Theoretical Study of the C2H5 + HO2 Reaction: Mechanism and Kinetics
Abstract
:1. Introduction
2. Calculation Methods
3. Results and Discussion
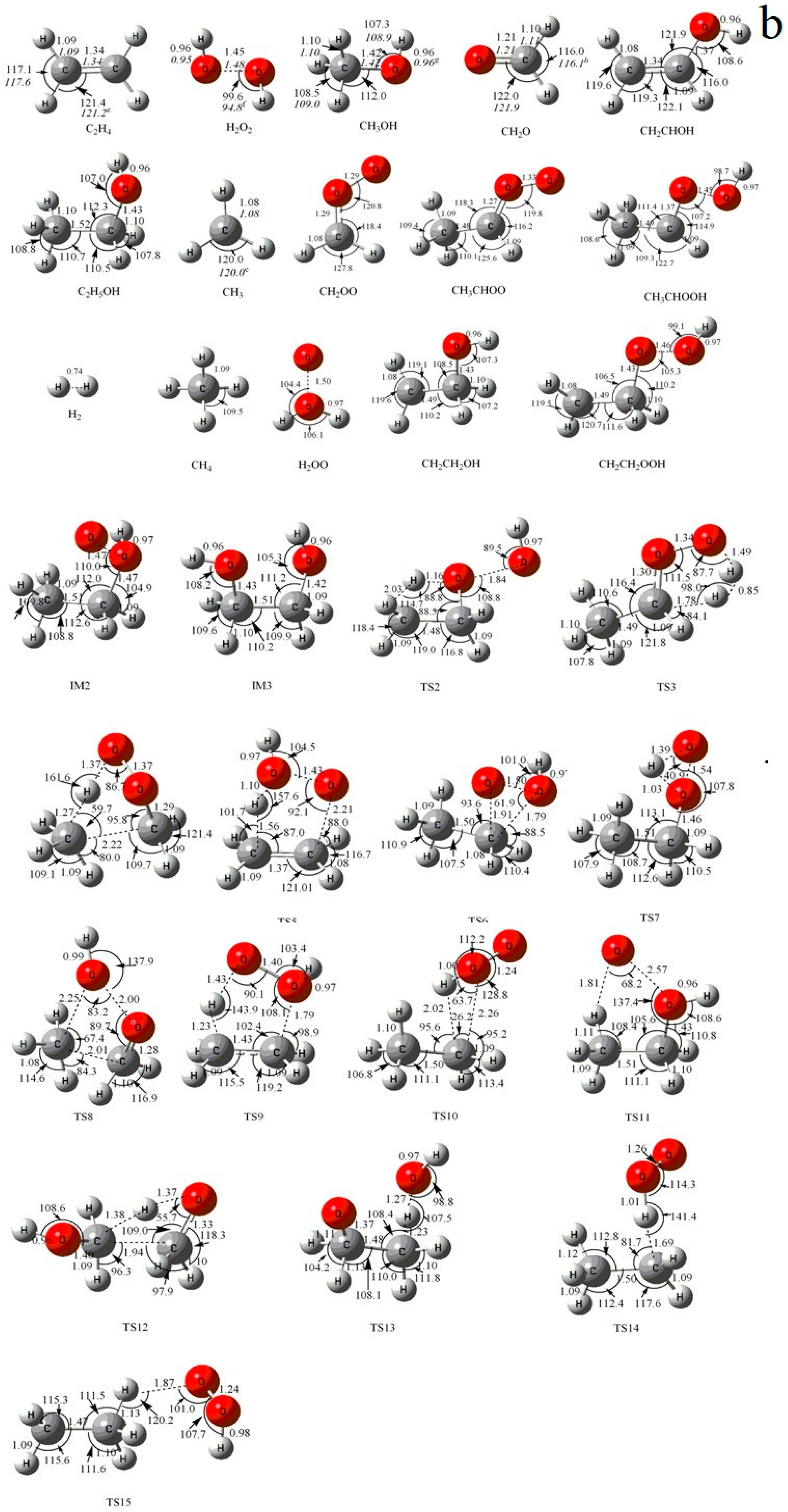
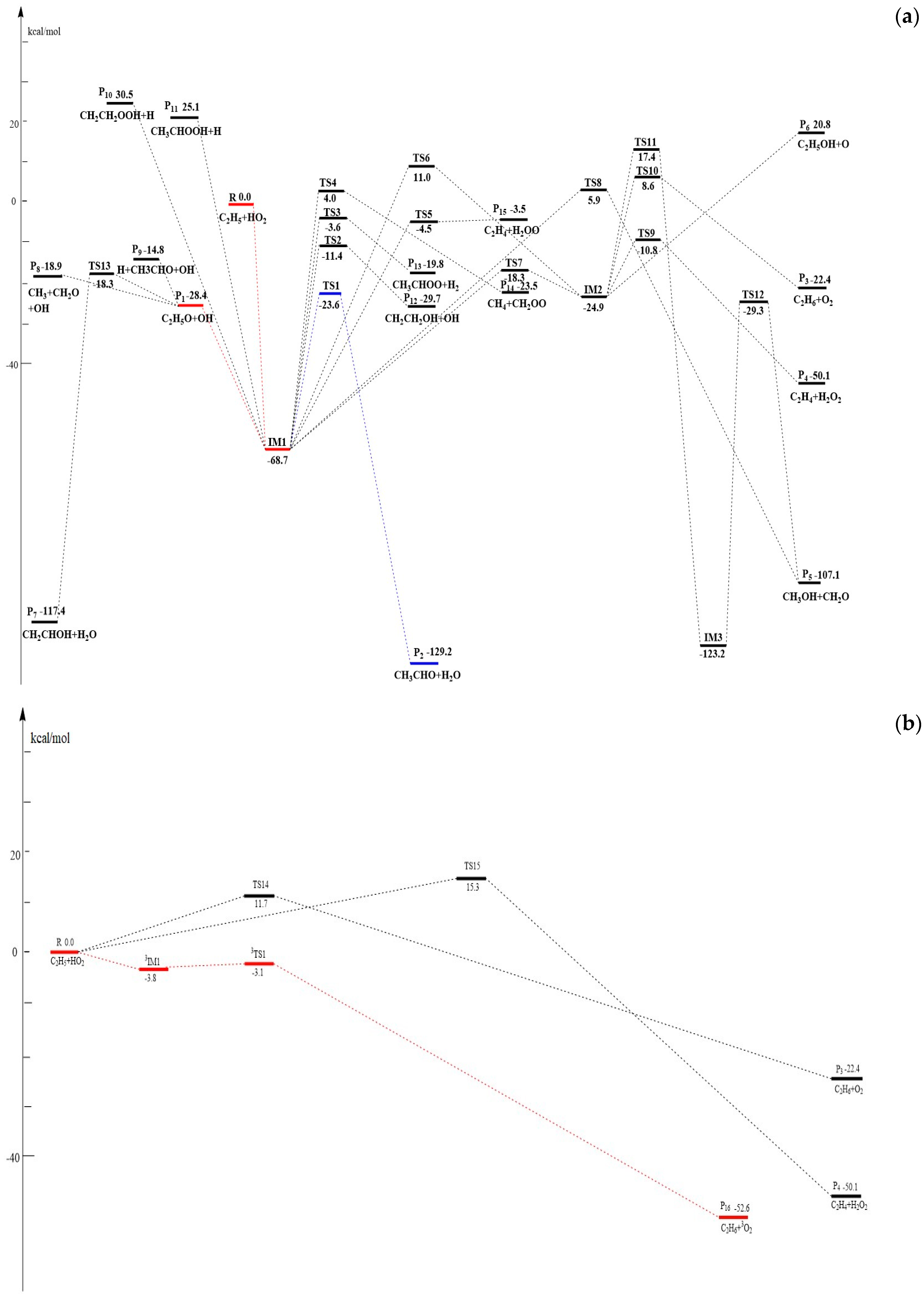
3.1. Potential Energy Surface and Reaction Mechanism
3.1.1. Association and Decomposition Channels
3.1.2. H-Abstraction Channels
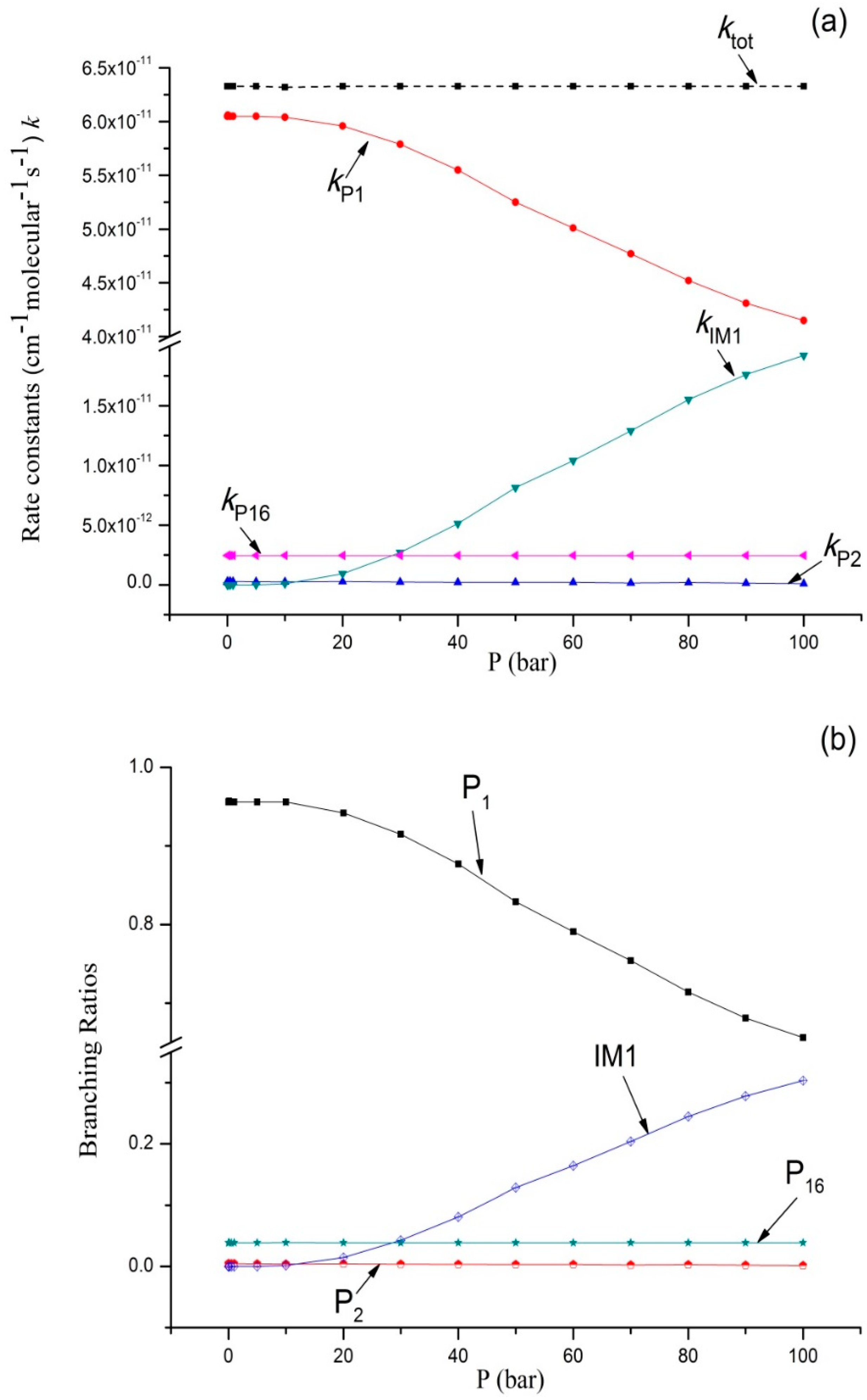
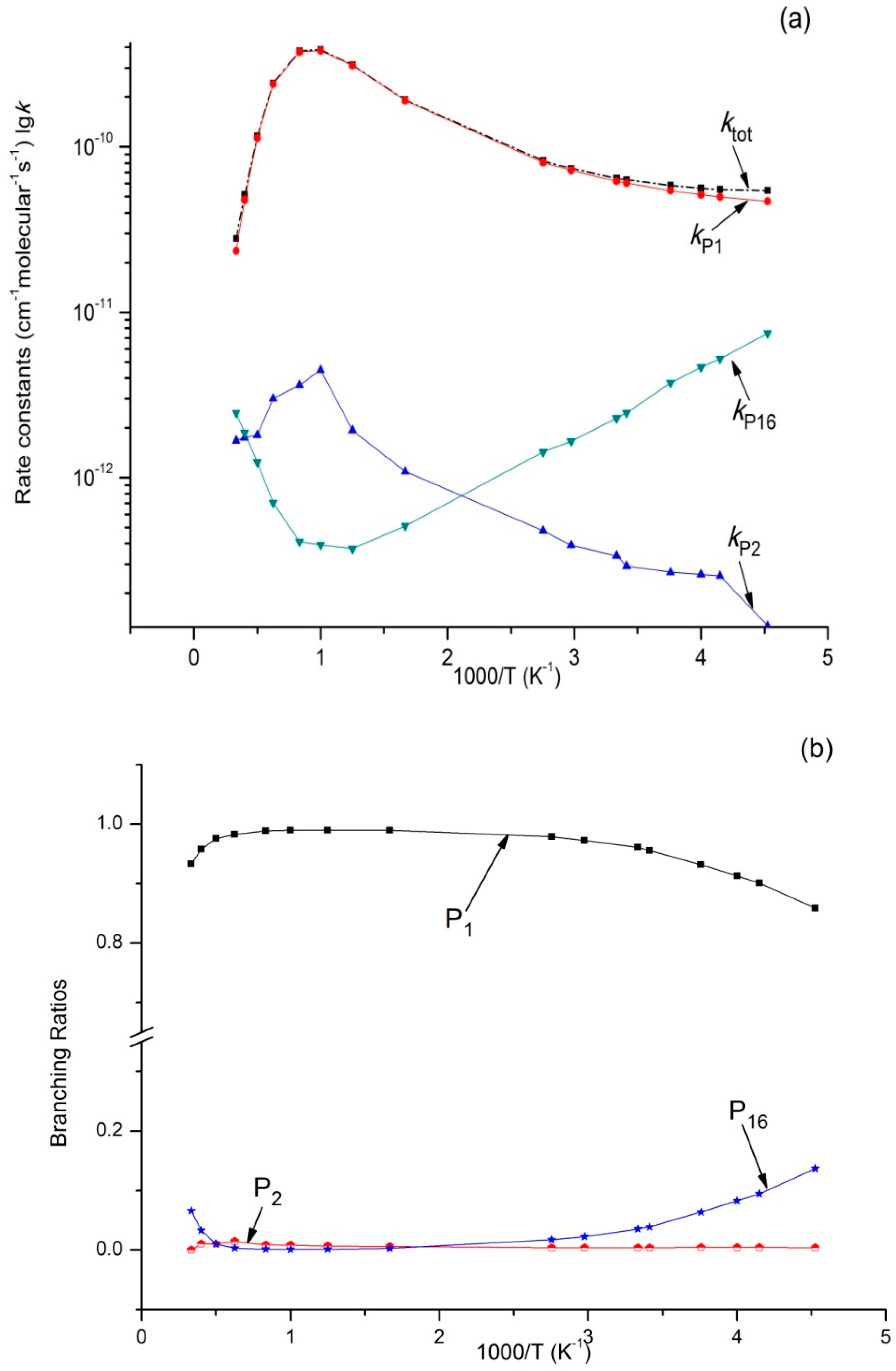
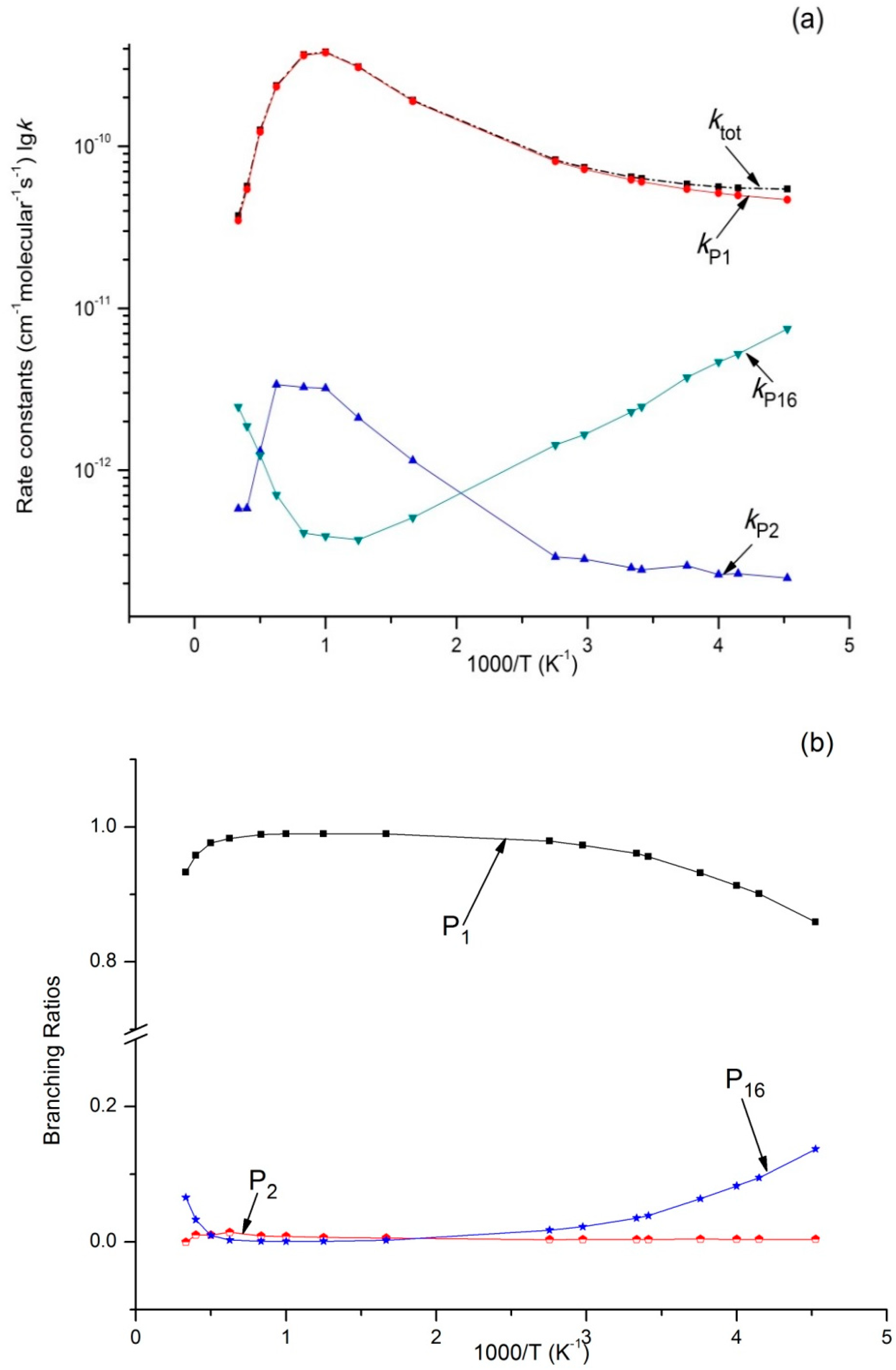
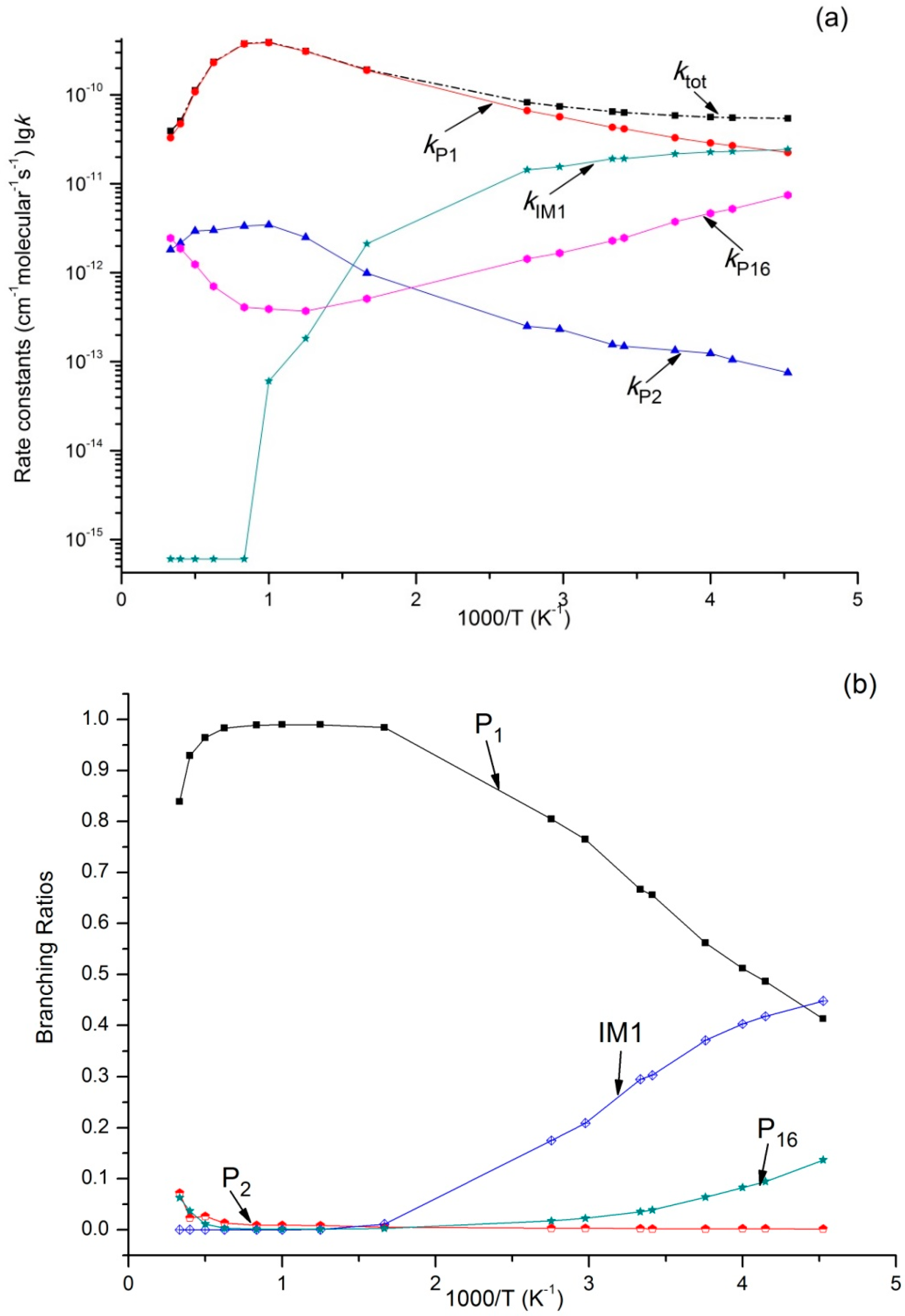
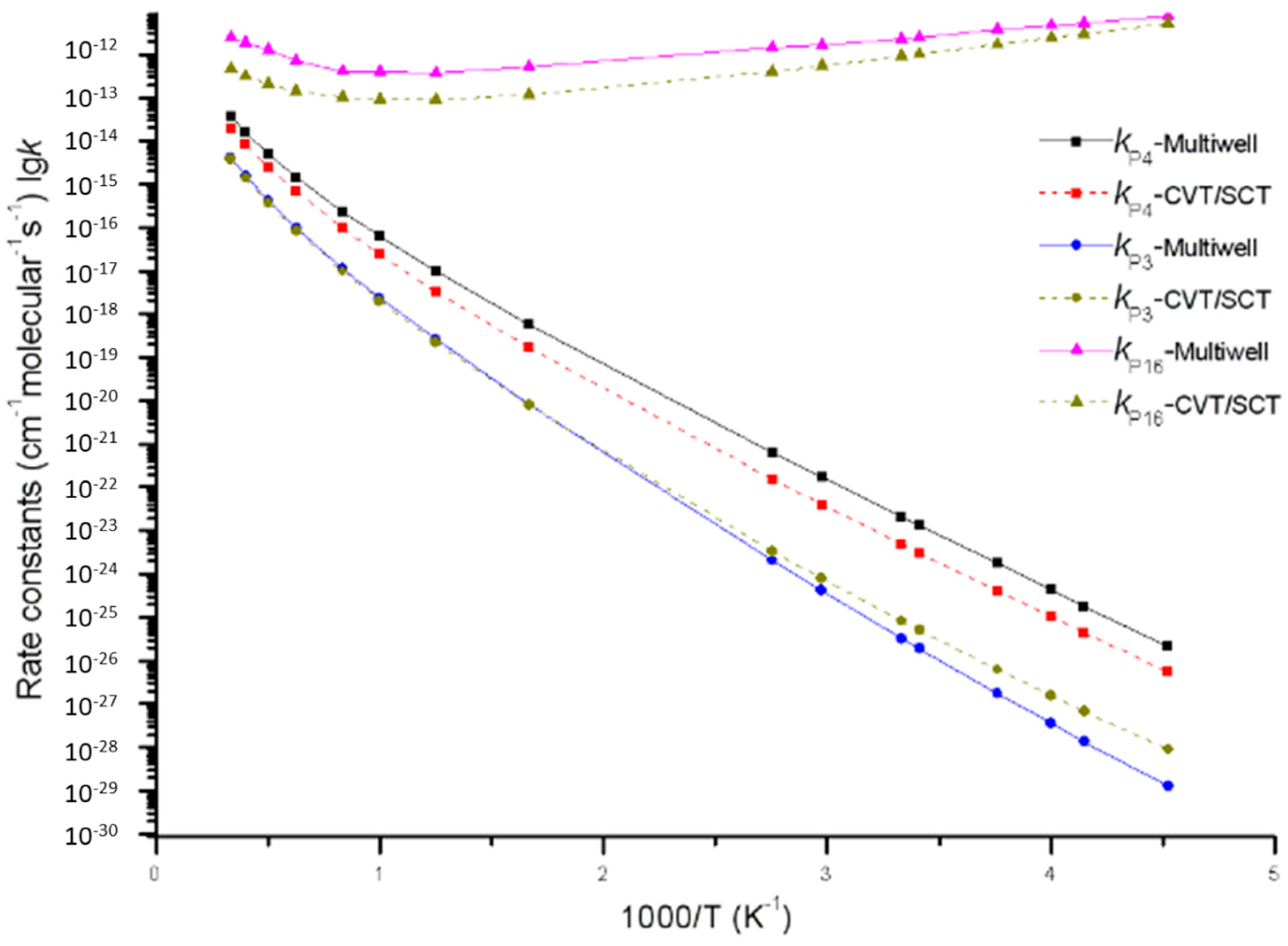
3.2. Kinetic Calculations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naik, C.V.; Dean, A.M. Detailed kinetic modeling of ethane oxidation. Combust. Flame 2006, 145, 16–37. [Google Scholar] [CrossRef]
- Kaiser, E.W. Mechanism of the reaction C2H5 + O2 from 298 To 680 K. J. Phys. Chem. A. 2002, 106, 1256–1265. [Google Scholar] [CrossRef]
- Ludwig, W.; Brandt, B.; Friedrichs, G.; Temps, F. Kinetics of the reaction C2H5 + HO2 by time-resolved mass spectrometry. J. Phys. Chem. A 2006, 110, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Stark, M.S. Addition of peroxyl radicals to alkenes and the reaction of oxygen with alkyl radicals. J. Am. Chem. Soc. 2000, 122, 4162–4170. [Google Scholar] [CrossRef]
- Platt, U.; Lebras, G.; Poulet, G.; Burrows, J.P.; Moortgat, G. Peroxy radicals from night-time reaction of NO3 with organic compounds. Nature 1990, 348, 147–149. [Google Scholar] [CrossRef]
- Lightfoot, P.D.; Cox, R.A.; Crowley, J.N.; Destriau, M.; Hayman, G.D.; Jenkin, M.E.; Moortgat, G.K.; Zabe, F. Organic peroxy radicals: Kinetics, spectroscopy and tropospheric chemistry. Atmos. Environ. Part A 1992, 26, 1805–1961. [Google Scholar] [CrossRef]
- Meloni, G.; Zou, P.; Klippenstein, S.J.; Ahmed, M.; Leone, S.R.; Taatjes, C.A.; Osborn, D.L. Energy-resolved photoionization of alkylperoxy radicals and the stability of their cations. J. Am. Chem. Soc. 2006, 128, 13559–13567. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.N.; OuYang, S.L.; Li, L. Theoretical study of ClOO + NO reaction: Mechanism and kinetics. Molecules 2017, 22, 2121. [Google Scholar] [CrossRef] [PubMed]
- Hrušák, J.; Friedrichs, H.; Schwarz, H.; Razafinjanahary, H.; Chermette, H. Electron affinity of hydrogen peroxide and the [H2, O2]•− potential energy surface. A comparative DFT and ab Initio study. J. Phys. Chem. 1996, 100, 100–110. [Google Scholar] [CrossRef]
- Tsang, W.; Hampson, R.F. Chemical kinetic data base for combustion chemistry. Part I. Methane and related compounds. J. Phys. Chem. Ref. Data 2009, 15, 1087–1279. [Google Scholar] [CrossRef]
- Dobis, O.; Benson, S. Reaction of the ethyl radical with oxygen at millitorr pressures at 243–368 K and a study of the Cl + HO2, ethyl + HO2, and HO2 + HO2 reactions. J. Am. Chem. Soc. 1993, 115, 8798–8809. [Google Scholar] [CrossRef]
- Holbrook, K.A.; Pilling, M.J.; Robertson, S.H. Unimolecular Reactions, 2nd ed.; John Wiley & Sons: Chichester, UK, 1996. [Google Scholar]
- Hehre, W.J.; Radom, L.; Schleyer, P.R.; Pople, J. AB INITIO Molecular Orbital Theory; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1986; Volume 9, pp. 399–406. [Google Scholar]
- Fukui, K. The path of chemical reactions—The IRC approach. Acc. Chem. Res. 1981, 14, 471–476. [Google Scholar] [CrossRef]
- Charles, D., Jr.; James, W.M., Jr.; Michael, P. Singlet biradicals as intermediates. Canonical variational transition-state theory results for trimethylene. J. Chem. Phys. 1988, 92, 4367–4371. [Google Scholar]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Frisch, M.J.; Truck, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.J.; Stratmann, R.E.; Burant, J.C.; et al. GAUSSIAN 03, Revision A1; Guassian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Holbrook, K.A.; Pilling, M.J.; Robertson, S.H. Unimolecular Reactions; Wiley: Chichester, UK, 1972; Volume 15, pp. 357–362. [Google Scholar]
- Barker, J.R. Multiple-Well, multiple-path unimolecular reaction systems. I. MultiWell computer program suite. Int. J. Chem. Kinet. 2001, 33, 232–245. [Google Scholar] [CrossRef]
- Barker, J.R.; Ortiz, N.F.; Preses, J.M.; Lohr, L.L.; Maranzana, A.; Stimac, P.J. MultiWell-2011 Software; University of Michigan: Ann Arbor, MI, USA, 2007; Available online: http://aoss.engin.umich.edu/multiwell/ (accessed on January 2011).
- Lubic, K.G.; Amano, T.; Uehara, H.; Kawaguchi, K.; Hirota, E. The V1 band of the DO2 radical by difference frequency laser and diode laser spectroscopy: The equilibrium sturcture of the hydroperoxyl radical. J. Chem. Phys. 1984, 81, 4826–4831. [Google Scholar] [CrossRef]
- Hellwege, K.H.; Hellwege, A.M. Structure data of free polyatomic molecules. In Landolt-Bornstein: Group II: Atomic and Molecular Physics; Springer Science & Business Media: Berlin, Germany, 1976; Volume 7. [Google Scholar]
- Hollenstien, H.; Gunthard, H.H. Solid State and gas infrared spectra and normal coordinate analysis of 5 isotopic species of acetaldehyde. Spec. Act. Part A 1971, 27A, 2027–2060. [Google Scholar] [CrossRef]
- Redington, R.L.; Olson, W.B.; Cross, P.C. Studies of hydrogen peroxide: The infrared spectrum and the internal rotation problem. J. Chem. Phys. 1962, 36, 1311–1326. [Google Scholar] [CrossRef]
- Venkateswarlu, P.; Gordy, W. Methyl alcohol II. molecular structure. J. Chem. Phys. 1955, 23, 1200–1202. [Google Scholar] [CrossRef]
- Gurvich, L.V.; Veyts, I.V.; Alcock, C.B. Thermodynamic Properties of Individual Substances, 4th ed.; Hemisphere Pub. Co.: New York, NY, USA, 1989. [Google Scholar]
- Hoy, A.R.; Bunker, P.R. A precise solution of the rotation beninding schrodinger equation for a triatomic molecule with application to the water molecule. J. Mol. Spectrosc. 1979, 74, 1–8. [Google Scholar] [CrossRef]
- Klippenstein, S.J. An efficient procedure for evaluating the number of available states within a variably defined reaction coordinate framework. J. Phys. Chem. 1994, 98, 11459–11464. [Google Scholar] [CrossRef]
- Klippenstein, S.J. A bond length reaction coordinate for unimolecular reactions. II. Microcanonical and canonical implementations with application to the dissociation of NCNO. J. Chem. Phys. 1991, 94, 6469–6482. [Google Scholar] [CrossRef]
- Werner, H.J.; Knowles, P.J. A second order multiconfiguration SCF procedure with optimum convergence. J. Chem. Phys. 1985, 82, 5053–5063. [Google Scholar] [CrossRef]
- Werner, H.J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Györffy, W.; Kats, D.; Korona, T.; Lindh, R.; et al. 2006 MOLPRO Quantum Chemistry Software, version 2006.1 a Package of ab Initio Programs. Available online: http://www.molpro.net (accessed on January 2011).
- Garrett, B.C.; Truhlar, D.G. Generalized transition state theory. Bond energy-bond order method for canonical variational calculations with application to hydrogen atom transfer reactions. J. Am. Chem. Soc. 1979, 101, 4534–4548. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G.; Grev, R.S. Improved treatment of threshold contributions in variational transition-state theory. J. Phys. Chem. 1980, 84, 1730–1748. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, N.-N.; Zhang, M.-Z.; Ou-Yang, S.-L.; Li, L. Theoretical Study of the C2H5 + HO2 Reaction: Mechanism and Kinetics. Molecules 2018, 23, 1919. https://doi.org/10.3390/molecules23081919
Wu N-N, Zhang M-Z, Ou-Yang S-L, Li L. Theoretical Study of the C2H5 + HO2 Reaction: Mechanism and Kinetics. Molecules. 2018; 23(8):1919. https://doi.org/10.3390/molecules23081919
Chicago/Turabian StyleWu, Nan-Nan, Ming-Zhe Zhang, Shun-Li Ou-Yang, and Liang Li. 2018. "Theoretical Study of the C2H5 + HO2 Reaction: Mechanism and Kinetics" Molecules 23, no. 8: 1919. https://doi.org/10.3390/molecules23081919




