All NMR spectra were recorded in deuterated dimethyl sulfoxide (DMSO) at 298 K on a Bruker Avance 500 (Billerica, MA, USA) or Bruker Avance Neo 500 spectrometer (Billerica, MA, USA). All chemical shifts (δ) were reported in ppm relative to the residual solvent signal. HRMS spectra were recorded on a Thermo Scientific Q Exactive Plus mass spectrometer (Waltham, MA, USA) using a heated electrospray ionization (HESI-II) probe ion source. TLC was performed on aluminum sheets coated with silica gel 60 F254 (Merck, 1.05554, Budapest, Hungary). Visualization was done under UV light (254 nm). Column chromatography was carried out using silica gel (Merck, 60 Å, 0.063‒0.200 mm, Budapest, Hungary). Melting points were determined by a Stuart SMP10 device (Staffordshire, UK), and they were uncorrected. All chemicals and solvents were of commercial grade and were used without further purification.
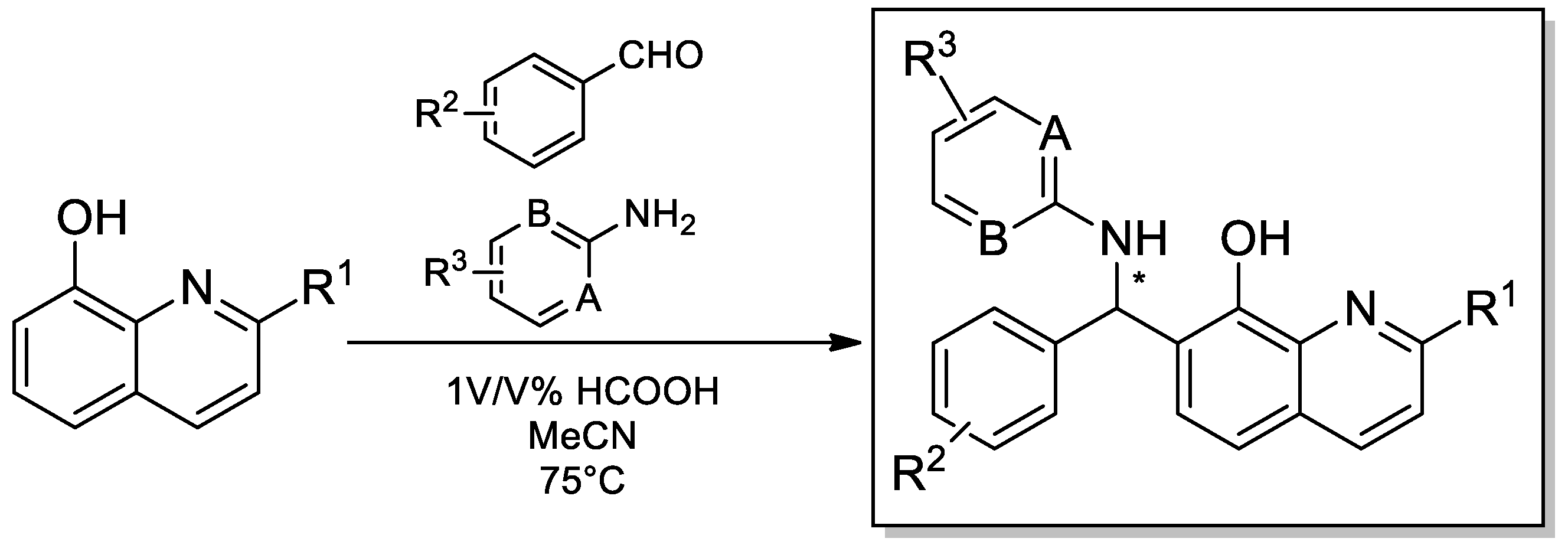
3.1. General Procedure for the Syntheses of Compounds 1–48
To a solution of 1 mmol aldehyde, 3× volume of acetonitrile and 1 equivalent volume of amine were added to 0.6 equivalent of quinoline derivatives and 1% (
v/
v) formic acid. The reaction mixture was stirred at higher temperature (75 °C). The reaction was monitored by TLC (eluent: hexane isomeric mixture:acetone). If the product precipitated, it was filtered, washed with hexane, and dried. If the reaction mixture was homogeneous, it was evaporated to dryness and was purified by column chromatography (eluent: hexane:acetone from 20:1 to 4:1,
v/
v). The crude product was crystallized from hexane/ethyl-acetate. The molecular structures were determined by means of 1D and 2D-NMR technologies (see
Supplementary Materials)
7-(Phenyl(phenylamino)methyl)quinolin-8-ol (1): white solid, yield: 26% (51 mg), melting point (m.p.): 141–143 °C, C22H18N2O; 1H-NMR (500 MHz, DMSO) δ 9.99 (s, 1H), 8.82 (s, 1H), 8.24 (d, J = 7.0 Hz, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.53–7.48 (m, 1H), 7.43–7.38 (m, 2H), 7.35 (d, J = 7.9 Hz, 1H), 7.32–7.23 (m, 2H), 7.24–7.14 (m, 1H), 7.01–6.92 (m, 2H), 6.67–6.59 (m, 2H), 6.49–6.42 (m, 2H), 6.14 (d, J = 5.3 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 149.77 (s), 148.25 (s), 147.97 (s), 142.87 (s), 138.11 (s), 136.04 (s), 128.75 (s), 128.34 (s), 127.51 (s), 127.36 (s), 126.82 (s), 126.29 (s), 125.45 (s), 121.69 (s), 117.54 (s), 118.06 (s), 112.94 (s), 54.16 (s); HRMS (ESI) m/z calcd. for C22H18N2O [M + H]+: 327.1492, found: 327.1493.
7-((Phenylamino)(4-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (2): white solid, yield: 29% (69 mg), m.p.: 83–85 °C, C23H17F3N2O; 1H-NMR (500 MHz, DMSO) δ 10.14 (s, 1H), 8.84 (d, J = 2.2 Hz, 1H), 8.26 (d, J = 8.2 Hz, 1H), 7.67 (d, J = 7.9 Hz, 2H), 7.62 (d, J = 7.8 Hz, 2H), 7.56 (d, J = 8.5 Hz, 1H), 7.52 (dd, J = 7.7, 3.7 Hz, 1H), 7.38 (d, J = 8.4 Hz, 1H), 7.00 (t, J = 7.4 Hz, 2 H), 6.64 (d, J = 7.8 Hz, 2H), 6.55 (d, J = 6.9 Hz, 1H), 6.50 (t, J = 7.0 Hz, 1H), 6.23 (d, J = 6.9 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 149.95 (s), 148.38 (s), 147.73 (s), 147.68 (s), 138.14 (s), 136.09 (s), 128.84 (s), 128.09 (s), 127.71 (s), 127.53 (q, J = 31.9 Hz), 126.16 (s), 125.29 (q, J = 3.3 Hz), 124.58 (s), 121.88 (s), 117.76 (s), 116.48 (s), 113.04 (s), 54.03 (s); HRMS (ESI) m/z calcd. for C23H17F3N2O [M + H]+: 395.1366, found: 395.1369.
7-((Phenylamino)(pyridin-2-yl)methyl)quinolin-8-ol (3): white solid, yield: 13% (26 mg), m.p.: 129–130 °C, C21H17N3O; 1H-NMR (500 MHz, DMSO) δ 10.16 (s, 1H), 8.86 (dd, J = 4.2, 1.5 Hz, 1H), 8.56 (dd, J = 4.8, 0.7 Hz, 1H), 8.25 (dd, J = 8.3, 1.5 Hz, 1H), 7.76 (td, J = 7.7, 1.8 Hz, 1H), 7.59–7.47 (m, 3H), 7.33 (d, J = 8.6 Hz, 1H), 7.27 (ddd, J = 7.4, 5.0, 0.8 Hz, 1H), 7.02 (dd, J = 8.1, 7.6 Hz, 2H), 6.70 (d, J = 7.8 Hz, 2H), 6.61 (d, J = 7.1 Hz, 1H), 6.50 (t, J = 7.3 Hz, 1H), 6.27 (d, J = 7.0 Hz, 1H); 13C-NMR (126 MHz, DMSO) δ 160.99 (s), 150.53 (s), 149.30 (s), 148.69 (s), 147.80 (s), 138.59 (s), 137.43 (s), 136.48 (s), 129.28 (s), 128.05 (s), 126.81 (s), 125.23 (s), 122.81 (s), 122.50 (s), 122.21 (s), 117.96 (s), 116.68 (s), 113.31 (s), 55.40 (s); HRMS (ESI) m/z calcd. for C21H17N3O [M + H]+: 328.1444, found: 328.1445.
Ethyl 4-((4-fluorophenyl)(8-hydroxyquinolin-7-yl)methylamino)benzoate (4): white solid, yield: 17% (42 mg), m.p.: 142–144 °C, C25H21FN2O3; 1H-NMR (500 MHz, DMSO) δ 10.17 (s, 1H), 8.87 (dd, J = 4.2, 1.5 Hz, 1H), 8.30 (dd, J = 8.3, 1.5 Hz, 1H), 7.64 (d, J = 8.9 Hz, 2H), 7.56 (dd, J = 8.3, 4.2 Hz, 1H), 7.51 (d, J = 8.6 Hz, 1H), 7.45–7.38 (m, 3H), 7.34 (d, J = 7.2 Hz, 1H), 7.20–7.14 (m, 2H), 6.69 (d, J = 8.8 Hz, 2H), 6.25 (d, J = 7.2 Hz, 1H), 4.18 (q, J = 7.0 Hz, 2H), 1.24 (t, J = 7.1 Hz, 3H).; 13C-NMR (126 MHz, CDCl3) δ 165.78 (s), 161.24 (d, J = 243.6 Hz), 151.80 (s), 150.01 (s), 148.42 (s), 138.11 (s), 136.11 (s), 130.78 (s), 129.38 (d, J = 8.1 Hz), 127.72 (s), 125.97 (s), 124.27 (s), 121.90 (s), 117.72 (s), 116.96 (s), 115.26 (s), 115.09 (s), 111.95 (s), 59.58 (s), 53.35 (s), 14.34 (s).
4-((4-Fluorophenyl)(8-hydroxyquinolin-7-yl)methylamino)benzoic acid (5): white solid, yield: 13% (30 mg), m.p.: 185–187 °C, C23H17FN2O3; 1H-NMR (500 MHz, DMSO) δ 12.01 (s, 1H), 10.22 (s, 1H), 8.85 (d, J = 2.2 Hz, 1H), 8.28 (d, J = 8.1 Hz, 1H), 7.69 (d, J = 7.9 Hz, 2H), 7.61 (d, J = 8.9 Hz, 2H), 7.59 (d, J = 8.4 Hz, 2H), 7.54 (dd, J = 8.0, 3.9 Hz, 1H), 7.48 (d, J = 8.5 Hz, 1H), 7.39 (d, J = 8.5 Hz, 1H), 7.31 (d, J = 6.9 Hz, 1H), 6.66 (d, J = 8.3 Hz, 2H), 6.31 (d, J = 6.7 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 167.37 (s), 151.48 (s), 150.16 (s), 149.13 (d, J = 297.5 Hz), 148.49 (s), 146.83 (s), 138.14 (s), 136.14 (s), 131.02 (s), 128.20 (s), 127.85 (s), 126.06 (s), 125.41 (d, J = 2.8 Hz), 123.73 (s), 122.01 (s), 118.04 (s), 117.84 (s), 111.92 (s), 53.82 (s).
7-(Phenyl(pyridin-2-ylamino)methyl)quinolin-8-ol (6): white solid, yield: 25% (49 mg), m.p.: 172–174 °C, C21H17N3O; 1H-NMR (500 MHz, DMSO) δ 9.92 (s, 1H), 8.85 (dd, J = 4.0, 1.3 Hz, 1H), 8.29 (dd, J = 8.3, 1.1 Hz, 1H), 7.92 (d, J = 4.1 Hz, 1H), 7.62 (d, J = 8.5 Hz, 1H), 7.54 (dd, J = 8.3, 4.2 Hz, 1H), 7.42–7.34 (m, 5 H), 7.29 (t, J = 7.6 Hz, 2 H), 7.20 (t, J = 7.3 Hz, 1 H), 6.86 (d, J = 8.5 Hz, 1 H), 6.68 (d, J = 8.4 Hz, 1 H), 6.55–6.37 (m, 1H); 13C-NMR (126 MHz, DMSO) δ 158.43 (s), 150.00 (s), 148.69 (s), 147.90 (s), 144.00 (s), 138.57 (s), 137.12 (s), 136.47 (s), 128.62 (s), 127.86 (s), 127.66 (s), 127.04 (s), 126.95 (s), 126.36 (s), 122.05 (s), 117.73 (s), 112.51 (s), 109.26 (s), 52.08 (s); HRMS (ESI) m/z calcd. for C21H17N3O [M + H]+: 328.1444, found: 328.1447.
7-(Isoxazol-3-ylamino)(pyridin-2-yl)methyl)quinolin-8-ol (7): white solid, yield: 27% (52 mg), m.p.: 145–146 °C, C18H14N4O2; 1H-NMR (500 MHz, DMSO) δ 10.02 (s, 1H), 8.82 (d, J = 2.1 Hz, 1H), 8.49 (d, J = 3.4 Hz, 1H), 8.31 (bs, 1H), 8.24 (d, J = 8.1 Hz, 1H), 7.73 (t, J = 7.5 Hz, 1H), 7.54 (d, J = 8.5 Hz, 1H), 7.52–7.46 (m, 2H), 7.33 (d, J = 8.5 Hz, 1H), 7.27–7.12 (m, 2H), 6.35 (d, J = 7.9 Hz, 1H), 6.09 (bs, 1H); 13C-NMR (126 MHz, CDCl3) δ 162.79 (s), 160.56 (s), 158.17 (s), 149.88 (s), 148.84 (s), 148.23 (s), 138.14 (s), 136.83 (s), 136.00 (s), 127.57 (s), 126.24 (s), 124.61 (s), 122.27 (s), 121.81 (s), 121.73 (s), 117.23 (s), 97.02 (s), 55.73 (s); HRMS (ESI) m/z calcd. for C18H14N4O2 [M + H]+: 319.1190, found: 319.1189.
7-((5-tert-Butylisoxazol-3-ylamino)(4-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (8): white solid, yield: 18% (48 mg), m.p.: 185–187 °C, C24H22F3N3O2; 1H-NMR (500 MHz, DMSO) δ 10.08 (s, 1H), 8.84 (bs, 1H), 8.28 (d, J = 8.2 Hz, 1H), 7.66 (d, J = 7.8 Hz, 2H), 7.59–7.50 (m, 4H), 7.40 (d, J = 8.4 Hz, 1H), 7.10 (d, J = 7.8 Hz, 1H), 6.31 (d, J = 7.7 Hz, 1H), 5.66 (s, 1H), 1.18 (s, 9H); 13C-NMR (126 MHz, DMSO) δ 179.26 (s), 163.57 (s), 150.17 (s), 148.89 (s), 148.27 (s), 138.57 (s), 136.54 (s), 128.11 (s), 127.84 (q, J = 31.9 Hz), 126.38 (s), 125.69 (q, J = 3.9 Hz), 124.93 (s), 124.78 (q, J = 272.2 Hz), 122.35 (s), 118.05 (s), 91.12 (s), 54.54 (s), 32.63 (s), 28.89 (s); HRMS (ESI) m/z calcd. for C24H22F3N3O2 [M + H]+: 442.1737, found: 442.1739.
7-((4-Fluorophenyl)(5-methylisoxazol-3-ylamino)methyl)quinolin-8-ol (9): white solid, yield: 13% (27 mg), m.p.: 153–155 °C, C20H16FN3O2; 1H-NMR (500 MHz, DMSO) δ 9.98 (s, 1H), 8.86 (dd, J = 3.9, 1.1 Hz, 1H), 8.30 (d, J = 8.3 Hz, 1H), 7.59 (d, J = 8.5 Hz, 1H), 7.55 (dd, J = 8.3, 4.2 Hz, 1H), 7.41 (d, J = 8.7 Hz, 1H), 7.38 (dd, J = 8.4, 5.9 Hz, 2H), 7.13 (t, J = 8.8 Hz, 2H), 7.01 (d, J = 8.3 Hz, 1H), 6.23 (d, J = 8.3 Hz, 1H), 5.73 (s, 1H), 2.20 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 167.92 (s), 164.03 (s), 161.48 (d, J = 242.6 Hz), 149.95 (s), 148.76 (s), 139.56 (s), 138.55 (s), 136.49 (s), 129.32 (d, J = 8.1 Hz), 127.96 (s), 126.27 (s), 125.66 (s), 122.18 (s), 117.87 (s), 115.36 (d, J = 21.2 Hz), 94.33 (s), 54.21 (s), 12.45 (s); HRMS (ESI) m/z calcd. for C20H16FN3O2 [M + H]+: 350.1299, found: 350.1300.
7-((5-Methylisoxazol-3-ylamino)(4-nitrophenyl)methyl)quinolin-8-ol (10): white solid, yield: 26% (59 mg), o.p.: 138–140 °C, C20H16N4O4; 1H-NMR (500 MHz, DMSO) δ 10.16 (s, 1H), 8.84 (s, 1H), 8.28 (d, J = 8.1 Hz, 1H), 8.16 (d, J = 8.2 Hz, 2H), 7.61 (d, J = 8.1 Hz, 2H), 7.56–7.48 (m, 2H), 7.40 (d, J = 8.4 Hz, 1H), 7.15 (d, J = 7.7 Hz, 1H), 6.34 (d, J = 7.7 Hz, 1H), 5.74 (s, 1H), 2.18 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 167.76 (s), 163.53 (s), 150.90 (s), 149.77 (s), 148.49 (s), 146.35 (s), 138.15 (s), 136.10 (s), 128.01 (s), 127.77 (s), 125.93 (s), 124.02 (s), 123.56 (s), 122.00 (s), 117.73 (s), 93.91 (s), 54.18 (s), 12.01 (s); HRMS (ESI) m/z calcd. for C20H16N4O4 [M + H]+: 377.1244, found: 377.1250.
7-((4-Fluorophenyl)(6-methylpyridin-2-ylamino)methyl)quinolin-8-ol (11): white solid, yield: 12% (26 mg), m.p.: 157–159 °C, C22H18FN3O; 1H-NMR (500 MHz, DMSO) δ 9.97 (s, 1H), 8.82 (d, J = 2.5 Hz, 1H), 8.26 (d, J = 8.0 Hz, 1H), 7.63 (d, J = 8.5 Hz, 1H), 7.50 (dd, J = 8.1, 4.0 Hz, 1H), 7.41–7.31 (m, 3H), 7.30–7.17 (m, 2H), 7.08 (t, J = 8.7 Hz, 2H), 6.80 (d, J = 8.4 Hz, 1H), 6.40 (t, J = 12.7 Hz, 1H), 6.33 (d, J = 6.9 Hz, 1H), 2.19 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 160.92 (d, J = 242.0 Hz), 157.50 (s), 155.70 (s), 149.53 (s), 148.29 (s), 139.82 (s), 138.13 (s), 137.24 (s), 136.04 (s), 128.95 (d, J = 8.0 Hz), 127.48 (s), 126.60 (s), 125.68 (s), 121.68 (s), 117.43 (s), 114.84 (d, J = 21.2 Hz), 111.29 (s), 105.17 (s), 51.11 (s), 24.23 (s); HRMS (ESI) m/z calcd. for C22H18FN3O [M + H]+: 360.1507, found: 360.1508.
7-((4-Isopropoxyphenyl)(6-methylpyridin-2-ylamino)methyl)quinolin-8-ol (12): white solid, yield: 25% (60 mg), m.p.: 132–134 °C, C25H25N3O2; 1H-NMR (500 MHz, DMSO) δ 9.80 (d, J = 117.5 Hz, 1H), 8.85 (dd, J = 4.1, 1.4 Hz, 1H), 8.29 (dd, J = 8.3, 1.3 Hz, 1H), 7.67 (d, J = 8.5 Hz, 1H), 7.53 (dd, J = 8.3, 4.2 Hz, 1H), 7.40 (d, J = 8.6 Hz, 1H), 7.26 (t, J = 8.2 Hz, 3H), 7.16 (d, J = 8.9 Hz, 1H), 6.82 (d, J = 8.7 Hz, 2H), 6.73 (d, J = 8.7 Hz, 1H), 6.41 (d, J = 8.3 Hz, 1H), 6.34 (d, J = 7.1 Hz, 1H), 4.53 (h, J = 6.0 Hz, 1H), 2.22 (s, 3H), 1.22 (d, J = 6.0 Hz, 6H); 13C-NMR (126 MHz, DMSO) δ 158.05 (s), 156.55 (s), 156.12 (s), 149.85 (s), 148.66 (s), 138.57 (s), 137.64(s), 136.45 (s), 135.71 (s), 128.73 (s), 127.80 (s), 127.12 (s), 126.66 (s), 122.00 (s), 117.73 (s), 115.62 (s), 111.54 (s), 105.42 (s), 69.45 (s), 51.58 (s), 24.68 (s), 22.33 (s); HRMS (ESI) m/z calcd. for C25H25N3O2 [M + H]+: 400.2020, found: 400.2018.
7-((6-Methylpyridin-2-ylamino)(pyridin-2-yl)methyl)quinolin-8-ol (13): white solid, yield: 16% (33 mg), m.p.: 170–173 °C, C21H18N4O; 1H-NMR (500 MHz, DMSO) δ 10.12 (s, 1H), 8.82 (s, 1H), 8.49 (s, 1H), 8.23 (d, J = 7.9 Hz, 1H), 7.71 (t, J = 7.1 Hz, 1H), 7.55–7.44 (m, 3H), 7.31 (d, J = 8.3 Hz, 1H), 7.27–7.18 (m, 3H), 6.76 (d, J = 7.1 Hz, 1H), 6.40 (t, J = 17.7 Hz, 1H), 6.33 (d, J = 6.7 Hz, 1H), 2.19 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 161.06 (s), 157.24 (s), 155.73 (s), 149.93 (s), 148.71 (s), 148.25 (s), 138.24 (s), 137.33 (s), 136.80 (s), 135.99 (s), 127.55 (s), 126.75 (s), 125.22 (s), 122.13 (s), 121.96 (s), 121.68 (s), 117.34 (s), 111.37 (s), 104.91 (s), 53.29 (s), 24.15 (s); HRMS (ESI) m/z calcd. for C21H18N4O [M + H]+: 343.1553, found: 343.1555.
7-(Phenyl(pyrimidin-2-ylamino)methyl)quinolin-8-ol (14): white solid, yield: 15% (30 mg), m.p.: 192–195 °C, C20H16N4O; 1H-NMR (500 MHz, DMSO) δ 9.98 (s, 1H), 8.85 (d, J = 2.9 Hz, 1H), 8.33–8.27 (m, 3H), 8.08 (d, J = 9.4 Hz, 1H), 7.76 (d, J = 8.5 Hz, 1H), 7.54 (dd, J = 8.2, 4.1 Hz, 1H), 7.44–7.36 (m, 3H), 7.28 (t, J = 7.6 Hz, 2H), 7.19 (t, J = 7.3 Hz, 1H), 7.00 (d, J = 9.4 Hz, 1H), 6.60 (t, J = 4.7 Hz, 1H); 13C-NMR (126 MHz, DMSO) δ 162.17 (s), 158.51 (s), 149.91 (s), 148.72 (s), 143.59 (s), 138.51 (s), 136.49 (s), 128.63 (s), 127.92 (s), 127.49 (s), 127.33 (s), 127.01 (s), 125.81 (s), 122.13 (s), 117.79 (s), 111.07 (s), 52.31 (s); HRMS (ESI) m/z calcd. for C20H16N4O [M + H]+: 329.1397, found: 329.1397.
7-((4-Nitrophenyl)(pyrimidin-2-ylamino)methyl)quinolin-8-ol (15): yellow solid, yield: 40% (90 mg), m.p.: 121–123 °C, C20H15N5O3; 1H-NMR (500 MHz, DMSO) δ 10.17 (s, 1H), 8.84 (d, J = 2.4 Hz, 1H), 8.29 (dd, J = 8.7, 6.1 Hz, 3H), 8.23 (d, J = 9.0 Hz, 1H), 8.15 (d, J = 8.4 Hz, 2H), 7.69 (d, J = 8.5 Hz, 1H), 7.62 (d, J = 8.4 Hz, 2H), 7.53 (dd, J = 8.0, 3.9 Hz, 1H), 7.40 (d, J = 8.5 Hz, 1H), 7.07 (d, J = 9.0 Hz, 1H), 6.62 (t, J = 4.3 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 161.62 (s), 158.17 (s), 151.08 (s), 149.79 (s), 148.46 (s), 146.32 (s), 138.11 (s), 136.11 (s), 128.16 (s), 127.79 (s), 126.68 (s), 123.95 (s), 123.56 (s), 121.96 (s), 117.69 (s), 111.07 (s), 51.78 (s); HRMS (ESI) m/z calcd. for C20H15N5O3 [M + H]+: 374.1248, found: 374.1250.
Ethyl 4-((8-hydroxyquinolin-7-yl)(4-(trifluoromethyl)phenyl)methylamino)benzoate (16): white solid, yield: 30% (84 mg), m.p.: 95–98 °C, C26H21F3N2O3; 1H-NMR (500 MHz, DMSO) δ 10.27 (s, 1H), 8.88 (dd, J = 4.1, 1.2 Hz, 1H), 8.31 (dd, J = 8.3, 1.1 Hz, 1H), 7.72 (d, J = 8.3 Hz, 2H), 7.66 (d, J = 8.7 Hz, 2H), 7.61 (d, J = 8.2 Hz, 2H), 7.57 (dd, J = 8.3, 4.2 Hz, 1H), 7.50 (d, J = 8.6 Hz, 1H), 7.44–7.39 (m, 2H), 6.71 (d, J = 8.7 Hz, 2H), 6.35 (d, J = 7.1 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 1.24 (t, J = 7.1 Hz, 3H); 13C-NMR (126 MHz, DMSO) δ 166.21 (s), 152.16 (s), 150.63 (s), 148.95 (s), 147.18 (s), 138.58 (s), 136.59 (s), 131.27 (s), 128.63 (s), 128.30 (s), 128.12–127.65(m), 126.48 (s), 125.87 (q, J = 3.2 Hz), 124.07 (s), 122.47 (s), 118.28 (s), 117.62 (s), 112.46 (s), 60.06 (s), 54.22 (s), 14.78 (s); HRMS (ESI) m/z calcd. for C26H21F3N2O3 [M + H]+: 467.1577, found: 467.1584.
Ethyl 4-((8-hydroxyquinolin-7-yl)(pyridin-2-yl)methylamino)benzoate (17): white solid, yield: 60% (144 mg), m.p.: 157–159 °C, C24H21N3O3; 1H-NMR (500 MHz, DMSO) δ 10.22 (s, 1H), 8.84 (s, 1H), 8.54 (s, 1H), 8.23 (d, J = 8.1 Hz, 1H), 7.75 (t, J = 7.5 Hz, 1H), 7.62 (d, J = 8.0 Hz, 2H), 7.53–7.50 (m, 1H), 7.48 (d, J = 8.3 Hz, 2H), 7.42 (d, J = 6.5 Hz, 1H), 7.32 (d, J = 8.4 Hz, 1H), 7.28–7.22 (m, 1H), 6.73 (d, J = 7.9 Hz, 2H), 6.34 (d, J = 6.5 Hz, 1H), 4.14 (q, J = 6.8 Hz, 2H), 1.20 (t, J = 6.6 Hz, 3H); 13C-NMR (126 MHz, CDCl3) δ 165.77 (s), 159.85 (s), 151.43 (s), 150.19 (s), 148.99 (s), 148.35 (s), 138.14 (s), 137.11 (s), 136.06 (s), 130.79 (s), 127.73(s), 126.24 (s), 123.92 (s), 122.56 (s), 122.11 (s), 121.89 (s), 117.63 (s), 116.94 (s), 111.95 (s), 59.58 (s), 54.84 (s), 14.33 (s); HRMS (ESI) m/z calcd. for C24H21N3O3 [M + H]+: 400.1656, found: 400.1661.
7-(Pyridin-2-yl(4-(trifluoromethyl)phenylamino)methyl)quinolin-8-ol (18): white solid, yield: 33% (78 mg), m.p.: 162–164 °C, C22H16F3N3O; 1H-NMR (500 MHz, DMSO) δ 10.22 (s, 1H), 8.84 (d, J = 3.4 Hz, 1H), 8.55 (d, J = 4.0 Hz, 1H), 8.23 (d, J = 8.2 Hz, 1H), 7.75 (t, J = 7.5 Hz, 1H), 7.54–7.47 (m, 3H), 7.34–7.30 (m, 4H), 7.29–7.23 (m, 1H), 6.79 (d, J = 8.2 Hz, 2H), 6.31 (d, J = 6.8 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 160.33 (s), 150.95 (s), 150.80 (s), 149.54 (s), 148.80 (s), 138.55 (s), 137.57 (s), 136.53 (s), 128.18 (s), 126.65 (s), 126.61 (s), 125.71 (q, J = 269.9 Hz), 124.33 (s), 123.01 (s), 122.57 (s), 122.33 (s), 118.10 (s), 116.35 (q, J = 31.8 Hz), 112.68 (s), 55.33 (s); HRMS (ESI) m/z calcd. for C22H16F3N3O [M + H]+: 396.1318, found: 396.1323.
7-((3-Fluoro-4-(trifluoromethyl)phenyl)(morpholino)methyl)quinolin-8-ol7-((4-nitrophenylamino) (pyridin-2-yl)methyl)quinolin-8-ol (19): yellow-green (viridian) solid, yield: 14% (31 mg), m.p.: 128–131 °C, C21H16N4O3; 1H-NMR (500 MHz, DMSO) δ 10.32 (s, 1H), 8.87–8.83 (m, 1H), 8.57 (d, J = 3.1 Hz, 1H), 8.25 (d, J = 8.2 Hz, 1H), 8.12 (d, J = 6.6 Hz, 1H), 7.94 (d, J = 8.6 Hz, 2H), 7.77 (t, J = 7.6 Hz, 1H), 7.53 (dd, J = 7.8, 3.8 Hz, 1H), 7.50–7.41 (m, 2H), 7.34 (d, J = 8.5 Hz, 1H), 7.31–7.25 (m, 1H), 6.78 (bs, 2H), 6.39 (d, J = 6.7 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 159.21 (s), 153.27 (s), 150.30 (s), 149.11 (s), 148.46 (s), 138.14 (s), 137.25 (s), 136.43 (s), 136.11 (s), 127.87 (s), 126.09 (s), 126.03 (s), 123.23 (s), 122.76 (s), 122.21 (s), 122.03 (s), 117.77 (s), 55.15 (s); HRMS (ESI) m/z calcd. for C21H16N4O3 [M + H]+: 373.1295, found: 373.1299.
7-((6-Methylpyridin-2-ylamino)(4-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (20): white solid, yield: 33% (81 mg), m.p.: 147–150 °C, C23H18F3N3O; 1H-NMR (500 MHz, DMSO) δ 10.06 (s, 1H), 8.83 (bs, 1H), 8.27 (d, J = 8.0 Hz, 1H), 7.66–7.59 (m, 3H), 7.56 (d, J = 7.4 Hz, 2H), 7.52 (d, J = 2.7 Hz, 1H), 7.40 (d, J = 8.3 Hz, 1H), 7.34 (d, J = 8.4 Hz, 1H), 7.26 (t, J = 7.1 Hz, 1H), 6.93 (d, J = 8.1 Hz, 1H), 6.46 (d, J = 8.0 Hz, 1H), 6.35 (d, J = 6.6 Hz, 1H), 2.19 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 157.41 (s), 155.70 (s), 149.70 (s), 148.68 (s), 148.38 (s), 138.15 (s), 137.27 (s), 136.07 (s), 127.79 (s), 127.62 (s), 127.16 (q, J = 31.7 Hz), 126.67 (s), 125.11 (dd, J = 6.9, 3.8 Hz), 124.96 (s), 124.39 (q, J = 271.7 Hz), 121.81 (s), 117.57 (s), 111.44 (s), 105.41 (s), 52.20 (s), 24.24 (s); HRMS (ESI) m/z calcd. for C23H18F3N3O [M + H]+: 410.1475, found: 410.1478.
7-((6-Methylpyridin-2-ylamino)(4-nitrophenyl)methyl)quinolin-8-ol (21): pale yellow solid, yield: 51% (118 mg), m.p.: 159–161 °C, C22H18N4O3; 1H-NMR (500 MHz, DMSO) δ 10.13 (s, 1H), 8.84 (d, J = 2.8 Hz, 1H), 8.28 (d, J = 8.1 Hz, 1H), 8.15 (d, J = 8.5 Hz, 2H), 7.64–7.56 (m, 3H), 7.53 (dd, J = 8.1, 4.0 Hz, 1H), 7.40 (t, J = 8.4 Hz, 2H), 7.33–7.19 (m, 1H), 6.96 (d, J = 8.3 Hz, 1H), 6.47 (d, J = 8.2 Hz, 1H), 6.36 (d, J = 7.1 Hz, 1H), 2.19 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 157.28 (s), 155.70 (s), 151.99 (s), 149.79 (s), 148.45 (s), 146.17 (s), 138.16 (s), 137.30 (s), 136.10 (s), 128.14 (s), 127.72 (s), 126.66 (s), 124.48 (s), 123.48 (s), 121.92 (s), 117.67 (s), 111.56 (s), 105.57 (s), 51.47 (s), 24.24 (s); HRMS (ESI) m/z calcd. for C22H18N4O3 [M + H]+: 387.1452, found: 387.1451.
2-Methyl-7-((6-methylpyridin-2-ylamino)(4-nitrophenyl)methyl)quinolin-8-ol (22): ivory-white solid, yield: 23% (55 mg), m.p.: 134–136 °C, C23H20N4O3; 1H-NMR (500 MHz, DMSO) δ 9.64 (s, 1H), 8.20–8.10 (m, 3H), 7.62 (d, J = 8.7 Hz, 2H), 7.53 (d, J = 8.5 Hz, 1H), 7.44 (d, J = 8.4 Hz, 1H), 7.41 (d, J = 8.6 Hz, 1H), 7.37 (d, J = 8.5 Hz, 1H), 7.30 (dd, J = 8.0, 7.4 Hz, 1H), 6.97 (d, J = 8.5 Hz, 1H), 6.50 (d, J = 8.3 Hz, H), 6.39 (d, J = 7.2 Hz, 1 H), 2.69 (d, J = 10.1 Hz, 3H), 2.22 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 157.73 (s), 157.60 (s), 156.12 (s), 152.53 (s), 149.34 (s), 146.59 (s), 137.87 (s), 137.74 (s), 136.58 (s), 128.52 (s), 126.23 (s), 125.95 (s), 124.69 (s), 123.91 (s), 123.17 (s), 117.96 (s), 111.99 (s), 106.04 (s), 51.87 (s), 25.16 (s), 24.69 (s); HRMS (ESI) m/z calcd. for C23H20N4O3 [M + H]+: 401.1608, found: 401.1609.
2-Methyl-7-((6-methylpyridin-2-ylamino)(4-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (23): beige solid, yield: 71% (180 mg), m.p.: 122–124 °C, C24H20F3N3O; 1H-NMR (500 MHz, DMSO) δ 9.54 (s, 1H), 8.15 (d, J = 8.4 Hz, 1H), 7.82 (d, J = 3.9 Hz, 1H), 7.65–7.59 (m, 3H), 7.55 (d, J = 7.9 Hz, 2H), 7.39 (d, J = 8.4 Hz, 1H), 7.33 (d, J = 8.5 Hz, 1H), 7.27 (d, J = 6.7 Hz, 1H), 7.07 (t, J = 18.0 Hz, 1H), 6.54–6.43 (m, 2H), 2.67 (s, 3H), 2.19 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 157.02 (s), 155.79 (s), 149.05 (s), 148.68 (s), 144.77 (s), 137.48 (s), 136.92 (s), 136.10 (s), 127.85 (s), 127.08 (dd, J = 63.3, 31.6 Hz), 126.25 (s), 125.75 (s), 124.97 (dd, J = 6.8, 3.8 Hz), 124.64 (s), 124.42 (q, J = 271.9 Hz), 122.59 (s), 117.34 (s), 117.14 (s), 112.75 (s), 52.45 (s), 24.69 (s), 16.92 (s); HRMS (ESI) m/z calcd. for C24H20F3N3O [M + H]+: 424.1631, found: 424.1631.
7-((6-Methylpyridin-2-ylamino)(4-(trifluoromethoxy)phenyl)methyl)quinolin-8-ol (24): white solid, yield: 34% (87 mg), m.p.: 99–101 °C, C23H18F3N3O2; 1H-NMR (500 MHz, DMSO) δ 10.00 (s, 1H), 8.82 (d, J = 2.0 Hz, 1H), 8.27 (d, J = 8.1 Hz, 1H), 7.63 (d, J = 8.4 Hz, 1H), 7.51 (dd, J = 7.6, 3.6 Hz, 1H), 7.45 (d, J = 8.1 Hz, 2H), 7.39 (d, J = 8.4 Hz, 1H), 7.30 (d, J = 8.6 Hz, 1H), 7.28–7.21 (m, 3H), 6.86 (d, J = 8.2 Hz, 1H), 6.43 (d, J = 8.1 Hz, 1H), 6.34 (d, J = 6.9 Hz, 1H), 2.19 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 157.44 (s), 155.70 (s), 149.71 (s), 148.31 (s), 146.90 (s), 143.21 (s), 138.17 (s), 137.25 (s), 136.05 (s), 128.89 (s), 127.57 (s), 126.57 (s), 125.30 (s), 121.73 (s), 120.78 (s), 117.42 (s), 116.02 (d, J = 260.6 Hz), 111.35 (s), 105.28 (s), 51.14 (s), 24.24 (s); HRMS (ESI) m/z calcd. for C23H18F3N3O2 [M + H]+: 426.1424, found: 426.1423.
7-((6-Methylpyridin-2-ylamino)(3-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (25): white solid, yield: 62% (152 mg), m.p.: 148–152 °C, C23H18F3N3O; 1H-NMR (500 MHz, DMSO) δ 10.07 (s, 1H), 8.83 (s, 1H), 8.27 (d, J = 8.2 Hz, 1H), 7.71 (s, 1H), 7.69–7.60 (m, 2H), 7.56–7.46 (m, 3H), 7.44–7.35 (m, 2H), 7.27 (t, J = 7.6 Hz, 1H), 6.93 (d, J = 8.6 Hz, 1H), 6.45 (d, J = 8.2 Hz, 1H), 6.35 (d, J = 6.9 Hz, 1H), 2.19 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 157.37 (s), 155.70 (s), 149.67 (s), 148.41 (s), 145.29 (s), 138.12 (s), 137.32 (s), 136.08 (s), 131.32 (s), 129.29 (s), 128.88 (d, J = 31.4 Hz), 127.60 (s), 126.44 (s), 125.22 (q, J = 48.0 Hz), 125.03 (s), 123.34 (s), 121.82 (s), 117.62 (s), 111.49 (s), 105.40 (s), 51.40 (s), 24.21 (s); HRMS (ESI) m/z calcd. for C23H18F3N3O [M + H]+: 410.1475, found: 410.1476.
7-((3-Methylpyridin-2-ylamino)(4-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (26): white solid, yield: 18% (44 mg), m.p.: 148–151 °C, C23H18F3N3O; 1H-NMR (500 MHz, DMSO) δ 10.09 (s, 1H), 8.83 (d, J = 3.1 Hz, 1H), 8.28 (d, J = 8.2 Hz, 1H), 7.81 (d, J = 4.1 Hz, 1H), 7.69 (d, J = 8.4 Hz, 1H), 7.62 (d, J = 7.9 Hz, 2 H), 7.58–7.48 (m, 3H), 7.39 (d, J = 8.4 Hz, 1H), 7.27 (d, J = 6.7 Hz, 1H), 7.11 (d, J = 8.1 Hz, 1H), 6.53–6.45 (m, 2H), 2.19 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 155.75 (s), 149.93 (s), 148.57 (s), 148.34 (s), 144.73 (s), 138.18 (s), 136.95 (s), 136.06 (s), 127.93 (s), 127.67 (s), 127.32 (s), 127.10 (q, J = 31.3 Hz), 125.00 (q, J = 2.9 Hz), 124.88 (s), 124.42 (q, J = 272.0 Hz), 121.79 (s), 117.48 (s), 117.17 (s), 112.75 (s), 52.33 (s), 16.95 (s); HRMS (ESI) m/z calcd. for C23H18F3N3O [M + H]+: 410.1475, found: 410.1479.
7-((4-Methylpyridin-2-ylamino)(4-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (27): mulatto solid, yield: 15% (37 mg), m.p.: 151–153 °C, C23H18F3N3O; 1H-NMR (500 MHz, DMSO) δ 10.06 (s, 1H), 8.86 (dd, J = 4.1, 1.4 Hz, 1H), 8.30 (dd, J = 8.3, 1.3 Hz, 1H), 7.80 (d, J = 5.2 Hz, 1H), 7.66 (d, J = 8.2 Hz, 2H), 7.59 (d, J = 8.5 Hz, 1H), 7.57–7.53 (m, 3H), 7.42 (d, J = 8.6 Hz, 1H), 7.37 (d, J = 8.4 Hz, 1H), 6.97 (d, J = 8.4 Hz, 1H), 6.54 (s, 1H), 6.36 (d, J = 5.1 Hz, 1H), 2.15 (s, H); 13C-NMR (126 MHz, DMSO) δ 158.49 (s), 150.16 (s), 149.13 (s), 148.81 (s), 147.58 (s), 147.46 (s), 138.59 (s), 136.52 (s), 128.27 (s), 128.05 (s), 127.60 (q, J = 31.5 Hz), 127.03 (s), 125.58(q, J = 3.5 Hz), 125.49 (s), 124.83 (q, J = 271.6 Hz), 122.24 (s), 117.96 (s), 114.48 (s), 109.35 (s), 51.91 (s), 21.09 (s); HRMS (ESI) m/z calcd. for C23H18F3N3O [M + H]+: 410.1475, found: 410.1477.
7-((3-Fluoro-5-(trifluoromethyl)phenyl)(6-methylpyridin-2-ylamino)methyl)quinolin-8-ol (28): white solid, yield: 35% (90 mg), m.p.: 163–165 °C, C23H17F4N3O; 1H-NMR (500 MHz, DMSO) δ 10.19 (s, 1H), 8.87 (dd, J = 4.0, 1.2 Hz, 1H), 8.31 (dd, J = 8.3, 1.1 Hz, 1H), 7.67 (d, J = 8.6 Hz, 1H), 7.60 (s, 1H), 7.56 (dd, J = 8.3, 4.2 Hz, 1H), 7.51 (d, J = 9.2 Hz, 2H), 7.45 (d, J = 8.4 Hz, 2H), 7.31 (t, J = 7.7 Hz, 1H), 6.95 (d, J = 8.7 Hz, 1H), 6.50 (d, J = 8.3 Hz, 1H), 6.40 (d, J = 7.2 Hz, 1H), 2.22 (s, 3H); 13C NMR (126 MHz, DMSO) δ 162.40 (d, J = 246.4 Hz), 157.62 (s), 156.15 (s), 150.19 (s), 149.34 (d, J = 6.8 Hz), 148.95 (s), 138.57 (s), 137.84 (s), 136.57 (s), 131.17 (qd, J = 32.7, 8.7 Hz), 128.16 (s), 126.69 (s), 124.86 (s), 123.82 (dq, J = 272.8, 2.6 Hz), 122.40 (s), 120.30–120.02 (m), 118.39 (d, J = 21.8 Hz), 118.22 (s), 112.16 (s), 111.39 (dq, J = 25.1, 3.4 Hz), 106.01 (s), 51.75 (s), 24.64 (s); HRMS (ESI) m/z calcd. for C23H17F4N3O [M + H]+: 428.1381, found: 428.1388.
7-((3,5-bis(Trifluoromethyl)phenyl)(6-methylpyridin-2-ylamino)methyl)quinolin-8-ol (29): white solid, yield: 43% (123 mg), m.p.: 144–146 °C, C24H17F6N3O; 1H-NMR (500 MHz, DMSO) δ 10.20 (s, 1H), 8.84 (bs, 1H), 8.28 (d, J = 8.1 Hz, 1H), 8.03 (s, 2H), 7.92 (s, 1H), 7.69 (d, J = 8.4 Hz, 1H), 7.58–7.48 (m, 2H), 7.43 (d, J = 8.4 Hz, 1H), 7.29 (t, J = 7.6 Hz, 1H), 6.98 (d, J = 8.3 Hz, 1H), 6.48 (d, J = 8.1 Hz, 1H), 6.38 (d, J = 6.9 Hz, 1H), 2.19 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 157.12 (s), 155.69 (s), 149.81 (s), 148.56 (s), 147.44 (s), 138.11 (s), 137.44 (s), 136.13 (s), 130.08 (q, J = 32.3 Hz), 127.78 (s), 127.68 (s), 126.08 (s), 124.14 (s), 123.39 (q, J = 272.5 Hz), 122.01 (s), 120.48 (s), 117.87 (s), 111.83 (s), 105.67 (s), 51.55 (s), 24.13 (s); HRMS (ESI) m/z calcd. for C24H17F6N3O [M + H]+: 478.1349, found: 478.1353.
7-((4-Fluoro-3-(trifluoromethyl)phenyl)(6-methylpyridin-2-ylamino)methyl)quinolin-8-ol (30): white solid, yield: 90% (231 mg), m.p.: 148–151 °C, C23H17F4N3O; 1H-NMR (500 MHz, DMSO) δ 10.10 (s, 1H), 8.83 (s, 1H), 8.27 (d, J = 8.1 Hz, 1H), 7.73 (d, J = 6.2 Hz, 1H), 7.65 (d, J = 8.7 Hz, 2H), 7.52 (dd, J = 8.0, 3.9 Hz, 1H), 7.45–7.36 (m, 3H), 7.27 (t, J = 7.7 Hz, 1H), 6.87 (d, J = 8.5 Hz, 1H), 6.44 (t, J = 9.4 Hz, 1H), 6.34 (t, J = 15.0 Hz, 1H), 2.19 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 159.53–156.77 (m), 157.71 (s), 156.16 (s), 150.13 (s), 148.90 (s), 141.39 (d, J = 3.4 Hz), 138.55 (s), 137.76 (d, J = 13.0 Hz), 136.55 (s), 134.27 (d, J = 8.5 Hz), 129.47–121.35 (m), 128.10(s), 126.68 (s), 125.77 (q, J = 4.7 Hz), 125.26 (s), 122.31 (s), 118.12 (s), 117.48 (d, J = 20.4 Hz), 116.78–116.78 (m), 112.03 (s), 105.88 (s), 51.42 (s), 24.65 (s); HRMS (ESI) m/z calcd. for C23H17F4N3O [M + H]+: 428.1381, found: 428.1385.
7-((2,4-bis(Trifluoromethyl)phenyl)(6-methylpyridin-2-ylamino)methyl)quinolin-8-ol (31): light rose solid, yield: 22% (63 mg), m.p.: decomposed 230 °C, C24H17F6N3O; 1H-NMR (500 MHz, DMSO) δ 9.83 (s, 1H), 8.87 (d, J = 2.9 Hz, 1H), 8.33 (d, J = 8.0 Hz, 1H), 8.16 (d, J = 8.2 Hz, 1H), 8.11 (d, J = 8.2 Hz, 1H), 8.01 (s, 1H), 7.63 (dd, J = 8.6, 4.1 Hz, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.36 (d, J = 7.6 Hz, 1H), 7.25 (t, J = 7.7 Hz, 1H), 6.92 (d, J = 8.0 Hz, 1H), 6.63 (d, J = 8.0 Hz, 1H), 6.37 (d, J = 7.2 Hz, 1H), 2.17 (s, 3H); HRMS (ESI) m/z calcd. for C24H17F6N3O [M + H]+: 478.1349, found: 478.1356.
7-((3,4-Difluorophenyl)(6-methylpyridin-2-ylamino)methyl)quinolin-8-ol (32): white solid, yield: 53% (120 mg), m.p.: 175–178 °C, C22H17F2N3O; 1H-NMR (500 MHz, DMSO) δ 10.10 (s, 1H), 8.86 (dd, J = 4.0, 1.2 Hz, 1H), 8.30 (dd, J = 8.2, 1.1 Hz, 1H), 7.63 (d, J = 8.5 Hz, 1H), 7.55 (dd, J = 8.3, 4.2 Hz, 1H), 7.42 (d, J = 8.6 Hz, 1H), 7.40–7.27 (m, 4 H), 7.24–7.15 (m, 1H), 6.83 (d, J = 8.8 Hz, 1H), 6.46 (d, J = 8.3 Hz, 1H), 6.38 (d, J = 7.2 Hz, 1H), 2.26 (d, J = 40.6 Hz, 3H); 13C-NMR (126 MHz, CDCl3) δ 157.32 (s), 155.72 (s), 149.61 (s), 149.19 (dd, J = 245.7, 13.0 Hz), 148.38 (s), 148.09 (dd, J = 244.0, 13.1 Hz), 141.68 (s), 138.14 (s), 137.30 (s), 136.07 (s),127.59 (s), 126.43 (s), 125.08 (s), 123.70 (s), 121.80 (s), 117.58 (s), 117.16 (d, J = 16.9 Hz), 115.74 (d, J = 17.3 Hz), 111.50 (s), 105.33 (s), 50.96 (s), 24.22 (s); HRMS (ESI) m/z calcd. for C22H17F2N3O [M + H]+: 378.1412, found: 378.1413.
7-((Pyrimidin-2-ylamino)(4-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (33): white solid, yield: 32% (76 mg), m.p.: 160–163 °C, C21H15F3N4O; 1H-NMR (500 MHz, DMSO) δ 10.10 (s, 1H), 8.83 (d, J = 2.3 Hz, 1H), 8.33–8.23 (m, 3H), 8.18 (d, J = 9.1 Hz, 1H), 7.70 (d, J = 8.5 Hz, 1H), 7.64 (d, J = 7.9 Hz, 2H), 7.57 (d, J = 7.8 Hz, 2H), 7.52 (dd, J = 8.0, 3.9 Hz, 1H), 7.39 (d, J = 8.5 Hz, 1H), 7.04 (d, J = 9.1 Hz, 1H), 6.60 (t, J = 4.3 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 162.48 (s), 158.95 (s), 150.50 (s), 149.22 (s), 148.76 (s), 138.91 (s), 136.90 (s), 128.58 (s), 128.51 (s), 128.14 (q, J = 31.7 Hz), 127.53 (s), 126.01 (q, J = 3.7 Hz), 125.23 (s), 125.17 (q, J = 272.1 Hz), 122.68 (s), 118.42 (s), 111.75 (s), 52.59 (s); HRMS (ESI) m/z calcd. for C21H15F3N4O [M + H]+: 397.1271, found: 397.1273.
7-((4-Methylpyrimidin-2-ylamino)(4-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (34): white solid, yield: 46% (113 mg), m.p.: 147–149 °C, C22H17F3N4O; 1H-NMR (500 MHz, DMSO) δ 10.12 (s, 1H), 8.86 (dd, J = 4.1, 1.5 Hz, 1H), 8.31 (dd, J = 8.3, 1.3 Hz, 1H), 8.16 (d, J = 5.0 Hz, 1H), 8.11 (d, J = 9.4 Hz, 1H), 7.75 (d, J = 8.6 Hz, 1H), 7.66 (d, J = 8.3 Hz, 2H), 7.60 (d, J = 8.2 Hz, 2H), 7.55 (dd, J = 8.3, 4.2 Hz, 1H), 7.42 (d, J = 8.6 Hz, 1H), 7.09 (d, J = 9.4 Hz, 1H), 6.52 (d, J = 5.0 Hz, 1H), 2.26 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 167.94 (s), 162.02 (s), 158.11 (s), 150.04 (s), 148.83 (s), 148.65 (s), 138.53 (s), 136.53 (s), 128.15 (s), 128.10 (s), 127.67 (q, J = 31.5 Hz), 127.22 (s), 125.61 (q, J = 3.5 Hz), 125.01 (s), 124.81 (q, J = 271.6 Hz), 122.29 (s), 118.03 (s), 110.84 (s), 52.09 (s), 24.11 (s).
7-((4-Methylpyrimidin-2-ylamino)(4-nitrophenyl)methyl)quinolin-8-ol (35): white solid, yield: 19% (44 mg), m.p.: 136–138 °C, C21H17N5O3; 1H-NMR (500 MHz, DMSO) δ 10.15 (s, 1H), 8.84 (d, J = 2.5 Hz, 1H), 8.28 (d, J = 7.9 Hz, 1H), 8.18–8.09 (m, 4H), 7.71 (d, J = 8.5 Hz, 1H), 7.62 (d, J = 8.4 Hz, 2H), 7.53 (dd, J = 8.1, 4.0 Hz, 1H), 7.40 (d, J = 8.5 Hz, 1H), 7.09 (d, J = 9.2 Hz, 1H), 6.51 (d, J = 4.8 Hz, 1H), 2.24 (s, 3 H); 13C-NMR (126 MHz, CDCl3) δ 168.00 (s), 161.51 (s), 158.09 (s), 151.33 (s), 149.69 (s), 148.45 (s), 146.26 (s), 138.10 (s), 136.10 (s), 128.10 (s), 127.75 (s), 126.73 (s), 124.09 (s), 123.53 (s), 121.94 (s), 117.68 (s), 110.53 (s), 51.69 (s), 23.69 (s); HRMS (ESI) m/z calcd. for C21H17N5O3 [M + H]+: 388.1404, found: 388.1409.
7-((4-Methylpyrimidin-2-ylamino)(4-(pentafluorothio)phenyl)methyl)quinolin-8-ol (36): white solid, yield: 68% (191 mg), m.p.: 164–165 °C, C21H17F5N4OS; 1H-NMR (500 MHz, DMSO) δ 10.16 (s, 1H), 8.87 (dd, J = 4.1, 1.5 Hz, 1H), 8.31 (dd, J = 8.3, 1.5 Hz, 1H), 8.17 (d, J = 5.0 Hz, 1H), 8.12 (d, J = 9.4 Hz, 1H), 7.84 (d, J = 8.9 Hz, 2H), 7.75 (d, J = 8.6 Hz, 1H), 7.59 (d, J = 8.5 Hz, 2H), 7.55 (dt, J = 12.4, 6.2 Hz, 1H), 7.43 (d, J = 8.6 Hz, 1H), 7.07 (d, J = 9.4 Hz, 1H), 6.52 (t, J = 6.2 Hz, 1H), 2.26 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 168.2–167.74 (m), 161.98 (s), 158.28–157.83 (m), 152.05–151.32 (m), 150.08 (s), 148.86 (s), 148.60 (s), 138.53 (s), 136.54 (s), 128.25(s), 128.16 (s), 127.13 (s), 126.36–126.19 (m), 124.73 (s), 122.34 (s), 118.09 (s), 110.92 (s), 51.90 (s), 24.11 (s); HRMS (ESI) m/z calcd. for C21H17F5N4OS [M + H]+: 469.1116, found: 469.1118.
7-((4-Fluorophenyl)(4-methylpyrimidin-2-ylamino)methyl)quinolin-8-ol (37): white solid, yield: 16% (35 mg), m.p.: 156–158 °C, C21H17FN4O; 1H-NMR (500 MHz, DMSO) δ 9.97 (s, 1H), 8.82 (d, J = 2.8 Hz, 1H), 8.27 (d, J = 8.2 Hz, 1H), 8.12 (d, J = 4.9 Hz, 1H), 7.98 (d, J = 9.5 Hz, 1H), 7.74 (d, J = 8.5 Hz, 1H), 7.51 (dd, J = 8.2, 4.1 Hz, 1H), 7.42–7.31 (m, 3H), 7.12–7.03 (m, 2H), 6.98 (t, J = 12.1 Hz, 1H), 6.47 (d, J = 4.9 Hz, 1H), 2.22 (s, H); 13C-NMR (126 MHz, CDCl3) δ 167.90 (s), 161.58 (s), 158.06 (s), 160.95 (d, J = 242.0 Hz), 149.37 (s), 148.30 (s), 139.53 (s), 138.07 (s), 136.05 (s), 128.87 (d, J = 8.1 Hz), 127.51 (s), 126.74 (s), 125.34 (s), 121.71 (s), 117.43 (s), 114.85 (d, J = 21.2 Hz), 110.20 (s), 51.25 (s), 23.63 (s); HRMS (ESI) m/z calcd. for C21H17FN4O [M + H]+: 361.1459, found: 361.1460.
7-((4-Iodophenyl)(4-methylpyrimidin-2-ylamino)methyl)quinolin-8-ol (38): white solid, yield: 18% (51 mg), m.p.: 139–142 °C, C21H17IN4O; 1H-NMR (500 MHz, DMSO) δ 9.99 (s, 1 H), 8.82 (s, 1 H), 8.27 (d, J = 8.2 Hz, 1 H), 8.12 (d, J = 4.3 Hz, 1 H), 7.96 (d, J = 9.3 Hz, 1 H), 7.70 (d, J = 8.4 Hz, 1 H), 7.61 (d, J = 7.8 Hz, 2 H), 7.52 (dd, J = 7.8, 3.8 Hz, 1 H), 7.37 (d, J = 8.4 Hz, 1 H), 7.15 (d, J = 7.6 Hz, 2 H), 6.91 (d, J = 9.3 Hz, 1 H), 6.48 (d, J = 4.5 Hz, 1 H), 2.22 (s, 3 H) 13C-NMR (126 MHz, DMSO) δ 167.89 (s), 162.01 (s), 158.01 (s), 149.90 (s), 148.77 (s), 143.75 (s), 138.51 (s), 137.34 (s), 136.50 (s), 129.86 (s), 128.00 (s), 127.23 (s), 125.35 (s), 122.20 (s), 117.91 (s), 110.69 (s), 92.81 (s), 51.91 (s), 24.06 (s); HRMS (ESI) m/z calcd. for C21H17IN4O [M + H]+: 469.0520, found: 469.0525.
7-((4-Bromophenyl)(4-methylpyrimidin-2-ylamino)methyl)quinolin-8-ol (39): ivory-white solid, yield: 29% (73 mg), m.p.: 143–145 °C, C21H17BrN4O; 1H-NMR (500 MHz, DMSO) δ 10.00 (s, 1H), 8.82 (s, 1H), 8.27 (d, J = 7.9 Hz, 1H), 8.12 (d, J = 4.5 Hz, 1H), 7.98 (d, J = 9.2 Hz, 1H), 7.71 (d, J = 8.5 Hz, 1H), 7.52 (dd, J = 7.8, 3.8 Hz, 1H), 7.44 (d, J = 8.1 Hz, 2H), 7.38 (d, J = 8.4 Hz, 1H), 7.30 (d, J = 8.0 Hz, 2H), 6.94 (d, J = 9.3 Hz, 1H), 6.48 (d, J = 4.6 Hz, 1H), 2.22 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 167.85 (s), 161.57 (s), 149.46 (s), 148.34 (s), 142.86 (s), 138.07 (s), 136.06 (s), 131.04 (s), 129.24 (s), 127.57 (s), 126.77 (s), 124.91 (s), 121.77 (s), 119.59 (s), 117.48 (s), 110.27 (s), 51.39 (s), 24.39 (s); HRMS (ESI) m/z calcd. for C21H17BrN4O [M + H]+: 421.0659, found: 421.0664.
7-((4-Chlorophenyl)(4-methylpyrimidin-2-ylamino)methyl)quinolin-8-ol (40): white solid, yield: 40% (90 mg), m.p.: 139–141 °C, C21H17ClN4O; 1H-NMR (500 MHz, DMSO) δ 10.04 (s, 1H), 8.86 (dd, J = 3.9, 1.3 Hz, 1H), 8.30 (d, J = 8.3 Hz, 1H), 8.15 (d, J = 4.9 Hz, 1H), 8.02 (d, J = 9.4 Hz, 1H), 7.75 (d, J = 8.5 Hz, 1H), 7.55 (dd, J = 8.2, 4.1 Hz, 1H), 7.44–7.37 (m, 3H), 7.34 (d, J = 8.5 Hz, 2H), 6.98 (d, J = 9.4 Hz, 1H), 6.51 (d, J = 5.0 Hz, 1H), 2.25 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 167.90 (s), 162.01 (s), 158.05 (s), 149.90 (s), 148.78 (s), 142.86 (s), 138.51 (s), 136.51 (s), 131.53 (s), 129.29 (s), 128.57 (s), 128.00 (s), 127.19 (s), 125.43 (s), 122.21 (s), 117.92 (s), 110.70 (s), 51.74 (s), 24.09 (s); HRMS (ESI) m/z calcd. for C21H17ClN4O [M + H]+: 377.1164, found: 377.1167.
7-((4-Methylpyrimidin-2-ylamino)(3-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (41): white solid, yield: 31% (76 mg), m.p.: 160–163 °C, C22H17F3N4O; 1H-NMR (500 MHz, DMSO) δ 10.08 (s, 1H), 8.91–8.73 (m, 1H), 8.27 (d, J = 8.2 Hz, 1H), 8.21–8.06 (m, 2H), 7.81–7.70 (m, 2H), 7.67 (d, J = 7.0 Hz, 1H), 7.59–7.44 (m, 3H), 7.40 (d, J = 8.5 Hz, 1H), 7.07 (d, J = 9.4 Hz, 1H), 6.49 (d, J= 4.7 Hz, 1H), 2.23 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 168.00 (s), 161.96 (s), 149.96 (s), 148.87 (s), 145.32 (s), 138.50 (s), 136.54 (s), 131.70 (s), 129.79 (s), 129.36 (q, J = 31.4 Hz), 128.07 (s), 126.93 (s), 125.18 (s), 124.74 (q, J = 272.43 Hz), 123.87 (q, J = 3.6 Hz), 123.68 (q, J = 3.7 Hz), 122.30 (s), 118.09 (s), 110.85 (s), 51.95 (s), 24.16 (s); HRMS (ESI) m/z calcd. for C22H17F3N4O [M + H]+: 411.1427, found: 411.1428.
7-((3,5-bis(Trifluoromethyl)phenyl)(4-methylpyrimidin-2-ylamino)methyl)quinolin-8-ol (42): white solid, yield: 59% (169 mg), m.p.: 128–130 °C, C23H16F6N4O; 1H-NMR (500 MHz, DMSO) δ 10.24 (s, 1H), 8.84 (d, J = 2.8 Hz, 1H), 8.36–8.23 (m, 2H), 8.15 (d, J = 4.8 Hz, 1H), 8.08 (s, 2H), 7.93 (s, 1H), 7.77 (d, J = 8.5 Hz, 1H), 7.53 (dd, J = 8.2, 4.1 Hz, 1H), 7.42 (d, J = 8.5 Hz, 1H), 7.13 (d, J = 9.4 Hz, 1H), 6.52 (d, J = 4.9 Hz, 1H), 2.24 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 167.63 (s), 161.38 (s), 157.64 (s), 149.65 (s), 148.57 (s), 146.83 (s), 138.06 (s), 136.14 (s), 130.19 (q, J = 32.9 Hz), 127.80 (s), 127.63 (s), 125.96 (s), 123.97 (s), 123.35 (q, J = 272.8 Hz), 122.02 (s), 120.80–120.55 (m), 117.93 (s), 110.72 (s), 51.60 (s), 23.62 (s); HRMS (ESI) m/z calcd. for C23H16F6N4O [M + H]+: 479.1301, found: 479.1304.
7-((2,4-bis(Trifluoromethyl)phenyl)(4-methylpyrimidin-2-ylamino)methyl)quinolin-8-ol (43): white solid, yield: 16% (46 mg), m.p.: 199–201 °C, C23H16F6N4O; 1H-NMR (500 MHz, DMSO) δ 9.97 (s, 1H), 8.81 (d, J = 2.4 Hz, 1H), 8.28 (d, J = 7.8 Hz, 1H), 8.08 (s, 1H), 8.03 (d, J = 6.0 Hz, 1H), 7.99–7.95 (m, 2H), 7.80 (bs, 1H), 7.52 (dd, J = 8.1, 3.9 Hz, 1H), 7.33 (dd, J = 11.7, 9.2 Hz, 2H), 7.21 (d, J = 4.7 Hz, 1H), 6.47 (d, J = 4.7 Hz, 1H), 2.19 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 169.04 (s), 161.45 (s), 158.49 (s), 150.79 (s), 148.79 (s), 138.45 (s), 136.53 (s), 131.63 (s), 129.90–129.72 (m), 128.66 (q, J = 31.4 Hz), 128.55 (q, J = 32.7 Hz), 128.26 (s), 127.05–126.81 (m), 124.01 (q, J = 275.4 Hz), 123.97 (q, J = 272.2 Hz), 123.57–123.27 (m), 122.34 (s), 117.30 (s), 116.90 (s), 110.80 (s), 49.76 (s), 24.05 (s); HRMS (ESI) m/z calcd. for C23H16F6N4O [M + H]+: 479.1301, found: 479.1307.
7-((4-Fluoro-3-(trifluoromethyl)phenyl)(4-methylpyrimidin-2-ylamino)methyl)quinolin-8-ol (44): white solid, yield: 25% (64 mg), m.p.: 89–92 °C, C22H16F4N4O; 1H-NMR (500 MHz, DMSO) δ 10.10 (s, 1H), 8.82 (bs, 1H), 8.26 (d, J = 5.6 Hz, 1H), 8.19–8.08 (m, 2H), 7.83–7.64 (m, 3H), 7.51 (dd, J = 5.6, 3.6 Hz, 1 H), 7.43–7.31 (m, 2H), 7.01 (d, J = 7.7 Hz, 1H), 6.49 (bs, 1H), 2.22 (s, 3H); 13C-NMR (126 MHz, CDCl3) δ 167.49 (bs), 161.44 (s), 157.95–157.18 (m), 157.63 (d, J = 252.6 Hz), 149.50 (s), 148.44 (s), 140.49 (s), 138.05 (s), 136.09 (s), 133.81 (d, J = 8.5 Hz), 127.67 (s), 126.23 (s), 125.27 (d, J = 3.4 Hz), 124.56 (s), 123.77 (s), 121.87 (s), 117.70 (s), 117.08 (d, J = 20.5 Hz), 110.49 (s), 51.15 (s), 23.62 (s); HRMS (ESI) m/z calcd. for C22H16F4N4O [M + H]+: 429.1333, found: 429.1337.
7-((3-Fluoro-5-(trifluoromethyl)phenyl)(4-methylpyrimidin-2-ylamino)methyl)quinolin-8-ol (45): ivory-white solid, yield: 15% (39 mg), m.p.: decomposed 92 °C, C22H16F4N4O; 1H-NMR (500 MHz, DMSO) δ 10.22 (s, 1H), 8.87 (d, J = 3.0 Hz, 1H), 8.31 (d, J = 8.1 Hz, 1H), 8.21 (d, J = 9.5 Hz, 1H), 8.18 (d, J = 4.8 Hz, 1H), 7.77 (d, J = 8.5 Hz, 1H), 7.65 (s, 1H), 7.58–7.52 (m, 3H), 7.44 (d, J = 8.5 Hz, 1H), 7.09 (d, J = 9.4 Hz, 1H), 6.55 (d, J = 4.9 Hz, 1H), 2.27 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 162.39 (d, J = 246.5 Hz), 161.86 (s), 150.03 (s), 148.97 (s), 148.74 (d, J = 6.8 Hz), 138.51 (s), 136.57 (s), 131.72–131.06 (m), 128.19 (s), 126.64 (s), 124.61 (s), 123.80 (dq, J = 272.7, 4.2 Hz), 122.42 (s), 120.28–119.83 (m), 118.40 (d, J = 22.0 Hz), 118.26 (s), 111.74–111.36 (m), 111.08 (s), 51.86 (s), 24.10 (s); HRMS (ESI) m/z calcd. for C22H16F4N4O [M + H]+: 429.1333, found: 429.1336.
7-((5-Bromopyrimidin-2-ylamino)(4-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (46): white solid, yield: 15% (43 mg), m.p.: 146–148 °C, C21H14BrF3N4O; 1H-NMR (500 MHz, DMSO) δ 10.12 (s, 1H), 8.84 (d, J = 2.2 Hz, 1H), 8.50 (d, J = 8.9 Hz, 1H), 8.41 (s, 2H), 8.28 (d, J = 7.9 Hz, 1H), 7.70–7.61 (m, 3H), 7.59–7.49 (m, 3H), 7.39 (d, J = 8.4 Hz, 1H), 6.96 (d, J = 8.8 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 160.07 (s), 149.81 (s), 148.45 (s), 147.38 (s), 138.07 (s), 136.11 (s), 127.82 (s), 127.75 (s), 127.19 (q, J = 31.8 Hz), 126.52 (s), 125.26 (q, J = 4.0 Hz), 124.77 (q, J = 272.0 Hz), 123.91 (s), 121.93 (s), 117.62 (s), 106.09 (s), 52.12 (s); HRMS (ESI) m/z calcd. for C21H14BrF3N4O [M + H]+: 475.0376, found: 475.0384.
7-((2-Hydroxyphenyl)(4-methylpyrimidin-2-ylamino)methyl)quinolin-8-ol (47): caesious solid, yield: 15% (32 mg), m.p.: decomposed 179 °C, C21H18N4O2; 1H-NMR (500 MHz, DMSO) δ 9.81 (s, 1 H), 9.49 (s, 1 H), 8.82 (d, J = 2.9 Hz, 1 H), 8.28 (d, J = 7.7 Hz, 1 H), 8.11 (d, J = 4.9 Hz, 1H), 7.59 (d, J = 8.5 Hz, 1H), 7.51 (dd, J = 8.3, 4.2 Hz, 1H), 7.49 (d, J = 9.2 Hz, 1H), 7.34 (d, J = 8.5 Hz, 1H), 7.24 (d, J = 7.2 Hz, 1H), 7.04 (t, J = 7.1 Hz, 1H), 6.99 (d, J = 9.0 Hz, 1H), 6.77 (d, J = 7.9 Hz, 1H), 6.72 (t, J = 7.4 Hz, 1H), 6.46 (d, J = 4.9 Hz, 1H), 2.23 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 167.64 (s), 161.85 (s), 157.94 (s), 155.36 (s), 150.18 (s), 148.47 (s), 138.47(s), 136.39 (s), 129.22 (s), 128.76 (s), 128.12 (s), 127.85 (s), 127.80 (s), 125.59 (s), 121.89 (s), 119.01 (s), 116.93 (s), 115.63 (s), 110.29 (s), 48.89 (s), 24.13 (s); HRMS (ESI) m/z calcd. for C21H18N4O2 [M + H]+: 359.1503, found: 359.1509.
2-Methyl-7-((4-methylpyrimidin-2-ylamino)(4-(trifluoromethyl)phenyl)methyl)quinolin-8-ol (48): white solid, yield: 25% (64 mg), m.p.: 123–125 °C, C23H19F3N4O; 1H-NMR (500 MHz, DMSO) δ 9.59 (s, 1H), 8.19–8.15 (m, 2H), 8.09 (d, J = 9.4 Hz, 1H), 7.69–7.64 (m, 3H), 7.59 (d, J = 8.3 Hz, 2H), 7.42 (d, J = 8.4 Hz, 1H), 7.36 (d, J = 8.6 Hz, 1H), 7.07 (d, J = 9.4 Hz, 1H), 6.52 (d, J = 5.0 Hz, 1H), 2.70 (s, 3H), 2.26 (s, 3H); 13C-NMR (126 MHz, DMSO) δ 167.96 (s), 162.04 (s), 158.23 (s), 157.51 (s), 149.14 (s), 148.73 (s), 137.80 (s), 136.56 (s), 128.09 (s), 127.52 (q, J = 31.6 Hz), 126.17 (s), 126.09 (s), 125.57 (q, J = 3.5 Hz), 124.81 (q, J = 271.9 Hz), 124.77 (s), 123.09 (s), 117.87 (s), 110.83 (s), 52.08 (s), 25.14 (s), 24.12 (s); HRMS (ESI) m/z calcd. for C23H19F3N4O [M + H]+: 425.1584, found: 425.1585.