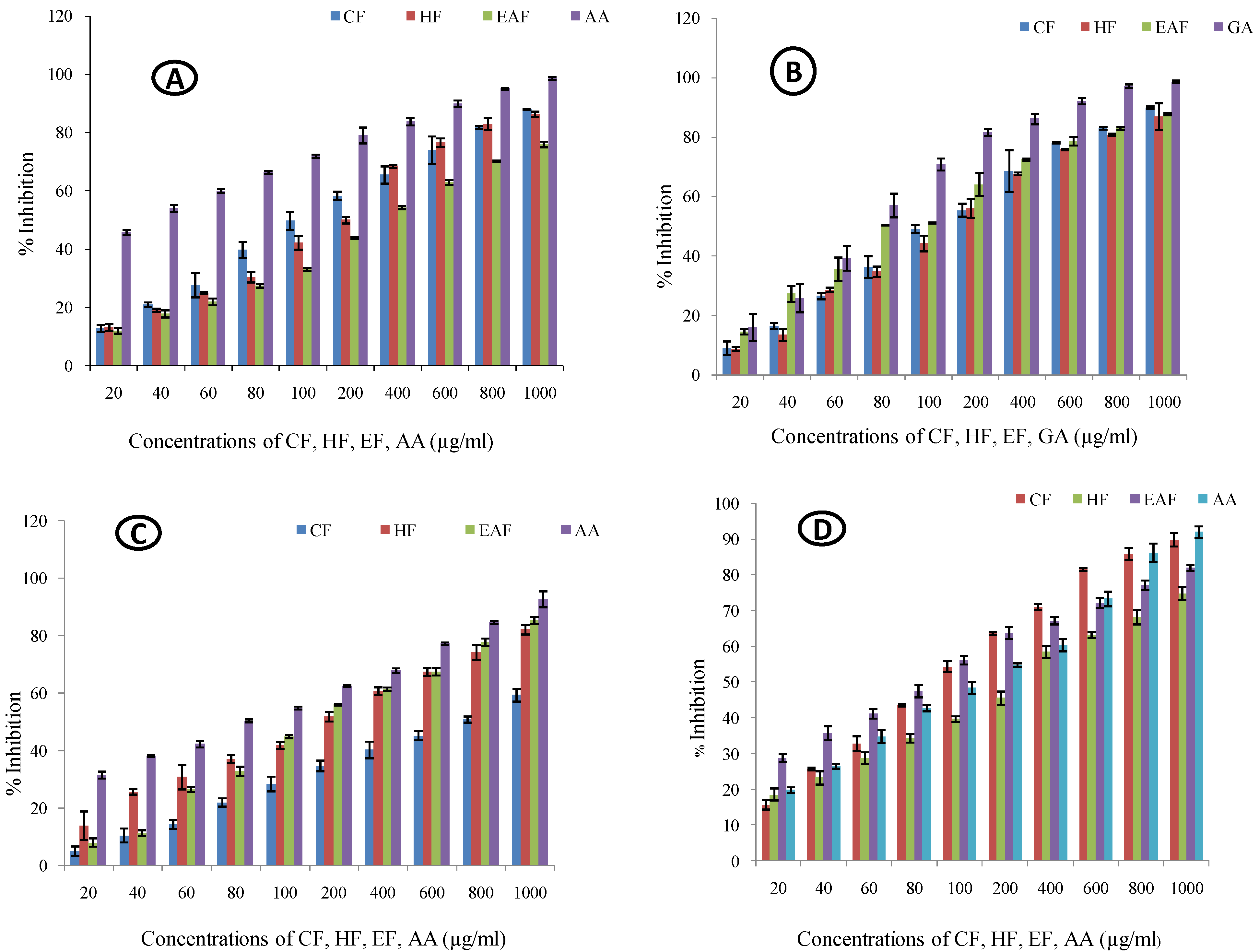

| Fractions | NO | LP | SS DDA | NSS DDA | |
|---|---|---|---|---|---|
| Chloroform Fraction | %I (20 µg/mL) | 12.749 ± 1.19 | 9.0 ± 2.266 | 4.862 ± 1.62 | 15.682 ± 1.318 |
| %I (1000 µg/mL) | 87.905 ± 0.224 | 89.91 ± 0.395 | 59.151 ± 2.122 | 89.803 ± 1.893 | |
| IC50 | 150.133 | 155.732 | 698.372 | 118.353 | |
| Fold Increase | 5.894 | 8.989 | 11.163 | 4.726 | |
| F-Ratio (9,20) | 295.564 * | 319.124 * | 247.581 * | 1298.927 * | |
| HSD | 7.61 | 8.03 | 5.773 | 3.647 | |
| Hexane Fraction | %I (20 µg/mL) | 13.106 ± 1.2 | 8.704 ± 0.617 | 13.758 ± 4.943 | 18.565 ± 1.674 |
| %I (1000 µg/mL) | 86.267 ± 0.895 | 86.844 ± 4.54 | 81.991 ± 1.689 | 74.753 ± 1.794 | |
| IC50 | 170.226 | 167.57 | 181.558 | 226.412 | |
| Fold Increase | 5.581 | 8.977 | 4.959 | 3.026 | |
| F-Ratio (9,20) | 1171.209 * | 505.111 * | 237.822 * | 460.541 * | |
| HSD | 4.033 | 6.251 | 7.27 | 4.608 | |
| Ethylacetate Fraction | %I (20 µg/mL) | 11.915 ± 0.917 | 14.54 ± 0.943 | 7.861 ± 1.441 | 28.745 ± 1.087 |
| %I (1000 µg/mL) | 75.930 ± 0.988 | 87.668 ± 0.317 | 85.22 ± 1.256 | 81.939 ± 0.823 | |
| IC50 | 267.067 | 116.029 | 191.907 | 96.817 | |
| Fold Increase | 5.372 | 5.029 | 9.84 | 1.85 | |
| F-Ratio (9,20) | 2410.284 * | 452.876 * | 1787.76 * | 506.233 * | |
| HSD | 2.337 | 5.865 | 3.194 | 4.063 | |
| Standard | %I (20 µg/mL) | 45.755 ± 0.852 | 15.924 ± 4.532 | 31.376 ± 1.214 | 19.789 ± 0.792 |
| %I (1000 µg/mL) | 98.599 ± 0.449 | 98.615 ± 0.395 | 92.577 ± 2.763 | 91.948 ± 1.585 | |
| IC50 | 25.648 | 95.185 | 83.86 | 132.597 | |
| Fold Increase | 1.154 | 5.192 | 1.95 | 3.646 | |
| F-Ratio (9,20) | 698.461 * | 317.475 * | 1028.868 * | 721.773 * | |
| HSD | 3.388 | 8.537 | 3.192 | 4.54 | |
| NO = Nitric oxide scavenging assay | LP = Lipid peroxidation assay | ||||
| SS DDA = Site specific deoxyribose deprivation assay | NSS DDA = Non site specific deoxyribose deprivation assay | ||||
| %I = Percent Inhibition | IC50 = 50% Inhibition concentration (µg/mL) | ||||
| Values are given as mean±standard error | HSD = Honestly significant difference | ||||
| * significant at p ≤ 0.001 level of significance | ANOVA = Analysis of variance | ||||
| Degree of Freedom | F-Ratio | ||||
|---|---|---|---|---|---|
| (TA-98) | (TA-98+S9) | (TA-100) | (TA-100+S9) | ||
| Chloroform Fraction | |||||
| F ratio Treatment | 1, 40 | 23.19 *** | 482.9 *** | 97.69 *** | 160.8 *** |
| F ratio Dose | 3, 40 | 1017 *** | 1384 *** | 3484 *** | 2267 *** |
| F ratio Treatment × Dose | 3, 40 | 3.171 ** | 13.14 *** | 0.748 | 25.08 *** |
| HSD | 154.2 | 131.8 | 115.7 | 116.2 | |
| Hexane Fraction | |||||
| F ratio Treatment | 1, 40 | 24.84 *** | 2.475 | 373.4 *** | 88.68 *** |
| F ratio Dose | 3, 40 | 611.1 *** | 398.5 *** | 1367 *** | 707.1 *** |
| F ratio Treatment × Dose | 3, 40 | 0.67 | 2.606 * | 3.294 ** | 18.11 *** |
| HSD | 297.4 | 159 | 142.2 | 187.3 | |
| Ethyl Acetate Fraction | |||||
| F ratio Treatment | 1, 40 | 42.24 *** | 2.399 * | 37.12 *** | 22.94 *** |
| F ratio Dose | 3, 40 | 1407 *** | 319.1 *** | 1607 *** | 791 *** |
| F ratio Treatment × Dose | 3, 40 | 4.286 ** | 0.018 | 1.502 | 3.663 ** |
| HSD | 153.2 | 370.1 | 164.5 | 240.4 | |
| No. | Chloroform Fraction | Hexane Fraction | Ethyl Acetate Fraction |
|---|---|---|---|
| 1 | 1-Dodecene | 3,7,11,15-Tetramethyl-2-hexa-decen-1-ol | 1-Dodecene |
| 2 | 1-Tetradecene | 14-Methylpentadecanoic acid, methyl ester | 1-Tetradecene |
| 3 | Docosanoic acid | 9-Octadecenoic acid | n-Tetradecane |
| 4 | 1-Nonadecene | 3,7,11,15-tetramethyl-2-hexadecen-1-ol, | 2,4-DI-Tert-butylphenol, |
| 5 | Neophytadiene | Linoleic acid | 9-(E)-Eicosene, |
| 6 | 3,7,11,15-Tetramethyl-2-hexa-decen-1-ol | Docosanoic acid | 9-Octadecenoic acid |
| 7 | Pentadecanoic acid | 1,2-Benzenedicarboxylic acid, ditridecyl ester | 3-(E)-Eicosene |
| 8 | 3-(E)-Eicosene, | 2-Hexyl-1-decanol | Neophytadiene |
| 9 | 3,7,11,15-Tetramethyl-, 2-hexadecen-1-ol, [R-[R*,R*-(E)]] phytol | 2,6,10,14,18,22-Tetracosa-hexaene | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
| 10 | Linoleic acid | Octadecyl Chloroacetate | 2-Methyl-7-octadecyne |
| 11 | 9-Octadecenoic acid | Vitamin E | Butyl-2-methylpropylphthalate |
| 12 | 9-(E)-Eicosene | 3,10-Epoxy-D:B-friedo-18,19-secolup-19-ene | Pentadecanoic acid |
| 13 | Heptadecyl trifluoroacetate | 3-Bromo-(3β)-cholest-5-ene | Pentadecyl trifluoroacetate |
| 14 | Hexatriacontane | Methyl commate C | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
| 15 | 2-Hexyl-1-decanol, | Methyl commate B | Linoleic acid |
| 16 | Docosyl pentafluoropropionate | Methyl commate D | Eicosanoic acid |
| 17 | Farnesol isomer a | Olean-12-en-28-al | 9-Tricosene |
| 18 | Octadecyl Chloroacetate | 1-Docosanol behenic alcohol | |
| 19 | 3,10-Epoxy-D:B-friedo-18,19-secolup-19-ene | 1,2-Benzenedicarboxylic acid, | |
| 20 | Cholest-5-ene | Nonadecyl pentafluoropropionate | |
| 21 | Methyl commate C | Vitamin E | |
| 22 | Methyl commate B | 3,10-Epoxy-(3β,10β)-D:B-friedo-18,19-secolup-19-ene | |
| 23 | Methyl commate D | 3β-Stigmast-5-en-3-ol | |
| 24 | Olean-12-en-28-al | Methyl commate C | |
| 25 | Methyl commate D | ||
| 26 | Urs-12-en-28-ol |
| NO | LP | SSDD | NSSDD | |
|---|---|---|---|---|
| NO | 1 | |||
| LP | 0.9979 * | 1 | ||
| SSDDA | 0.9971 * | 0.9946 * | 1 | |
| NSSDDA | 0.9983 * | 0.9980 * | 0.9923 * | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, V.; Kohli, S.K.; Arora, S.; Bhardwaj, R.; Kazi, M.; Ahmad, A.; Raish, M.; Ganaie, M.A.; Ahmad, P. Antioxidant and Antimutagenic Activities of Different Fractions from the Leaves of Rhododendron arboreum Sm. and Their GC-MS Profiling. Molecules 2018, 23, 2239. https://doi.org/10.3390/molecules23092239
Gautam V, Kohli SK, Arora S, Bhardwaj R, Kazi M, Ahmad A, Raish M, Ganaie MA, Ahmad P. Antioxidant and Antimutagenic Activities of Different Fractions from the Leaves of Rhododendron arboreum Sm. and Their GC-MS Profiling. Molecules. 2018; 23(9):2239. https://doi.org/10.3390/molecules23092239
Chicago/Turabian StyleGautam, Vandana, Sukhmeen Kaur Kohli, Saroj Arora, Renu Bhardwaj, Mohsin Kazi, Ajaz Ahmad, Mohammad Raish, Majid Ahmad Ganaie, and Parvaiz Ahmad. 2018. "Antioxidant and Antimutagenic Activities of Different Fractions from the Leaves of Rhododendron arboreum Sm. and Their GC-MS Profiling" Molecules 23, no. 9: 2239. https://doi.org/10.3390/molecules23092239
APA StyleGautam, V., Kohli, S. K., Arora, S., Bhardwaj, R., Kazi, M., Ahmad, A., Raish, M., Ganaie, M. A., & Ahmad, P. (2018). Antioxidant and Antimutagenic Activities of Different Fractions from the Leaves of Rhododendron arboreum Sm. and Their GC-MS Profiling. Molecules, 23(9), 2239. https://doi.org/10.3390/molecules23092239







