Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms
Abstract
:1. Introduction
2. Methods
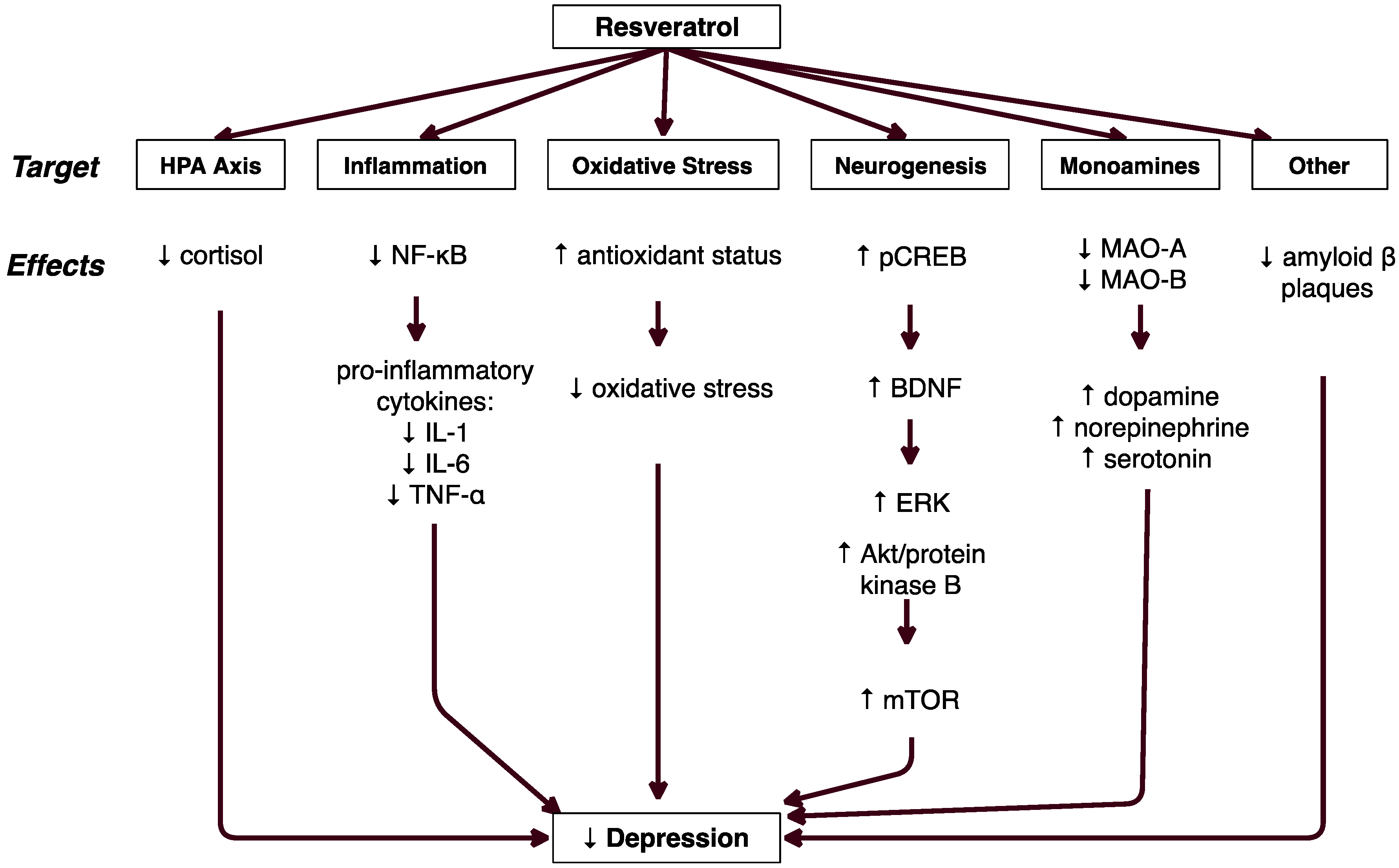
3. Results
3.1. Resveratrol′s Effect on Depressive Behaviors
3.2. HPA Axis Regulation
3.3. Decreased Inflammation
3.4. Decreased Oxidative Stress
3.5. Decreased Amyloid Beta Cytotoxicity
3.6. Increased Neurogenesis
3.6.1. Brain-Derived Neurotrophic Factor (BDNF)
3.6.2. cAMP Response Element Binding Protein
3.6.3. Extracellular Regulated Kinase Pathway
3.6.4. Neural Stem Cells
3.6.5. Mammalian Target of Rapamycin (mTOR) Pathway
3.6.6. Canonical Pathway of Wnt-β-Catenin
3.7. Serotonin, Norepinephrine, & Dopamine
4. Discussion
Author Contributions
Conflicts of Interest
References
- Depression Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed on 15 August 2018).
- Collins, P.Y.; Patel, V.; Joestl, S.S.; March, D.; Insel, T.R.; Daar, A.S.; Bordin, I.A.; Costello, E.J.; Durkin, M.; Fairburn, C.; et al. Grand challenges in global mental health. Nature 2011, 475, 27–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratt, L.A.; Brody, D.J.; Gu, Q. Antidepressant Use in Persons Aged 12 and over: United States, 2005–2008; US Department of Health and Human Services, Centers for Disease Control, NCHS Data Brief: Hyattsville, MD, USA, 2011; pp. 1–7.
- National Center for Health Statistics. Health, United States, 2010: With Special Feature on Death and Dying; U.S. Government Printing Office: Washington, DC, USA, 2011; p. 107. Available online: https://www.cdc.gov/nchs/data/hus/hus10.pdf#074 (accessed on 15 August 2018).
- Liu, L.; Zhang, Q.; Cai, Y.; Sun, D.; He, X.; Wang, L.; Yu, D.; Li, X.; Xiong, X.; Xu, H.; et al. Resveratrol counteracts lipopolysaccharide-induced depressive-like behaviors via enhanced hippocampal neurogenesis. Oncotarget 2016, 7, 56045–56059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurley, L.L.; Akinfiresoye, L.; Kalejaiye, O.; Tizabi, Y. Antidepressant effects of resveratrol in an animal model of depression. Behav. Brain Res. 2014, 268, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wang, Z.; You, W.; Zhang, X.; Li, S.; Barish, P.A.; Vernon, M.M.; Du, X.; Li, G.; Pan, J.; et al. Antidepressant-like effect of trans-resveratrol: Involvement of serotonin and noradrenaline system. Eur. Neuropsychopharm. 2010, 20, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.M. Ssri antidepressant medications: Adverse effects and tolerability. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr. Neurosci. 2017, 20, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Quincozes-Santos, A.; Bobermin, L.D.; Latini, A.; Wajner, M.; Souza, D.O.; Goncalves, C.A.; Gottfried, C. Resveratrol protects c6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS ONE 2013, 8, e64372. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Bath, S.; Elbarbry, F. An organ system approach to explore the antioxidative, anti-inflammatory, and cytoprotective actions of resveratrol. Oxid. Med. Cell. Longev. 2015, 2015, 803971. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.D.; Geetha, T.; Griffin, G.D.; Broderick, T.L.; Babu, J.R. Neuroprotective effects of resveratrol in alzheimer disease pathology. Front. Aging Neurosci. 2014, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.F.; Xu, Y.Y.; Qin, G.; Cheng, J.Q.; Chen, F.H. Resveratrol ameliorates the anxiety- and depression-like behavior of subclinical hypothyroidism rat: Possible involvement of the hpt axis, hpa axis, and wnt/beta-catenin pathway. Front. Endocrinol. (Lausanne) 2016, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.F.; Abdel-Rahman, R.F.; Farid, O.A.H.A.; El-Marasy, S.A.; Hessin, A.F. Combined hepatoprotective and antidepressant effects of resveratrol in an acute model of depression. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 191–197. [Google Scholar] [CrossRef]
- Ali, S.H.; Madhana, R.M.; Athira, K.V.; Kasala, E.R.; Bodduluru, L.N.; Pitta, S.; Mahareddy, J.R.; Lahkar, M. Resveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in mice. Steroids 2015, 101, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Du, J.K.; Hu, X.; Yu, Q.; Li, D.X.; Wang, C.N.; Zhu, X.Y.; Liu, Y.J. Protective effects of resveratrol on mitochondrial function in the hippocampus improves inflammation-induced depressive-like behavior. Physiol. Behav. 2017, 182, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Finnell, J.E.; Lombard, C.M.; Melson, M.N.; Singh, N.P.; Nagarkatti, M.; Nagarkatti, P.; Fadel, J.R.; Wood, C.S.; Wood, S.K. The protective effects of resveratrol on social stress-induced cytokine release and depressive-like behavior. Brain Behav. Immun. 2017, 59, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, J.F.; Peng, L.; Cheng, J.Q.; Pan, C.X.; Tang, J.; Chen, F.H.; Li, J. Antidepressant-like effect of resveratrol: Involvement of antioxidant effect and peripheral regulation on hpa axis. Pharmacol. Biochem. Behav. 2013, 114–115, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Liu, L.; Liu, H.; Liu, S.; Xue, H.; Wang, X.; Yuan, L.; Wang, Z.; Liu, D. Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and creb/bdnf signaling in mice. Eur. J. Pharmacol. 2015, 768, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, Z.; Wang, Q.; Lin, M.; Wu, S.; Yan, Q.; Wu, F.; Yu, X.; Xie, X.; Li, G.; et al. Piperine potentiates the antidepressant-like effect of trans-resveratrol: Involvement of monoaminergic system. Metab. Brain Dis. 2013, 28, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.; Parihar, V.K.; Hattiangady, B.; Mishra, V.; Shuai, B.; Shetty, A.K. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature, and reduced glial activation. Sci. Rep. 2015, 5, 8075. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Liu, Y.-M.; Shen, J.-D.; Chen, J.-J.; Pei, Y.-Y.; Fang, X.-Y. Resveratrol ameliorates the depressive-like behaviors and metabolic abnormalities induced by chronic corticosterone injection. Molecules 2016, 21, 1341. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xie, K.; Yang, X.; Gu, J.; Ge, L.; Wang, X.; Wang, Z. Resveratrol reverses the effects of chronic unpredictable mild stress on behavior, serum corticosterone levels and bdnf expression in rats. Behav. Brain Res. 2014, 264, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Q.; Gu, J.; Wang, X.; Xie, K.; Xian, X.; Wang, J.; Jiang, H.; Wang, Z. Resveratrol prevents impaired cognition induced by chronic unpredictable mild stress in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 49, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, T.; Liu, H.; Wang, X.; Bo, S.; Xie, Y.; Bai, X.; Wu, L.; Wang, Z.; Liu, D. Resveratrol exerts antidepressant properties in the chronic unpredictable mild stress model through the regulation of oxidative stress and mtor pathway in the rat hippocampus and prefrontal cortex. Behav. Brain Res. 2016, 302, 191–199. [Google Scholar] [CrossRef] [PubMed]
- López, M.C.; Fontenla, J.A.; Uriarte, E.; Santana, L.; Sobarzo-Sánchez, E. Comparison of the antidepressive effects of trans-resveratrol and 5-methoxy-7h-dibenzo[de,h]quinolin-7-one. Curr. Top. Med. Chem. 2014, 14, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Cao, L.; Wu, F.; Wang, L.; Wang, G.; Yu, Y.; Zhang, M.; Chen, L.; Wang, W.; Lv, W.; et al. The effect of trans-resveratrol on post-stroke depression via regulation of hypothalamus-pituitary-adrenal axis. Neuropharmacology 2015, 97, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Sakr, H.F.; Abbas, A.M.; Elsamanoudy, A.Z.; Ghoneim, F.M. Effect of fluoxetine and resveratrol on testicular functions and oxidative stress in a rat model of chronic mild stress-induced depression. J. Physiol. Pharmacol. 2015, 66, 515–527. [Google Scholar] [PubMed]
- Wang, Z.; Gu, J.; Wang, X.; Xie, K.; Luan, Q.; Wan, N.; Zhang, Q.; Jiang, H.; Liu, D. Antidepressant-like activity of resveratrol treatment in the forced swim test and tail suspension test in mice: The hpa axis, bdnf expression and phosphorylation of erk. Pharmacol. Biochem. Behav. 2013, 112, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, Y.; Zhang, T.; Bo, S.; Bai, X.; Liu, H.; Li, T.; Liu, S.; Zhou, Y.; Cong, X.; et al. Resveratrol reverses chronic restraint stress-induced depression-like behaviour: Involvement of bdnf level, erk phosphorylation and expression of bcl-2 and bax in rats. Brain Res. Bull. 2016, 125, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.; An, J.; Yuan, G.; Hao, X.; Zhang, Y. Resveratrol ameliorates depressive disorder through the netrin1-mediated extracellular signal-regulated kinase/camp signal transduction pathway. Mol. Med. Rep. 2018, 17, 4611–4618. [Google Scholar] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl.) 1985, 85, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Guven, N.; Dietis, N. Stress-based animal models of depression: Do we actually know what we are doing? Brain Res. 2016, 1652, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Pang, T.Y. Is dysregulation of the hpa-axis a core pathophysiology mediating co-morbid depression in neurodegenerative diseases? Front. Psychiatry 2015, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.; Mocking, R.J.; Ruhe, H.G.; Visser, I.; Koeter, M.W.; Assies, J.; Bockting, C.L.; Olff, M.; Schene, A.H. Longitudinal hypothalamic-pituitary-adrenal axis trait and state effects in recurrent depression. Psychoneuroendocrinology 2012, 37, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Vythilingam, M.; Vermetten, E.; Anderson, G.M.; Luckenbaugh, D.; Anderson, E.R.; Snow, J.; Staib, L.H.; Charney, D.S.; Bremner, J.D. Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biol. Psychiatry 2004, 56, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, N.; Daiber, A.; Forstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Colaianna, M.; Tucci, P.; Zotti, M.; Morgese, M.G.; Schiavone, S.; Govoni, S.; Cuomo, V.; Trabace, L. Soluble beta amyloid(1-42): A critical player in producing behavioural and biochemical changes evoking depressive-related state? Br. J. Pharmacol. 2010, 159, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X.P.; Yang, S.G.; Wang, Y.J.; Zhang, X.; Du, X.T.; Sun, X.X.; Zhao, M.; Huang, L.; Liu, R.T. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 2009, 30, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Zheng, W.H.; Bastianetto, S.; Chabot, J.G.; Quirion, R. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: Involvement of protein kinase c. Br. J. Pharmacol. 2004, 141, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Jang, J. Protective effect of resveratrol on β-amyloid-induced oxidative pc12 cell death. Free Radic. Biol. Med. 2003, 34, 1100–1110. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y. Involvement of brain-derived neurotrophic factor in late-life depression. Am. J. Geriatr. Psychiatry 2013, 21, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mrnas in the hippocampus. J. Neurosci. 1995, 15, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.J.; De Kloet, E.R.; Vreugdenhil, E. Corticosterone effects on bdnf expression in the hippocampus. Implications for memory formation. Stress 2000, 3, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.; Rizavi, H.S.; Pandey, G.N. Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: Differential regulation of specific exons by antidepressants and corticosterone. Neuroscience 2006, 139, 1017–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, W.; Wang, W.; Gong, T.; Zhang, H.; Tao, W.; Xue, L.; Sun, Y.; Wang, F.; Chen, G. Pka-creb-bdnf signaling regulated long lasting antidepressant activities of yueju but not ketamine. Sci. Rep. 2016, 6, 26331. [Google Scholar] [CrossRef] [PubMed]
- Nibuya, M.; Nestler, E.J.; Duman, R.S. Chronic antidepressant administration increases the expression of camp response element binding protein (creb) in rat hippocampus. J. Neurosci. 1996, 16, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Pinnock, S.B.; Blake, A.M.; Platt, N.J.; Herbert, J. The roles of bdnf, pcreb and wnt3a in the latent period preceding activation of progenitor cell mitosis in the adult dentate gyrus by fluoxetine. PLoS ONE 2010, 5, e13652. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.C.; Cryan, J.F.; Dalvi, A.; Lucki, I.; Blendy, J.A. Camp response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J. Neurosci. 2002, 22, 3262–3268. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.; Zhang, H. Altered erk1/2 signaling in the brain of learned helpless rats: Relevance in vulnerability to developing stress-induced depression. Neural Plast. 2016, 2016, 7383724. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.; Rizavi, H.S.; Roberts, R.C.; Conley, R.C.; Tamminga, C.A.; Pandey, G.N. Reduced activation and expression of erk1/2 map kinase in the post-mortem brain of depressed suicide subjects. J. Neurochem. 2001, 77, 916–928. [Google Scholar] [CrossRef] [PubMed]
- First, M.; Gil-Ad, I.; Taler, M.; Tarasenko, I.; Novak, N.; Weizman, A. The effects of fluoxetine treatment in a chronic mild stress rat model on depression-related behavior, brain neurotrophins and erk expression. J. Mol. Neurosci. 2011, 45, 246–255. [Google Scholar] [CrossRef] [PubMed]
- First, M.; Gil-Ad, I.; Taler, M.; Tarasenko, I.; Novak, N.; Weizman, A. The effects of reboxetine treatment on depression-like behavior, brain neurotrophins, and erk expression in rats exposed to chronic mild stress. J. Mol. Neurosci. 2013, 50, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Abelaira, H.M.; Reus, G.Z.; Neotti, M.V.; Quevedo, J. The role of mtor in depression and antidepressant responses. Life Sci. 2014, 101, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Chandran, A.; Iyo, A.H.; Jernigan, C.S.; Legutko, B.; Austin, M.C.; Karolewicz, B. Reduced phosphorylation of the mtor signaling pathway components in the amygdala of rats exposed to chronic stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Lie, D.C.; Colamarino, S.A.; Song, H.J.; Desire, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, S.M.; Choi, C.I.; Cho, C.H.; Kim, H.J.; Jun, H.; Jang, M.H. Wnt signaling in neuropsychiatric disorders: Ties with adult hippocampal neurogenesis and behavior. Neurosci. Biobehav. Rev. 2014, 47, 369–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okerlund, N.D.; Cheyette, B.N. Synaptic wnt signaling-a contributor to major psychiatric disorders? J. Neurodev. Disord. 2011, 3, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.J.; Moller, M.; Harvey, B.H. A review of biomarkers in mood and psychotic disorders: A dissection of clinical vs. Preclinical correlates. Curr. Neuropharmacol. 2015, 13, 324–368. [Google Scholar] [CrossRef] [PubMed]
- Berton, O.; Nestler, E.J. New approaches to antidepressant drug discovery: Beyond monoamines. Nat. Rev. Neurosci. 2006, 7, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Tye, K.M.; Mirzabekov, J.J.; Warden, M.R.; Ferenczi, E.A.; Tsai, H.C.; Finkelstein, J.; Kim, S.Y.; Adhikari, A.; Thompson, K.R.; Andalman, A.S.; et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013, 493, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Ramis, M.R.; Aparicio, S.; Ruiz, L.; Esteban, S.; Miralles, A.; Moranta, D. Improving effect of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. Age (Dordr.) 2015, 37, 9777. [Google Scholar] [CrossRef] [PubMed]
- Gartside, S.E.; Leitch, M.M.; McQuade, R.; Swarbrick, D.J. Flattening the glucocorticoid rhythm causes changes in hippocampal expression of messenger rnas coding structural and functional proteins: Implications for aging and depression. Neuropsychopharmacology 2003, 28, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Jarcho, M.R.; Slavich, G.M.; Tylova-Stein, H.; Wolkowitz, O.M.; Burke, H.M. Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol. Psychol. 2013, 93, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.J.; Liu, M.Y.; Li, H.; Liu, X.; Chen, C.; Han, Z.; Wu, H.Y.; Jing, X.; Zhou, H.H.; Suh, H.; et al. The different roles of glucocorticoids in the hippocampus and hypothalamus in chronic stress-induced hpa axis hyperactivity. PLoS ONE 2014, 9, e97689. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.S.; Koakoski, G.; Ferreira, D.; Oliveira, T.A.; Rosa, J.G.; Gusso, D.; Giacomini, A.C.; Piato, A.L.; Barcellos, L.J. Diazepam and fluoxetine decrease the stress response in zebrafish. PLoS ONE 2014, 9, e103232. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Kim, Y.K. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World J. Psychiatry 2016, 6, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.O.; del Rey, A. The cytokine-hpa axis feed-back circuit. Z. Rheumatol. 2000, 59 (Suppl. 2), II/26–II/30. [Google Scholar] [CrossRef]
- Busch, F.; Mobasheri, A.; Shayan, P.; Lueders, C.; Stahlmann, R.; Shakibaei, M. Resveratrol modulates interleukin-1beta-induced phosphatidylinositol 3-kinase and nuclear factor kappab signaling pathways in human tenocytes. J. Biol. Chem. 2012, 287, 38050–38063. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Samuel, L.J.; Miller, E.R.; Szanton, S.L. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom. Med. 2014, 76, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Shelton, R.C.; Claiborne, J.; Sidoryk-Wegrzynowicz, M.; Reddy, R.; Aschner, M.; Lewis, D.A.; Mirnics, K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry 2011, 16, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledo, J.H.; Azevedo, E.P.; Beckman, D.; Ribeiro, F.C.; Santos, L.E.; Razolli, D.S.; Kincheski, G.C.; Melo, H.M.; Bellio, M.; Teixeira, A.L.; et al. Cross talk between brain innate immunity and serotonin signaling underlies depressive-like behavior induced by alzheimer’s amyloid-beta oligomers in mice. J. Neurosci. 2016, 36, 12106–12116. [Google Scholar] [CrossRef] [PubMed]
- Ledo, J.H.; Azevedo, E.P.; Clarke, J.R.; Ribeiro, F.C.; Figueiredo, C.P.; Foguel, D.; De Felice, F.G.; Ferreira, S.T. Amyloid-beta oligomers link depressive-like behavior and cognitive deficits in mice. Mol. Psychiatry 2013, 18, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Swardfager, W.; Lanctot, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A meta-analysis of cytokines in alzheimer’s disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Morgese, M.G.; Tucci, P.; Colaianna, M.; Zotti, M.; Cuomo, V.; Schiavone, S.; Trabace, L. Modulatory activity of soluble beta amyloid on hpa axis function in rats. Curr. Pharm. Des. 2014, 20, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol. Med. 2004, 5, 11–25. [Google Scholar] [CrossRef]
- Park, H.R.; Kong, K.H.; Yu, B.P.; Mattson, M.P.; Lee, J. Resveratrol inhibits the proliferation of neural progenitor cells and hippocampal neurogenesis. J. Biol. Chem. 2012, 287, 42588–42600. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; Cameron, H.A. Adult neurogenesis and mental illness. Neuropsychopharmacology 2015, 40, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Neto, F.L.; Borges, G.; Torres-Sanchez, S.; Mico, J.A.; Berrocoso, E. Neurotrophins role in depression neurobiology: A review of basic and clinical evidence. Curr. Neuropharmacol. 2011, 9, 530–552. [Google Scholar] [CrossRef] [PubMed]
- Young, C.B.; Chen, T.; Nusslock, R.; Keller, J.; Schatzberg, A.F.; Menon, V. Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl. Psychiatry 2016, 6, e810. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Dalla, C.; Pitychoutis, P.M.; Kokras, N.; Papadopoulou-Daifoti, Z. Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 2010, 106, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Gea, A.; Beunza, J.J.; Estruch, R.; Sanchez-Villegas, A.; Salas-Salvado, J.; Buil-Cosiales, P.; Gomez-Gracia, E.; Covas, M.I.; Corella, D.; Fiol, M.; et al. Alcohol intake, wine consumption and the development of depression: The predimed study. BMC Med. 2013, 11, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Howe, P.R.; Wong, R.H. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Pennisi, M.; Bertino, G.; Motta, M.; Borzi, A.M.; Vicari, E.; Bella, R.; Drago, F.; Malaguarnera, M. Resveratrol in patients with minimal hepatic encephalopathy. Nutrients 2018, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Floel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Animal Model | Intervention | Dosage/Route | Behavioral Outcomes w/RSV Txt | Clinical Outcomes w/RSV Txt |
|---|---|---|---|---|---|
| Ahmed and colleagues, 2014 [17] | reserpine-injected adult male Wistar rats | vehicle (saline + DMSO) reserpine reserpine + reserpine + RSV reserpine + resperpine + FLX × 3 days | RSV: 15, 30, 60 FLX: 24 | ↑ ambulation in OFT a,a (30/60) ↓ latency in OOFT a,a,a (15/30/60) FST ↓ immobility time in FST a,a,a (15/30/60) | ↑ NT levels: brain 5-HT a,a,a (15/30/60), dopamine a,a,a (15/30/60), and Norepinephrine a (60) |
| Ali and colleagues, 2015 [18] | CORT-injected male Swiss albino mice | CORT (con) CORT + RSV CORT + FLX Vehicle (con) 21 days | CORT: 40, s.c. RSV: 80, oral FLX: 15, oral Vehicle, 0, oral | ↑ sucrose preference c ↓ immobility time in FST b ↓ immobility time in TST a | ↓ serum CORT b ↑ hippocampal BDNF a |
| Chen and colleagues, 2017 [19] | LPS-injected male ICR mice | vehicle (saline) LPS LPS + RSV tests after 24 h | RSV: 0.3, i.c.v. | ↑ sucrose preference a ↓ immobility time in FST a | ↓ hippocampal superoxide a ↓ hippocampal apoptosis a ↑ hippocampal ATP production a ↑ hippocampal mitochondrial membrane potential a |
| Finnel and colleagues, 2017 [20] | social stress-exposed male Sprague-Dawley rats & Long-Evans retired breeders | RSV + social stress Vehicle + social stress 5 days social stress, Txt 7 days pre-social stress & during | RSV: 10, 30, i.p. Vehicle: 0, i.p. | ↑ sucrose preference c (30) | ↓ TNF-α c (10/30) ↓ IL-1β a,b (10/30) ↓ IL-6 c (10/30) ↓ IL-2 c (30)↓ IL-4 c (30) |
| Ge and colleagues, 2013 [21] | CUMS-exposed male Sprague-Dawley rats | CUMS + Vehicle CUMS + RSV CUMS + FLX CUMS 21 days, Txt days 14–21 | RSV: 15, i.p. FLX: 2, i.p. | ↑ sucrose preference a ↓ immobility time in FST c ↓ immobility time in TST c | ↓ serum CORT c ↓ serum MDA c ~ serum CRH d |
| Ge and colleagues, 2015 [22] | LPS-injected adult male Kunming mice | Vehicle (saline) Vehicle + LPS RSV + Vehicle RSV + LPS | RSV: 80, i.p. LPS: 0.83, i.p. | ↓ immobility time in FST b ↑ swimming time in FST b ↓ immobility time in TST a ↑ sucrose preference a ~ locomotor activity d | ↓ hippocampal & PFC IL-1β a ↓ hippocampal TNF-α a ↓ PFC TNF-α b ↓ hippocampal & PFC pNF-κB p65 a ↑ PFC pCREB a ↑ hippocampal BDNF a |
| Ge and colleagues, 2016 [16] | SCH male Sprague-Dawley rats | SCH + Vehicle SCH + RSV SCH + LT4 Txt post SCH for 16 days | RSV: 15, i.g. LT4: 60 *, i.g. | ↑ sucrose preference b ↑ locomotor activity a ↓ immobility time in FST b ↓ immobility time in TST b | ↓ adrenal gland wt to body wt ratio b ↓ plasma CORT b ↓ CRH mRNA expresión b ↓ GSK-3β b ↑ pGSK-3β b ↑ pGSK-3β/ GSK-3β ratio b ↑ β-catenin b ↓ p β-catenin b ↓ p β-catenin/ β-catenin ratio b ↑ cyclin D1 & c-myc b ↓ TSH a ↓ TRH mRNA expression b |
| Huang and colleagues, 2013 [23] | male ICR mice | RSV FLX RSV + piperine | RSV: 1.25, 2.5, 10, 20, 40, 160, oral FLX: 10 i.p. piperine: 2.5 i.p. | ↓ immobility time in FST a,a,a,a(2.5/10/40/160) ↓ immobility time in TST a,a,a,a(2.5/10/40/160) ~ locomotor activityd ↓ reserpine-induced hypothermia a,a,a,a,a(1.25/2.5/5/10/20) and ptosis a (20) | ↑ 5-HT a,b (10/20), norepinephrine a (20), dopamine a (20) in FC ↓ 5-HIAA/5-HT in FC b (20) ↓ MAO-A in FC a,b,b (5/10/20) and hippocampus a,b (10/20) ↓ MAO-B in FC a (20) |
| Hurley and colleagues, 2014 [6] | Adult male Wistar kyoto rats | RSV v Vehicle Txt 20 min post-injection (acute) & 18–20 h post-injection (chronic) × 7 days | RSV: 0 (saline), 10, 40, i.p. | ↓ immobility time in FST a,c (acute, 10/40) ↓ immobility time in FST a,c (chronic, 10/40) ~ sucrose preference d (acute) ↑ sucrose preference a,c (chronic, 10/40) ~ locomotor activity d (acute/chronic, 10/40) | ↑ hippocampal BDNF b (10/40) ~PFC BDNF d (acute/chronic, 10/40) |
| Kodali and colleagues, 2015 [24] | Late/middle-age male Fischer 344 rats | RSV v Vehicle 4 weeks txt, 4 weeks waiting period, behavioral tests | RSV: 40, i.p. | ↓ immobility time in FST a | ↑ BrdU+ cells a ↑ hippocampal neurogenesis b ↑ DCX newly born neurons c ↑ hippocampal microvasculature b and CA1 subfield microvasculature a ↓ astrocyte hypertrophy c ↑ hippocampal resting microglia a |
| Li and colleagues, 2016 [25] | CORT-injected male ICR mice | CORT RSV FLX Pioglitazone × 3 weeks | CORT: 40 s.c. RSV: 50, 100, oral FLX: 20, oral pioglitazone: 10, oral | ↑ sucrose preference b,c (50/100) ↓ immobility time in FST b,c (50/100) ~ locomotor activity d | ↓ serum CORT b,b (50/100) |
| Liu and colleagues, 2014 [26] | CUMS-exposed male Wistar rats | Vehicle RSV (80) DES (10) CUMS + vehicle CUMS + RSV (20, 40, 80) CUMS + DES × 5 weeks | RSV: 20, 40, 80, i.p. DES: 10, i.p. Vehicle: 1% ethanol, i.p. | ↑ sucrose preference a,a,b (20/40/80) ↓ immobility time in FST a,a (40/80) ↑ locomotor activity a (80) | ↓ serum CORT a (80) ↑ hippocampal BDNF b (80) ↑ amygdala BDNF b,b (40/80) ↑ hippocampal and amygdala pCREB/CREB ratio b,a (80) ↑ hippocampal pERK a,b (40/80) ↑ amygdala pERK a,a (40/80) |
| iu and colleagues, 2014 [27] | CUMS-exposed male Wistar rats | Vehicle RSV CUMS + vehicle CUMS + RSV × 5 weeks | vehicle (1% ethanol) RSV: 80 i.p. | ↓ escape latency in Morris water maze b ↑ exploration time in novel object recognition test a | ↓ serum CORT a ↑ PFC BDNF a ↑ hippocampal BDNF a ↓ hippocampal and PFC p CREB/CREB ratio a ↑ p ERK/ERK ratio a |
| Liu and colleagues, 2016 [5] | LPS-injected adult male C57/BL6 mice | Saline + DMSO Saline + RSV LPS + DMSO LPS + RSV × 14 days | RSV: 20, i.p. LPS: 1, i.p. | ↓ immobility time in FST b ↓ immobility time in TST a | ↓ microglia w/ activated morphologies ↓ Ib-A1 immunoreactivity b ↑ BrdU+ cells b ↑ DCX+ neurons b ↑ type-1 RGL cells a ↑ symmetric division of RGL cells b ↓ hippocampal NF-κB expression b |
| Liu and colleagues, 2016 [28] | CUMS-exposed male Wistar rats | Vehicle RSV CUMS + vehicle CUMS + RSV CUMS + ketamine × 4 weeks | RSV: 80, i.p. Ketamine: 20, i.p. Vehicle: 1% ethanol, i.p. | ↑ sucrose preference a ↓ immobility time in FST b ↑ locomotor activity a | ↓ MDA in hippocampus & PFC c ↑ SOD in hippocampus c & PFC b ↑ phosphorylated mTOR in hippocampus a & PFC a ↑ p-Akt in hippocampus a & PFC a |
| López and colleagues, 2014 [29] | male CD1 mice | vehicle (saline) DMSO 1% DMSO 10% RSV OXO 4 buproprion citalopram DES imipramine moclobemide nisoxetine nomifensine | RSV: 2.5, 5, 10, i.p. OXO 4: 1, i.p. buproprion: 10 i.p. citalopram: 20 i.p. DES: 35 i.p. imipramine: 35 i.p. moclobemide: 35 i.p. nisoxetine: 2.5 i.p. nomifensine: 2.5 i.p. | ↓ immobility time in FST a(10) | N/A |
| Pang and colleagues, 2015 [30] | middle cerebral artery occluded male Sprague-Dawley rats | sham/vehicle MCAO MCAO + RES MCAO + imipramine × 7 days pre-surgery; tested either day 8 or days 20–21 | RSV: 10, 20, 40, oral imipramine: 10 i.p. | ↑ sucrose preference a,b (20/40) ↓ immobility time in FST a,c (20/40) ~ locomotor activity d | ↑ hippocampal BDNF a ↑ hippocampal β-actin a ↓ adrenal gland index b (40) ↓ CRF expression in FC, hippocampus, and hypothalamus b,c (20/40) ↓ glucocorticoid receptor expression in FC b,c (20/40), hippocampus b,c (20/40), and hypothalamus a (40) ↑ BDNF expression in FC a,a (20/40), hippocampus b,b (20/40), and hypothalamus b,b (20/40) |
| Sakr and colleagues, 2015 [31] | CUMS- exposed male Sprague-Dawley rats | CUMS + watercontrol CUMS + FLX water CUMS + RSV CUMS + FLX/RSV × 4 weeks ctrl + water ctrl + FLX ctrl + RSV ctrl + FLX/RSV | FLX: 10, oral RSV: 20, oral | ↑ sucrose preference a ↑ immobility time in FST a | ↓ serum CORT a ↓ 5-HT in cerebral cortex and hippocampus a ↑ serum testosterone a ↑ testicular SOD a ↑ testicular CAT a ↑ testicular GSH a ↓ testicular MDA a |
| Wang and colleagues, 2013 [32] | male Kunming mice | Vehicle RSV FLX × 21 days; tests followed | RSV: 20, 40, 80, i.p. FLX: 10, i.p. Vehicle: 1% ethanol, i.p., 0.9% NaCl, i.p. | ↓ immobility time in FST a,b,c (20/40/80) ↓ immobility time in TST a,c,c (20/40/80) ~ locomotor activity d | ↓ serum CORT b(80) ↑ PFC BDNF c,c,c (20/40/80) ↑ hippocampal BDNF c,c (40/80) ↑ pERK 1/2 in PFC a,a,c & hippocampus a,b,c (20/40/80) |
| Wang and colleagues, 2016 [33] | CRS-exposed male Wistar rats | RSV FLX CRS exposed 30 min after injection × 21 days | RSV: 80, i.p. FLX: 10, i.p. | ↑ sucrose preference a ↓ immobility time in FST a ~ locomotor activity d | ↑ hippocampal BDNF a ↑ PFC BDNF a ↑ BDNF/GFAP immunoreaction in hippocampus c ↑ pERK/ERK ratio in hippocampus b and PFC b ↑ bcl-2 hippocampal & PFC mRNA a ↓ hippocampal BAX mRNA b |
| Wang and colleagues, 2018 [34] | ouabain- exposed female J20 mice | ouabain + PBS (control) ouabain + RSV × 10 weeks | RSV: 10, oral | ↑ distance moved a ↑ path efficiency a ↓ time to recognize novel object a ↓ Rankin score a | ↓ plasma IL-1β, IL-17A, IL-8 and TNF-α a ↓ serum H3R a ↓ hippocampal plasma IL-1β, IL-17A, IL-8 and TNF-α a ↓ hippocampal CAT, SOD, GSH and NEG a ↓ hippocampal COX-2 expression a ↓ hippocampal neuron apoptosis a ↑ hippocampal neuron P53 and Bcl-2 a ↑ hippocampal neuron NETRIN1 and NRG3 a ↓ hippocampal neuron cAMP a |
| Xu and colleagues, 2010 [7] | male ICR mice | RSV Moclobemide (MAOI) Imipramine (TCA) Fluoxetine (SSRI) Treated w/PCPA or vehicle prior to FST & TST; treated w/apomorphine or vehicle after RSV Txt; behavioral tests 30 min post-RSV | RSV: 20, 40, 80, i.g. Moclobemide: 20, i.p. Imipramine: 20, i.p. Fluoxetine: 10, i.p. PCPA: 300, i.p. Apomorphine: 16, s.c. Vehicle | ↓ immobility time in FST a,b,c (20/40/80) ↓ immobility time in TST a,a,a (20/40/80) ~ locomotor activity d | ↑ 5-HT b (80), norepinephrine a (80) & dopamine a (80) in FC ↓ 5-HIAA/5-HT ratio in FC b (80) ↑ hippocampal 5-HT a,b (40/80) & norepinephrine a (80) ↓ hippocampal 5-HIAA/5-HT ratio a (80) ↓ MAO-A activity a,b,c (20,40,80) ↓ MAO-B activity b (80) |
| Author, Year | Animal Model | Txt Duration | Dosages (mg/kg/Day) | Comparative Effectiveness of RSV |
|---|---|---|---|---|
| Ahmed and colleagues, 2014 [17] | reserpine-injected adult male Wistar rats | 3 day | RSV: 15, 30, 60, oral FLX: 24, oral | >FLX: Liver GSH (60), liver MDA (30/60) =FLX: FST (60), 5-HT (15/30/60), norepinephrine (60), dopamine (15/30/60), brain MDA (60) < FLX: OFT |
| Ali and colleagues, 2015 [18] | CORT-injected male Swiss albino mice | 21 days | RSV: 80, oral FLX: 15, oral | =FLX: Sucrose preference, immobility time in FST, immobility time in TST, serum CORT, hippocampal BDNF |
| Ge and colleagues, 2013 [21] | CUMS-exposed male Sprague-Dawley rats | 21 days | RSV: 15, i.p. FLX: 2, i.p. | =FLX: Sucrose preference, immobility time in FST, immobility time in TST, serum MDA, serum CORT <FLX: CRH mRNA expression |
| Huang and colleagues, 2013 [23] | male ICR mice | 4 days | RSV: 1.25, 2.5, 10, 20, 40, 80, oral + piperine: 2.5 i.p. FLX: 10 i.p. Imipramine: 10 i.p. | =FLX: Immobility time in FST (10/20), immobility time in TST (10/20) =Imipramine: locomotor activity; reserpine-induced hypothermia (10,20) and ptosis (20); FC 5-HT (10,20), norepinephrine (20), dopamine (20), and 5-HIAA/5-HT ratio (20) |
| Li and colleagues, 2016 [25] | CORT-injected male ICR mice | 21 days | RSV: 50,100, oral FLX: 20, oral | =FLX: Sucrose preference (50/100), immobility time in FST (50/100), CORT (50/100) |
| Liu and colleagues, 2014 [26] | CUMS-exposed male Wistar rats | 5 weeks | RSV: 20, 40, 80, i.p. DES: 10, i.p. | =sucrose preference (20/40/80), immobility time in FST (40/80), crossing and grooming in OFT (80), serum CORT (80) BDNF in hippocampus (80) and amygdala (40/80), p-CREB in hippocampus (80) and amygdala (80), p-ERK in hippocampus (40/80) and amygdala (40/80) |
| Liu and colleagues, 2016 [28] | CUMS-exposed male Wistar rats | 4 weeks | RSV: 80, i.p. Ketamine: 20, i.p | =Ketamine: Sucrose preference; immobility time in FST; OFT; PFC and hippocampal MDA and SOD; phosphoylated mTOR and Akt |
| López and colleagues, 2014 [29] | male CD1 mice | <1 day | RSV: 2.5, 5, 10, i.p. buproprion: 10 i.p. citalopram: 20 i.p. desipramine: 35 i.p. imipramine: 35 i.p. moclobemide: 35 i.p. nisoxetine: 2.5 i.p. nomifensine: 2.5 i.p. | ↓ immobility time in FST (10) |
| Pang and colleagues, 2015 [30] | middle cerebral artery occluded male Sprague-Dawley rats | 14 days | RSV: 10, 20, 40, oral Imipramine: 10 i.p. | =Imipramine: Sucrose preference (20/40); FST (20/40); CRF expression in hypothalamus (20/40), hippocampus (20/40) and FC (20/40); GR expression in hypothalamus (40), hippocampus (20/40), and FC (20/40); BDNF expression in hypothalamus (40), hippocampus (20/40) and FC (40) <Imipramine: adrenal gland index |
| Sakr and colleagues, 2015 [31] | CUMS-exposed male Sprague-Dawley rats | 28 days | RSV: 20, oral FLX: 10, oral | >FLX: MDA, SOD, CAT, GSH <FLX: Sucrose preference, immobility time in FST, serum testosterone, serum CORT, hippocampal 5-HT |
| Wang and colleagues, 2013 [32] | male Kunming mice | 21 days | RSV: 20, 40, 80, i.p. FLX: 10, i.p. | =FLX: Immobility time in FST (20/40/80), immobility time in TST (20/40/80), serum CORT (80), BDNF mRNA in hippocampus (40/80) and PFC (20/40/80), BDNF protein expression in hippocampus (20/40/80) and PFC (20/40/80) |
| Wang and colleagues, 2016 [33] | CRS-exposed male Wistar rats | 21 days | RSV: 80, i.p. FLX: 10, i.p. | =FLX: Sucrose preference; immobility time in FST; OFT; hippocampal and PFC BDNF mRNA and protein expression; hippocampal and PFC phosphorylated ERK mRNA and protein expression |
| Xu and colleagues, 2010 [7] | male ICR mice | 4 days | RSV: 20, 40, 80, i.g. FLX: 10, i.p. Moclobemide: 20, i.p. Imipramine: 20, i.p. | >FLX: Dopamine in FC (80); 5-HIAA/5-HT ratio in FC (80), hippocampus (80) and hypothalamus (80); noradrenaline in FC (80) and hippocampus (80); MAO-A (20/40/80); MAO-B (80) =FLX: FST (20/40/80); TST (40/80); 5-HT in FC (80), hippocampus (40/80) and hypothalamus (80) >Imipramine: Dopamine in FC (80); 5-HIAA/5-HT ratio in FC (80), hippocampus (80) and hypothalamus (80); MAO-A (20/40/80); MAO-B (80) =Imipramine: FST (20/40/80); TST (40/80); apomorphine-induced hypothermia (20/40/80); 5-HT in FC (80), hippocampus (40/80) and hypothalamus (80); noradrenaline in FC (80) and hippocampus (80) =Moclobemide: MAO-A (20/40/80) >Moclobemide: MAO-B (80) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, A.; Beidler, J.; Hong, M.Y. Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms. Molecules 2018, 23, 2197. https://doi.org/10.3390/molecules23092197
Moore A, Beidler J, Hong MY. Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms. Molecules. 2018; 23(9):2197. https://doi.org/10.3390/molecules23092197
Chicago/Turabian StyleMoore, Alyssa, Joshua Beidler, and Mee Young Hong. 2018. "Resveratrol and Depression in Animal Models: A Systematic Review of the Biological Mechanisms" Molecules 23, no. 9: 2197. https://doi.org/10.3390/molecules23092197




