Antinociceptive Effects of Cardamonin in Mice: Possible Involvement of TRPV1, Glutamate, and Opioid Receptors
Abstract
:1. Introduction
2. Results
2.1. Evaluation of the Antinociceptive Activity
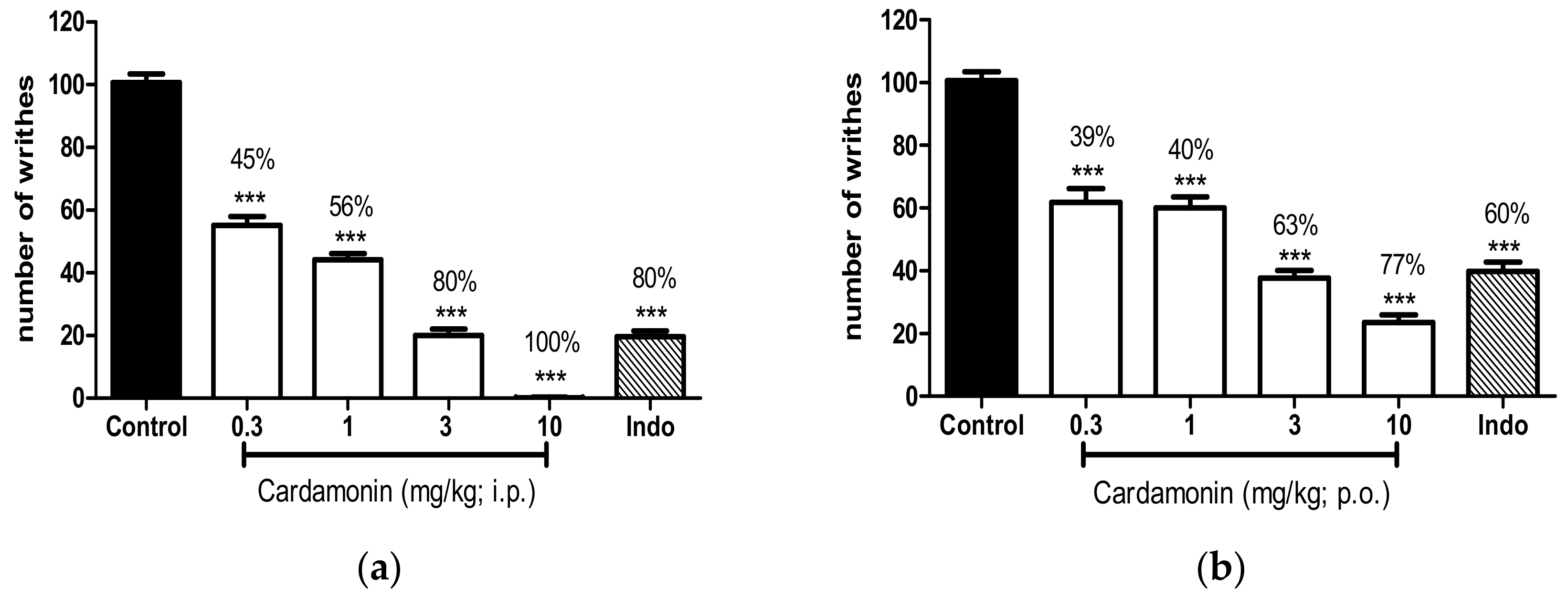
2.1.1. Acetic Acid–Induced Abdominal Writhing Test
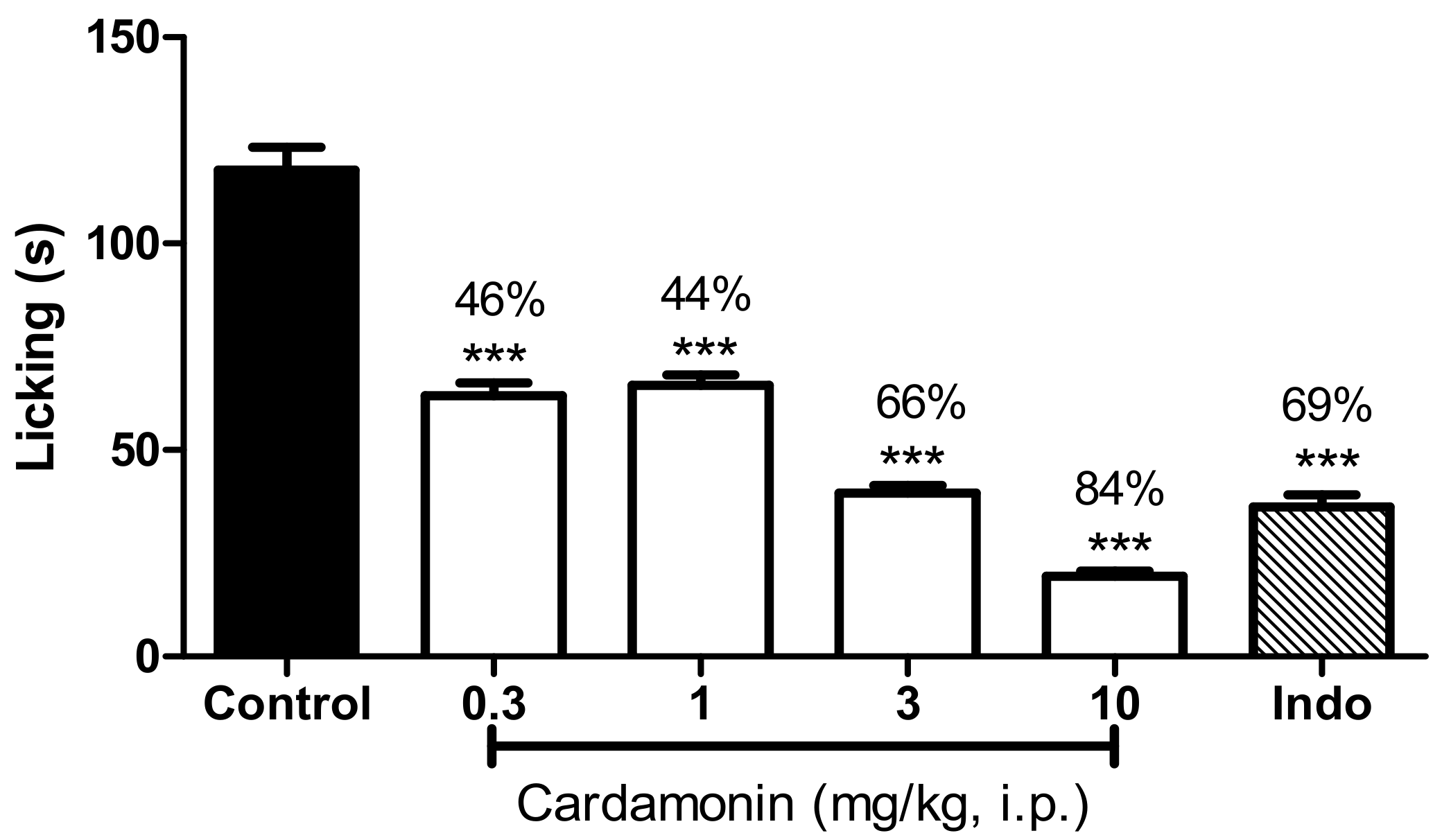
2.1.2. Formalin-Induced Paw Licking Test
2.1.3. Hot Plate Test
2.2. Investigation of the Mechanisms of Action
2.2.1. Involvement of the TRPV1 Receptor
2.2.2. Involvement of the Glutamate Receptor
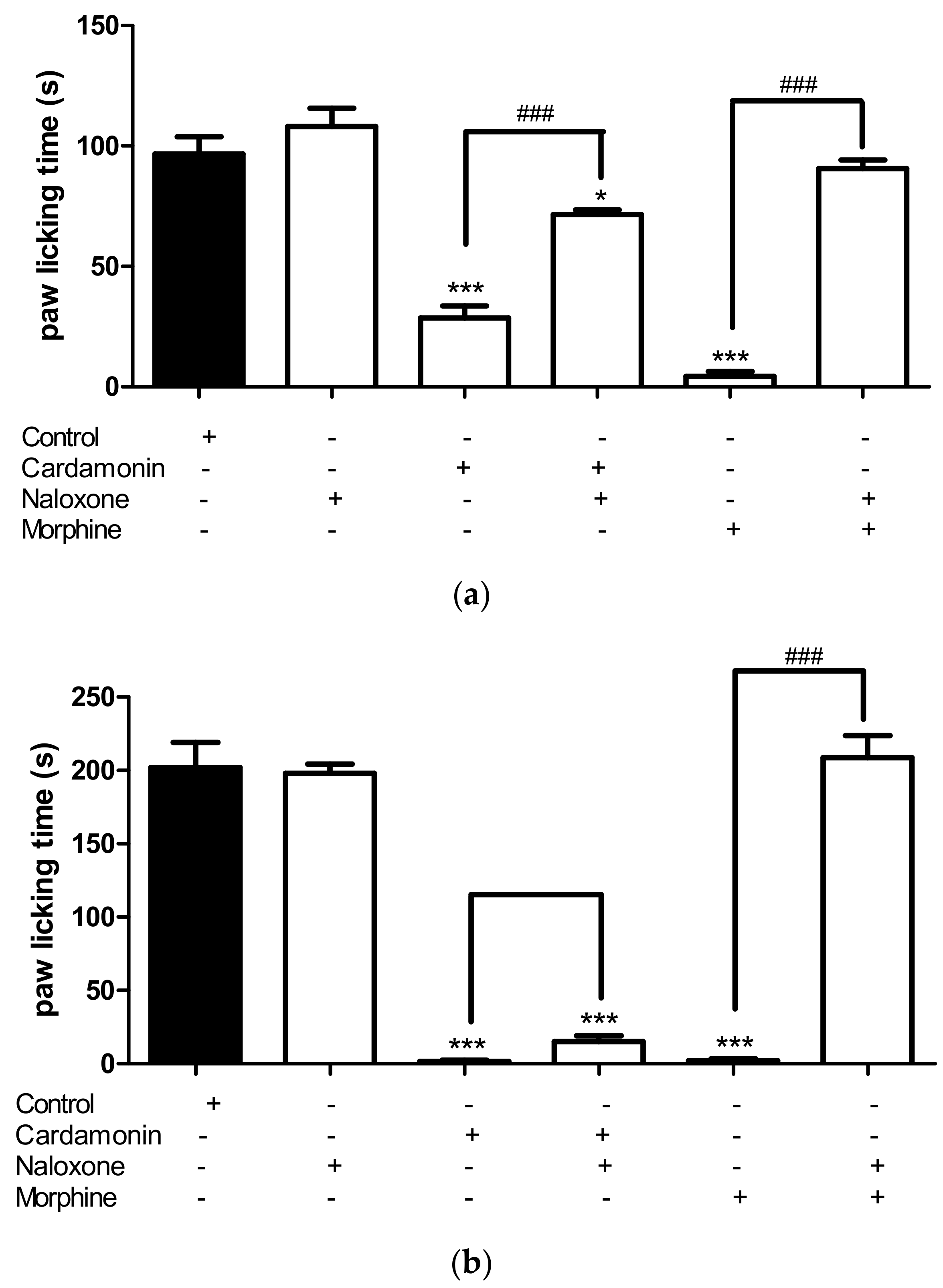
2.2.3. Involvement of the Opioid Receptors
2.3. Toxicity Assessment
2.4. Motor Coordination Evaluation
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Experimental Animals
4.4. Drugs and Chemicals
4.5. Evaluation of the Antinociceptive Activity
4.5.1. Acetic Acid–Induced Abdominal Writhing Test
4.5.2. Formalin-Induced Paw Licking Test
4.5.3. Hot Plate Test
4.6. Investigation of the Mechanisms of Action
4.6.1. Involvement of the TRPV1 Receptor
4.6.2. Involvement of the Glutamate Receptor
4.6.3. Involvement of the Opioid Receptors
4.7. Toxicity Assessment
4.8. Motor Coordination Test
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bheemasankara, R.C.; Namosiva, R.T.; Suryaprakasam, S. Cardamonin and alpinetin from the seeds of Amomum subulatum. Planta Medica 1976, 29, 391–392. [Google Scholar]
- Pascoal, A.C.; Ehrenfried, C.A.; Lopez, B.G.; de Araujo, T.M.; Pascoal, V.D.; Gilioli, R.; Anhe, G.F.; Ruiz, A.L.; Carvalho, J.E.; Stefanello, M.E. Antiproliferative Activity and Induction of Apoptosis in PC-3 Cells by the Chalcone Cardamonin from Campomanesia adamantium (Myrtaceae) in a Bioactivity-Guided Study. Molecules 2014, 19, 1843. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Sun, C.-Y.; Lu, F.-R.; Shu, X.-R.; Yang, D.; Chen, L.; She, X.-M.; Gregg, N.M.; Guo, T.; Hu, Y. Cardamonin exerts potent activity against multiple myeloma through blockade of NF-κB pathway in vitro. Leuk. Res. 2012, 36, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fang, Q.; Shi, D.; Niu, P.; Chen, Y.; Deng, J. mTOR inhibition of cardamonin on antiproliferation of A549 cells is involved in a FKBP12 independent fashion. Life Sci. 2014, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, J.; Xia, Y.F.; Huang, W.Z.; Wang, Z.T.; Dai, Y. Cardamonin protects septic mice from acute lung injury by preventing endothelial barrier dysfunction. J. Biochem. Mol. Toxicol. 2012, 26, 282–290. [Google Scholar] [CrossRef] [PubMed]
- El-Naga, R.N. Pre-treatment with cardamonin protects against cisplatin-induced nephrotoxicity in rats: Impact on NOX-1, inflammation and apoptosis. Toxicol. Appl. Pharmacol. 2014, 274, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Raweh, S.M.; Sirat, H.M.; Jamil, S.; Mohd Yasin, Y.H.; Jalil, J.; Jamal, J.A. Inhibitory effect of compounds from Zingiberaceae species on human platelet aggregation. Phytomedicine 2008, 15, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-T.; Lau, C.-W.; Chan, F.L.; Yao, X.; Chen, Z.-Y.; He, Z.-D.; Huang, Y. Vasorelaxant effects of cardamonin and alpinetin from Alpinia henryi K. Schum. J. Cardiovasc. Pharmacol. 2001, 37, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Shi, D.-H.; Zheng, W.; Xu, X.-J.; Yu, Y.-H. Antiproliferation of cardamonin is involved in mTOR on aortic smooth muscle cells in high fructose-induced insulin resistance rats. Eur. J. Pharmacol. 2010, 641, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yamamoto, N.; Yamashita, Y.; Ashida, H. The chalcones cardamonin and flavokawain B inhibit the differentiation of preadipocytes to adipocytes by activating ERK. Arch. Biochem. Biophys. 2014, 554, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Ryu, M.; Jeong, Y.; Chung, Y.-H.; Kim, D.-E.; Cho, H.-S.; Kang, S.; Han, J.-S.; Chang, M.-Y.; Lee, C.-K.; et al. Cardamonin suppresses melanogenesis by inhibition of Wnt/β-catenin signaling. Biochem. Biophys. Res. Commun. 2009, 390, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Choi, J.K.; Kim, H.J.; Nakahata, N.; Lim, K.M.; Kim, S.Y.; Lee, C.H. Novel inhibitory effects of cardamonin on thromboxane A2-induced scratching response: Blocking of Gh/transglutaminase-2 binding to thromboxane A2 receptor. Pharmacol. Biochem. Behav. 2014, 126, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Israf, D.A.; Lajis, N.H.; Shaari, K.; Mohamed, H.; Wahab, A.A.; Ariffin, K.T.; Hoo, W.Y.; Aziz, N.A.; Kadir, A.A.; et al. Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. Eur. J. Pharmacol. 2006, 538, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israf, D.A.; Khaizurin, T.A.; Syahida, A.; Lajis, N.H.; Khozirah, S. Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-κB nuclear translocation and κ-B phosphorylation in RAW 264.7 macrophage cells. Mol. Immunol. 2007, 44, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, Y.-L.; Lee, K.-H.; Vidyadaran, S.; Lajis, N.H.; Akhtar, M.N.; Israf, D.A.; Syahida, A. Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-γ/LPS-stimulated BV2 microglia via NF-κB signalling pathway. Int. Immunopharmacol. 2012, 12, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S. Graminex Pollen: Phenolic Pattern, Colorimetric Analysis and Protective Effects in Immortalized Prostate Cells (PC3) and Rat Prostate Challenged with LPS. Molecules 2018, 23, e1145. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Yamamoto, N.; Murakami, A. Cardamonin suppresses nitric oxide production via blocking the IFN-γ/STAT pathway in endotoxin-challenged peritoneal macrophages of ICR mice. Life Sci. 2011, 89, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Schmidtko, A.; Tegeder, I.; Geisslinger, G. No NO, no pain? The role of nitric oxide and cGMP in spinal pain processing. Trends Neurosci. 2009, 32, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Lee, H.J.; Choi, J.K.; Kim, H.J.; Kang, J.H.; Lee, E.J.; Kim, Y.R.; Kang, J.H.; Yoo, J.K.; Cho, H.Y.; et al. Novel anti-nociceptive effects of cardamonin via blocking expression of cyclooxygenase-2 and transglutaminase-2. Pharmacol. Biochem. Behav. 2014, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Cardosa, M.; Osman, Z.J.; Nicholas, M.; Tonkin, L.; Williams, A.; Aziz, K.A.; Ali, R.M.; Dahari, N.M. Self-management of chronic pain in Malaysian patients: Effectiveness trial with 1-year follow-up. Transl. Behav. Med. 2012, 2, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Zaki, L.R.M.; Hairi, N.N. Chronic pain and pattern of health care utilization among Malaysian elderly population: National Health and Morbidity Survey III (NHMS III, 2006). Maturitas 2014, 79, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Kowaluk, E.A.; Arneric, S.P. Emerging molecular approaches to pain therapy. J. Med. Chem. 1999, 42, 1481. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Wang, Y.; Cudnik, M.; Smith, D.A.; Pakiela, J.; Emerman, C.L. The Effectiveness and Adverse Events of Morphine versus Fentanyl on a Physician-staffed Helicopter. J. Emerg. Med. 2012, 43, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Boettger, M.K.; Reif, A.; Schmitt, A.; Uceyler, N.; Sommer, C. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol. Pain 2010, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.M.; Pereira, M.A.; Ardenghi, J.V.; Mora, T.C.; Bresciani, L.F.; Yunes, R.A.; Monache, F.D.; Cechinel Filho, V. Filicene obtained from Adiantumcuneatum interacts with the cholinergic, dopaminergic, glutamatergic, GABAergic, and tachykinergic systems to exert antinociceptive effect in mice. Pharmacol. Biochem. Behav. 2009, 93, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Deraedt, R.; Jouquey, S.; Delevallee, F.; Flahaut, M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur. J. Pharmacol. 1980, 61, 17–24. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ueno, A.; Naraba, H.; Oh-ishi, S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001, 69, 2911–2919. [Google Scholar] [CrossRef]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [PubMed]
- Omote, K.; Kawamata, T.; Kawamata, M.; Namiki, A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res. 1998, 787, 161–164. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkubo, T.; Takahashi, H.; Inoki, R. Modified formalin test: Characteristic biphasic pain response. Pain 1989, 38, 347–352. [Google Scholar] [CrossRef]
- Abbott, F.V.; Melzack, R. Brainstem lesions dissociate neural mechanisms of morphine analgesia in different kinds of pain. Brain Res. 1982, 251, 149–155. [Google Scholar] [CrossRef]
- Nemirovsky, A.; Chen, L.; Zelman, V.; Jurna, I. The antinociceptive effect of the combination of spinal morphine with systemic morphine or buprenorphine. Anesthesia Analg. 2001, 93, 197–203. [Google Scholar] [CrossRef]
- Sakurada, T.; Matsumura, T.; Moriyama, T.; Sakurada, C.; Ueno, S.; Sakurada, S. Differential effects of intraplantar capsazepine and ruthenium red on capsaicin-induced desensitization in mice. Pharmacol. Biochem. Behav. 2003, 75, 115–121. [Google Scholar] [CrossRef]
- Beirith, A.; Santos, A.R.S.; Calixto, J.B. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 2002, 924, 219–228. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Tengku Mohamad, T.A.; Shaik Mossadeq, W.M.; Moin, S.; Yusof, M.; Mokhtar, A.F.; Zakaria, Z.A.; Israf, D.A.; Lajis, N. Antinociceptive activity of the essential oil of Zingiber zerumbet. Planta Medica 2010, 76, 107. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, A.S.; Akhtar, M.N.; Zakaria, Z.A.; Perimal, E.K.; Khalid, S.; Mohd, P.A.; Khalid, M.H.; Israf, D.A.; Lajis, N.H.; Sulaiman, M.R. Antinociceptive activity of a synthetic chalcone, flavokawin B on chemical and thermal models of nociception in mice. Eur. J. Pharmacol. 2010, 647, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.M.; Mohamad, A.S.; Makhtar, N.; Khalid, M.H.; Khalid, S.; Perimal, E.K.; Mastuki, S.N.; Zakaria, Z.A.; Lajis, N.; Israf, D.A. Antinociceptive activity of methanolic extract of Acmella uliginosa (Sw.) Cass. J. Ethnopharmacol. 2011, 133, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ming-Tatt, L.; Khalivulla, S.I.; Akhtar, M.N.; Mohamad, A.S.; Perimal, E.K.; Khalid, M.H.; Akira, A.; Lajis, N.; Israf, D.A.; Sulaiman, M.R. Antinociceptive activity of a synthetic curcuminoid analogue, 2, 6-bis-(4-hydroxy-3-methoxybenzylidene) cyclohexanone, on nociception-induced models in mice. Basic Clin. Pharmacol. Toxicol. 2012, 110, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Perimal, E.K.; Akhtar, M.N.; Mohamad, A.S.; Khalid, M.H.; Ming, O.H.; Khalid, S.; Tatt, L.M.; Kamaldin, M.N.; Zakaria, Z.A.; Israf, D.A. Zerumbone-induced antinociception: Involvement of the l-arginine-nitric oxide-cGMP-PKC-K+ ATP channel pathways. Basic Clin. Pharmacol. Toxicol. 2011, 108, 155–162. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



| Treatment | Dose | Latency Time (s) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (mg/kg) | 0 min | 30 min | 60 min | 90 min | 120 min | 150 min | 180 min | 210 min | |
| Control | 6.17 ± 0.17 | 6.83 ± 0.31 | 6.67 ± 0.33 | 6.67 ± 0.21 | 6.83 ± 0.31 | 6.67 ± 0.21 | 6.83 ± 0.31 | 6.33 ± 0.21 | |
| Cardamonin (i.p.) | 0.3 | 6.34 ± 0.16 | 6.99 ± 0.52 | 7.01 ± 0.27 | 8.26 ± 0.33 | 8.28 ± 0.56 | 9.08 ± 1.09 * | 8.36 ± 0.59 | 6.83 ± 0.55 |
| 1 | 7.05 ± 0.22 | 6.84 ± 0.56 | 7.03 ± 0.16 | 7.11 ± 0.28 | 8.04 ± 0.67 | 8.97 ± 0.44 * | 8.50 ± 0.37 | 7.22 ± 0.26 | |
| 3 | 6.95 ± 0.25 | 6.83 ± 0.25 | 7.59 ± 0.25 | 7.65 ± 0.16 | 8.37 ± 0.41 | 8.92 ± 0.24 * | 7.52 ± 0.51 | 6.71 ± 0.36 | |
| 10 | 6.63 ± 0.20 | 7.45 ± 0.46 | 8.08 ± 0.46 | 9.14 ± 0.72 * | 10.09 ± 0.89 *** | 9.21 ± 0.83 ** | 8.07 ± 0.33 | 7.48 ± 0.15 | |
| Naloxone (i.p.) + Cardamonin (i.p.) | 1 + 5 | 7.30 ± 0.24 | 8.70 ± 0.28 | 8.54 ± 0.32 | 8.60 ± 0.25 | 11.33 ± 0.59 ### | 11.62 ± 0.50 ## | 9.40 ± 0.58 | 8.45 ± 0.66 |
| Morphine (s.c.) | 5 | 7.50 ± 0.34 | 18.33 ± 0.67 *** | 17.00 ± 0.82 *** | 16.33 ± 0.42 *** | 16.33 ± 0.99 *** | 15.33 ± 0.62 *** | 15.17 ± 0.40 *** | 14.83 ± 0.40 *** |
| Naloxone (i.p.) + Morphine (s.c.) | 5 + 5 | 7.30 ± 0.29 | 8.93 ± 0.82 ### | 11.18 ± 0.74 ### | 10.76 ± 0.61 ### | 10.10 ± 0.84 ### | 9.01 ± 0.83 ### | 7.64 ± 0.30 ### | 6.92 ± 0.30 ### |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ping, C.P.; Tengku Mohamad, T.A.S.; Akhtar, M.N.; Perimal, E.K.; Akira, A.; Israf Ali, D.A.; Sulaiman, M.R. Antinociceptive Effects of Cardamonin in Mice: Possible Involvement of TRPV1, Glutamate, and Opioid Receptors. Molecules 2018, 23, 2237. https://doi.org/10.3390/molecules23092237
Ping CP, Tengku Mohamad TAS, Akhtar MN, Perimal EK, Akira A, Israf Ali DA, Sulaiman MR. Antinociceptive Effects of Cardamonin in Mice: Possible Involvement of TRPV1, Glutamate, and Opioid Receptors. Molecules. 2018; 23(9):2237. https://doi.org/10.3390/molecules23092237
Chicago/Turabian StylePing, Chung Pui, Tengku Azam Shah Tengku Mohamad, Muhammad Nadeem Akhtar, Enoch Kumar Perimal, Ahmad Akira, Daud Ahmad Israf Ali, and Mohd Roslan Sulaiman. 2018. "Antinociceptive Effects of Cardamonin in Mice: Possible Involvement of TRPV1, Glutamate, and Opioid Receptors" Molecules 23, no. 9: 2237. https://doi.org/10.3390/molecules23092237





