Residue Depletion of Florfenicol and Florfenicol Amine in Broiler Chicken Claws and a Comparison of Their Concentrations in Edible Tissues Using LC–MS/MS
Abstract
:1. Introduction
2. Results
2.1. In-House Validation of the Analytical Methodologies
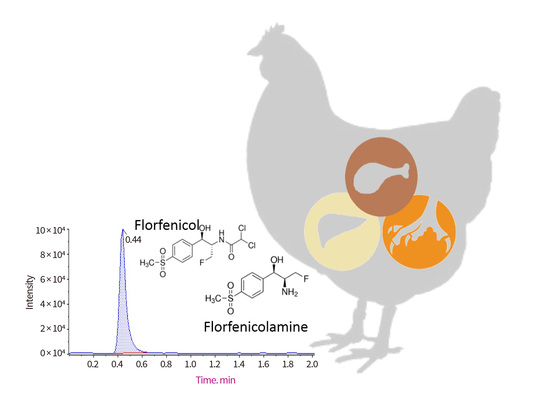
2.2. Detection and Quantification of FF and FFA Concentrations in Samples of Claws, and Muscle and Liver Tissues
3. Discussion
4. Materials and Methods
4.1. Experimental Animals: Controlled and Treated Groups
4.2. Quantification of FF and FFA in Broiler Claws
4.2.1. Standard Solutions and Reagents
4.2.2. Sample Collection and Processing
4.2.3. Extraction Procedure
4.2.4. Instrumental Analysis
4.3. In-House Validation of the Analytical Methods
4.4. Depletion Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization FAO. Perspectivas Alimentarias: Resumenes de Mercado. Noviembre de 2017; Food Outlook; Food and Agriculture Organization: Rome, Italy, 2017; p. 12. [Google Scholar]
- Livestock and Poultry: World Markets and Trade; Foreign Agricultural Service, United States Department of Agriculture: Washington, DC, USA, 2018; p. 30.
- Ramírez, H.; Mauricio, M. Aprovechamiento de los Subproductos o Residuos en la Industria Avícola Para la Producción de Harinas de Origen Animal; RevistaVirtualPro: Bogotá, Colombia, 2008; Number 82. [Google Scholar]
- Bolan, N.S.; Szogi, A.A.; Chuasavathi, T.; Seshadri, B.; Rothrock, M.J.; Panneerselvam, P. Uses and management of poultry litter. World’s Poult. Sci. J. 2010, 66, 673–698. [Google Scholar] [CrossRef]
- United States of America Poultry and Egg Export Council. Chicken-Feet Exportѕ Help Sаvаnnаh Lead U.S. Port Growth; United States of America Poultry and Egg Export Council: Stone Mountain, GA, USA, 2014. [Google Scholar]
- Asociación de Productores Avícolas de Chile A.G. Análisis de Exportaciones de Garras de Pollos Periodo Anual 2014; Asociación de Productores Avícolas de Chile A.G.: Santiago, Chile, 2015. [Google Scholar]
- Pokrant, E.; Medina, F.; Maddaleno, A.; San Martín, B.; Cornejo, J. Determination of sulfachloropyridazine residue levels in feathers from broiler chickens after oral administration using liquid chromatography coupled to tandem mass spectrometry. PLoS ONE 2018, 13, e0200206. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, J.; Lapierre, L.; Iragüen, D.; Pizarro, N.; Hidalgo, H.; Martín, B.S. Depletion study of three formulations of flumequine in edible tissues and drug transfer into chicken feathers. J. Vet. Pharmacol. Ther. 2011, 34, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, J.; Pokrant, E.; Krogh, M.; Briceño, C.; Hidalgo, H.; Maddaleno, A.; Araya-Jordán, C.; Martín, B.S. Determination of oxytetracycline and 4-epi-oxytetracycline residues in feathers and edible tissues of broiler chickens using liquid chromatography coupled with tandem mass spectrometry. J. Food Prot. 2017, 80, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, J.; Pokrant, E.; Araya, D.; Briceño, C.; Hidalgo, H.; Maddaleno, A.; Araya-Jordán, C.; San Martin, B. Residue depletion of oxytetracycline (OTC) and 4-epi-oxytetracycline (4-epi-OTC) in broiler chicken’s claws by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Food Addit. Contam. Part A 2017, 34, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, S.; Alley, M.; Hess, J.; Moran, E., Jr. Influence of strain-cross, sex and feeding programs on broiler chicken paw (feet) yield and quality. In Proceedings of the XVIIth European Symposium on the Quality of Poultry Meat, Doorwerth, The Netherlands, 23–26 May 2005; pp. 342–349. [Google Scholar]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Odarda, O.; Viola, H. Análisis del Comercio Agrícola de China en 2014; Consejería Agrícola (MAGyP), Embajada de Argentina en la República Popular China: Beijing, China, 2015; p. 29. [Google Scholar]
- General Consulate and Commercial Promotion Center of the Argentine Republic in Shanghai. El Mercado de Carne Aviar en China; General Consulate and Commercial Promotion Center of the Argentine Republic in Shanghai: Shanghai, China, 2016; p. 25. [Google Scholar]
- Chang, S.K.; Davis, J.L.; Cheng, C.N.; Shien, R.H.; Hsieh, M.K.; Koh, B.W.; Chou, C.C. Pharmacokinetics and tissue depletion of florfenicol in Leghorn and Taiwan Native chickens. J. Vet. Pharmacol. Ther. 2010, 33, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Martínez, M.A.; Martínez, M.; Ríos, A.; Caballero, V.; Ares, I.; Martínez-Larrañaga, M.R. Plasma and Tissue Depletion of Florfenicol and Florfenicol-amine in Chickens. J. Agric. Food Chem. 2008, 56, 11049–11056. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-K.; Lim, J.-H.; Kim, M.-S.; Hwang, Y.-H.; Yun, H.-I. Pharmacokinetics of florfenicol and its major metabolite, florfenicol amine, in rabbits. J. Vet. Pharmacol. Ther. 2007, 30, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Jia, L.; Yao, Y.; Xu, D.; Chen, S.; Xie, X.; Pei, Y.; Bao, W.; Dai, G.; Wang, J.; et al. Simultaneous determination of thiamphenicol, florfenicol and florfenicol amine in eggs by reversed-phase high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B 2011, 879, 2351–2354. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, D.; Wu, X.; Coats, J.R. Bioavailability and pharmacokinetics of florfenicol in broiler chickens. J. Vet. Pharmacol. Ther. 2003, 26, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Hamed, E.; Hassanin, O. Residue withdrawal of florfenicol from the serum and edible tissues of broiler chickens. J. Am. Sci. 2012, 8, 514–524. [Google Scholar]
- European Commission. Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Union 2010, 15, 1–72. [Google Scholar]
- Codex Committee on Residues of Veterinary Drugs in Foods. Maximum Residue Limits (MRLs) and Risk Management Recommendations (RMRs) for Residues of Veterinary Drugs in Foods; Codex Committee on Residues of Veterinary Drugs in Foods: Chicago, IL, USA, 2017; Volume CAC/MRL 2-2015, pp. 1–43. [Google Scholar]
- Codex Committee on Residues of Veterinary Drugs in Foods. Global Survey to Provide Information to the CCRVDF to Move Compounds from the Database on Countries’ Needs for MRLs to the JECFA Priority List and Database on Countries’ Needs for MRLs; Codex Committee on Residues of Veterinary Drugs in Foods: Chicago, IL, USA, 2016. [Google Scholar]
- Imran, M.; Habib, F.E.; Tawab, A.; Rauf, W.; Rahman, M.; Khan, Q.M.; Asi, M.R.; Iqbal, M. LC–MS/MS based method development for the analysis of florfenicol and its application to estimate relative distribution in various tissues of broiler chicken. J. Chromatogr. B 2017, 1063, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Habib, F.E.; Majeed, S.; Tawab, A.; Rauf, W.; Rahman, M.; Umer, M.; Iqbal, M. LC-MS/MS-based determination of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in poultry meat from the Punjab-Pakistan. Food Addit. Contam. Part A 2018, 35, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- CVMP. Guideline on Approach towards Harmonisation of Withdrawal Periods; European Medicines Agency, Committee for Veterinary Medicinal Products: London, UK, 2016; pp. 1–37. [Google Scholar]
- European Commission. 2002/657/EC: Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Union 2002, L221, 8–36. [Google Scholar]
- Food and Drug Administration. VICH GL48(R). Guidance for Industry—Studies to Evaluate the Metabolism and Residue Kinetics of Veterinary Drugs in Food-Producing Animals: Marker Residue Depletion Studies to Establish Product Withdrawal Periods; Food and Drug Administration: Rome, Italy, 2015; pp. 1–15. [Google Scholar]
- Odore, R.; De Marco, M.; Gasco, L.; Rotolo, L.; Meucci, V.; Palatucci, A.T.; Rubino, V.; Ruggiero, G.; Canello, S.; Guidetti, G.; et al. Cytotoxic effects of oxytetracycline residues in the bones of broiler chickens following therapeutic oral administration of a water formulation. Poult. Sci. 2015, 94, 1979–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornejo, J.; Pokrant, E.; Riquelme, R.; Briceño, C.; Maddaleno, A.; Araya-Jordán, C.; San Martin, B. Single-laboratory validation of an LC-MS/MS method for determining florfenicol (FF) and florfenicol amine (FFA) residues in chicken feathers and application to a residue-depletion study. Food Addit. Contam. Part A 2016, 34, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Martínez-Larrañaga, M. Residuos de Medicamentos de uso Veterinario In Toxicología Alimentaria; Díaz de Santos: Madrid, Spain, 2012; pp. 394–412. ISBN 978-84-9969-208-1. [Google Scholar]
- Tang, Y.; Fang, L.; Xu, C.; Zhang, Q. Antibiotic resistance trends and mechanisms in the foodborne pathogen, Campylobacter. Anim. Health Res. Rev. 2017, 18, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Congreso Nacional de la República de Chile. Ley N°20.380 Sobre la Protección de los Animales; Congreso Nacional de la República de Chile: Santiago, Chile, 2009; pp. 1–6. [Google Scholar]
- European Parliament and the Council of the European Union. Directive 2010/63/EU of 22 September 2010 of the European Parliament and of the Council on the protection of animals used for scientific purposes. Off. J. Eur. Union 2018, 276, 33–79. [Google Scholar]
- European Parliament and the Council of the European Union. Council Regulation (EC) No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Off. J. Eur. Union 2009, L303, 1–30. [Google Scholar]
- Zhang, S.; Liu, Z.; Guo, X.; Cheng, L.; Wang, Z.; Shen, J. Simultaneous determination and confirmation of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in chicken muscle by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2008, 875, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Hormazabal, V.; Steffenak, I.; Yndestad, M. Simultaneous determination of residues of florfenicol and the metabolite florfenicol amine in fish tissues by high-performance liquid chromatography. J. Chromatogr. B 1993, 616, 161–165. [Google Scholar] [CrossRef]
- Li, J.; Ding, S.; Zhang, S.; Li, C.; Li, X.; Liu, Z.; Liu, J.; Shen, J. Residue Depletion of Florfenicol and Its Metabolite Florfenicol Amine in Swine Tissues after Intramuscular Administration. J. Agric. Food Chem. 2006, 54, 9614–9619. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. VICH GL49(R). Guidance for Industry—Studies to Evaluate the Metabolism and Residue Kinetics of Veterinary Drugs in Food-Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies; Food and Drug Administration: Rome, Italy, 2015; pp. 1–23. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| Sampling Point | Days after Ceasing Treatment | Age of Birds (Days) | Average Concentration of FF + FFA in Claws Samples (ng·g−1) | Average Concentration of FF + FFA in Muscle Samples (ng·g−1) | Average Concentration of FF + FFA in Liver Samples (ng·g−1) |
|---|---|---|---|---|---|
| 1 | 5 | 15 | 651.9 | 68.9 | <LOD |
| 2 | 10 | 20 | 596.9 | <LOQ | <LOD |
| 3 | 20 | 30 | 161.0 | <LOD | <LOD |
| 4 | 25 | 35 | 97.9 | - | - |
| 5 | 30 | 40 | 102.4 | - | - |
| 6 | 35 | 45 | <LOD | - | - |
| 7 | 40 | 50 | <LOD | - | - |
| Analyte | Precursor Ion (Q1 Mass; Da) | Fragment Ion (Q3 Mass; Da) | Time (ms) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| FF1 | 356 | 336 | 100 | −50 | −5 | −15 | −8 |
| FF2 | 356 | 185 | 100 | −50 | −5 | −17 | −12 |
| FFA1 | 248 | 230 | 200 | 45 | 5 | 22 | 25 |
| FFA2 | 248 | 130 | 200 | 45 | 2 | 30 | 10 |
| CAF-d5 (IS) | 326 | 157 | 100 | 25 | 10 | −25 | −20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokrant, E.; Riquelme, R.; Maddaleno, A.; San Martín, B.; Cornejo, J. Residue Depletion of Florfenicol and Florfenicol Amine in Broiler Chicken Claws and a Comparison of Their Concentrations in Edible Tissues Using LC–MS/MS. Molecules 2018, 23, 2211. https://doi.org/10.3390/molecules23092211
Pokrant E, Riquelme R, Maddaleno A, San Martín B, Cornejo J. Residue Depletion of Florfenicol and Florfenicol Amine in Broiler Chicken Claws and a Comparison of Their Concentrations in Edible Tissues Using LC–MS/MS. Molecules. 2018; 23(9):2211. https://doi.org/10.3390/molecules23092211
Chicago/Turabian StylePokrant, Ekaterina, Ricardo Riquelme, Aldo Maddaleno, Betty San Martín, and Javiera Cornejo. 2018. "Residue Depletion of Florfenicol and Florfenicol Amine in Broiler Chicken Claws and a Comparison of Their Concentrations in Edible Tissues Using LC–MS/MS" Molecules 23, no. 9: 2211. https://doi.org/10.3390/molecules23092211