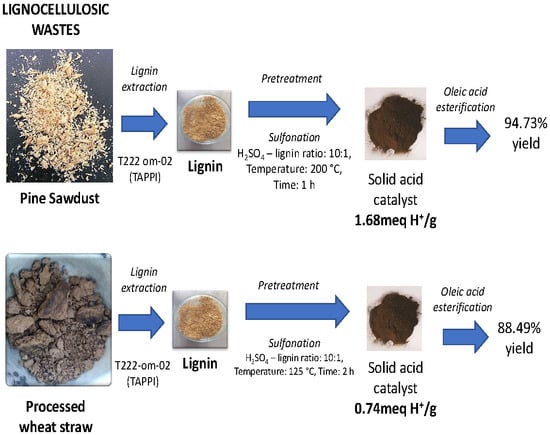
Optimization of Lignin-Based Biocatalyst Production from Pine Sawdust and Wheat Straw
Abstract
:1. Introduction
2. Results
2.1. Biocatalyst from Pine Sawdust
2.2. Biocatalyst from Wheat Straw
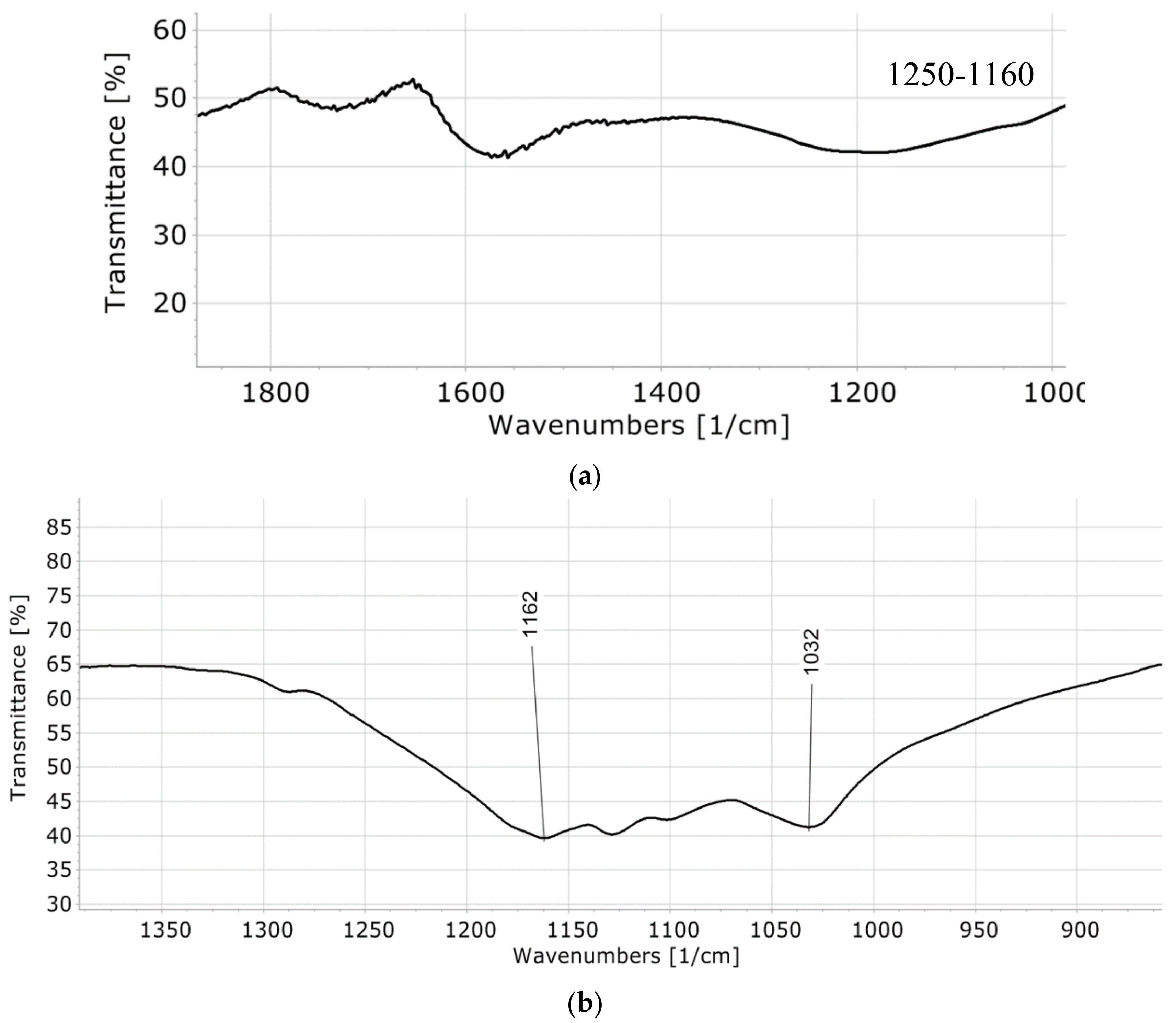
3. Discussion
4. Materials and Methods
4.1. Lignin Extraction
4.2. Optimization of Sulfonation Rections
4.3. Global Yield of Biocatalyst Synthesis
4.4. Activity Test of the Biocatalysts
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Farías-Sánchez, J.C.; López-Miranda, J.; Castro-Montoya, A.J.; Saucedo-Luna, J.; Carrillo-Parra, A.; López-Albarrán, P.; Pineda-Pimentel, M.G.; Rutiaga-Quiñones, J.G. Comparison of five pretreatments for the production of fermentable sugars obtained from Pinus Pseudostrobus L. Wood. EXCLI J. 2015, 14, 430–438. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Montero, G.; Coronado, M.; Torres, R.; Vázquez, A.M.; Ayala, J.R.; León, J.A.; Sagaste, C.A. Power generation estimation from wheat straw in Mexico. WIT Trans. Ecol Environ. 2015, 195, 101–110. [Google Scholar] [CrossRef]
- Chouchene, A.; Jeguireim, M.; Favre-Reguillon, A.; Trouvé, G.; Le Buzit, G.; Khiari, B.; Zagrouba, F. Energetic valorization of olive mill wastewater impregnated on low cost absorbent: Sawdust versus olive solid waste. Energy 2012, 39, 74–81. [Google Scholar] [CrossRef]
- Vázquez-Guerrero, A.; Alfaro-Cuevas-Villanueva, R.; Rutiaga-Quiñones, J.; Cortés-Martínez, R. Fluoride removal by aluminum-modified pine sawdust: Effect of competitive ions. Ecol. Eng. 2016, 94, 365–379. [Google Scholar] [CrossRef]
- Moset, V.; Neves Xavier, C.D.A.; Feng, L.; Wahid, R.; Bjarne Moller, H. Combined low thermal alkali addition and mechanical pre-treatment to improve biogas yield from wheat straw. J. Clean. Prod. 2017, 172, 1391–1398. [Google Scholar] [CrossRef]
- Wei, M.; Chen, L.; Feng, L.; Lian, X.M.; Chen, Z.Q.; Wei, S.J.; Yan, P.S. Energy and protein requirements for maintenance of Southern Yellow cattle fed a corn silage or straw-based diet. Livest. Sci. 2018, 207, 75–82. [Google Scholar] [CrossRef]
- Frankó, B.; Galbe, M.; Walberg, O. Bioethanol production from forestry residues: A comparative techno-economic analysis. Appl. Energy. 2016, 184, 727–736. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable resources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Liu, G.; Bang, J. Evaluation of electricity generation from lignin residue and biogas in cellulosic ethanol production. Bioresour. Technol. 2017, 243, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Fang, Z.; Smith, R.L.; Wu, Z.; Liu, M. Properties, chemical characteristics and application of lignin and its derivatives. In Production of Biofuels and Chemicals from Lignin; Fang, Z., Smith, R.L., Eds.; Springer: Singapore, 2016; p. 19. [Google Scholar]
- Pua, F.; Fang, Z.; Zakaria, S.; Guo, F.; Chia, C. Direct production of biodiesel from high-acid value Jatropha oil with solid acid catalyst derived from lignin. Biotechnol. Biofuels 2011, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Xiu, Z.; Liang, Z. Synthesis of biodiesel from acidified soybean soapstock using a lignin-derived carbonaceous catalyst. Appl. Energy 2012, 98, 47–52. [Google Scholar] [CrossRef]
- Hu, S.; Jiang, F.; Hsieh, Y. 1D lignin based solid acid catalyst for cellulose hydrolysis into glucose and nanocellulose. ACS Sustain. Chem. Eng. 2015, 3, 2566–25674. [Google Scholar] [CrossRef]
- Zhu, J.; Gan, L.; Li, B.; Yang, X. Synthesis and characteristics of lignin-derived solid acid catalyst for microcrystalline cellulose hydrolisis. Korean J. Chem. Eng. 2017, 34, 110–117. [Google Scholar] [CrossRef]
- Shulga, G.; Betkers, T.; Shakels, V. Effect of the modification of lignocellulosic materials with a lignin-polymer complex on their mulching properties. BioResources 2007, 2, 572–582. [Google Scholar] [CrossRef]
- Kumar, P.; Barret, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Liang, F.; Song, Y.; Huang, C.; Zhang, J.; Chen, B. Preparation and performance evaluation of a lignin-based solid acid hydrolysis lignin. Catal. Commun. 2013, 40, 93–97. [Google Scholar] [CrossRef]
- Adhikari, S.; Hood, Z.; Gallego, N.; Contescu, C. Lignin-derived carbon fibers as efficient heterogeneous solid acid catalysts for esterification of oleic acid. MRS Adv. 2018, 481. [Google Scholar] [CrossRef]
- Ullah, S.; Pakkanen, H.; Lehto, J.; Alén, R. A comparable study on the how-water treatment of wheat Straw and okra stalk prior to delignification. Biomass. Convers. Biorefin. 2018, 8, 413–421. [Google Scholar] [CrossRef]
- Miranda, I.; Mirra, I.; Gominho, J.; Pereira, H. Fractioning og bark of Pinus pinea by milling and chemical characterization of the different fractions. Maderas Cienc. Tecnol. 2017, 19, 185–194. [Google Scholar] [CrossRef]
- Helfferich, F.G. Ion Exchange; Dover Publications: New York, NY, USA, 1995. [Google Scholar]
- Liu, X.; Huang, M.; Ma, H.; Zhang, Z.; Gao, J.; Zhu, Y.; Han, X.; Guo, X. Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Canakci, M.; Van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Trans. ASAE 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Cavalcanti, E.D.C.; Aguieiras, E.C.G.; da Silva, P.R.; Duarte, J.G.; Cipolatti, E.P.; Fernandez-Lafuente, R.; da Silva, J.A.C.; Freire, D.M.G. Improved production of biolubricants from soybean oil and different polyols via esterification reaction catalyzed by immobilized lipase from Candida Rugosa. Fuel 2018, 215, 705–713. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Ratio (mL/g) of Concentrated H2SO4 to Lignin (X1) | Temperature (°C) (X2) | Time (h) (X3) | Total Acid Sites (TAS) (meq H+/g) | |
|---|---|---|---|---|
| Pine Sawdust | Wheat Straw | |||
| 6:1 | 100 | 1.5 | 0.97 ± 0.13 | 0.68 ± 0.13 |
| 6:1 | 150 | 1.0 | 1.02 ± 0.06 | n.d. |
| 6:1 | 150 | 2.0 | 2.01 ± 0.06 | 0.59 ± 0.19 |
| 6:1 | 200 | 1.5 | 1.37 ± 0.25 | 0.45 ± 0.13 |
| 8:1 | 100 | 1.0 | 0.91 ± 0.13 | n.d. |
| 8:1 | 100 | 2.0 | 1.42 ± 0.00 | 0.77 ± 0.06 |
| 8:1 | 150 | 1.5 | 0.94 ± 0.13 | 0.63 ± 0.19 |
| 8:1 | 200 | 1.0 | 1.96 ± 0.06 | 0.58 ± 0.13 |
| 8:1 | 200 | 2.0 | 1.53 ± 0.13 | 0.58 ± 0.06 |
| 10:1 | 100 | 1.5 | 1.21 ± 0.25 | 0.67 ± 0.06 |
| 10:1 | 150 | 1.0 | 1.10 ± 0.13 | 0.77 ± 0.25 |
| 10:1 | 150 | 2.0 | 0.98 ± 0.06 | 0.82 ± 0.19 |
| 10:1 | 200 | 1.5 | 1.44 ± 0.19 | 0.60 ± 0.06 |
| Source | Sum of Squares | df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A:X1 | 0.1024 | 1 | 0.1024 | 2.84 | 0.1094 |
| B:X2 | 0.7921 | 1 | 0.7921 | 21.94 | 0.0002 |
| C:X3 | 0.225625 | 1 | 0.225625 | 6.25 | 0.0223 |
| AC | 0.621612 | 1 | 0.621612 | 17.22 | 0.0006 |
| BB | 0.261446 | 1 | 0.261446 | 7.24 | 0.0149 |
| BC | 0.437112 | 1 | 0.437112 | 12.11 | 0.0027 |
| CC | 0.332231 | 1 | 0.332231 | 9.20 | 0.0071 |
| Total error | 0.649729 | 18 | 0.036096 | ||
| Total (corr.) | 3.28665 | 25 |
| Source | Sum of Squares | df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A:X1 | 0.0466617 | 1 | 0.0466617 | 26.67 | 0.0001 |
| B:X2 | 0.0647717 | 1 | 0.0647717 | 37.01 | 0.0000 |
| C:X3 | 0.000127019 | 1 | 0.000127019 | 0.07 | 0.7911 |
| AB | 0.0128 | 1 | 0.0128 | 7.31 | 0.0156 |
| BB | 0.0137366 | 1 | 0.0137366 | 7.85 | 0.0128 |
| BC | 0.000725595 | 1 | 0.000725595 | 0.41 | 0.5287 |
| CC | 0.0128658 | 1 | 0.0128658 | 7.35 | 0.0154 |
| Total error | 0.0279983 | 16 | 0.00174989 | ||
| Total (corr.) | 0.2362 | 23 |
| Raw Material | Sulfonation Conditions | Total Acid Sites of Lignin-Based Catalyst | Reference |
|---|---|---|---|
| Kraft lignin | Temperature: 200 °C Time: 2 h H2SO4 to biomass ratio: 10:1 (g/mL) | 2.21 mmol/g | Pua et al. [11] |
| Lignin from Xanthoceras Sorbifolia | Temperature: 150 °C Time: 2 h H2SO4 to biomass ratio: 5:10 (g/mL) | 1.71 mmol/g | Guo et al. [12] |
| Lignin based activated carbon fibers | Temperature: 110 °C or 150 °C Time: 20 h H2SO4 to biomass ratio: 5:10 (mg/mL) | 0.3–2.43 mmol/g | Hu et al. [13] |
| Mason pine alkali lignin | Temperature: 180 °C Time: 12 h H2SO4 to biomass ratio: 20:1 (g/mL) | 1.46–3.52 mmol/g | Zhu et al. [14] |
| Lignin from 40–60 mesh pine powder (Pinus tabuliformis) | Temperature: 50 °C Time: 5 h Sulfuryl chloride+tetrachloroethane to biomass ratio: 75:10 (g/mL) | 2.22 mmol/g | Liang at al. [17] |
| Alcell lignin | Temperature: 150 °C Time: 12 h H2SO4 to biomass ratio: 50:1 (g/mL) | 1.86 mmol/g | Adhikari et al. [18] |
| Lignin from pine sawdust | Temperature: 200 °C Time: 1 h H2SO4 to biomass ratio: 10:1 (g/mL) | 1.68 mmol/g | This study |
| Lignin from processed wheat straw | Temperature: 125 °C Time: 2 h H2SO4 to biomass ratio: 10:1 (g/mL) | 0.74 mmol/g | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escalante, F.M.E.; Carranza-Hernández, A.; García-Zamora, A.; Aguilar-Garnica, E. Optimization of Lignin-Based Biocatalyst Production from Pine Sawdust and Wheat Straw. Molecules 2018, 23, 1877. https://doi.org/10.3390/molecules23081877
Escalante FME, Carranza-Hernández A, García-Zamora A, Aguilar-Garnica E. Optimization of Lignin-Based Biocatalyst Production from Pine Sawdust and Wheat Straw. Molecules. 2018; 23(8):1877. https://doi.org/10.3390/molecules23081877
Chicago/Turabian StyleEscalante, Froylán M.E., Alejandra Carranza-Hernández, Adelina García-Zamora, and Efrén Aguilar-Garnica. 2018. "Optimization of Lignin-Based Biocatalyst Production from Pine Sawdust and Wheat Straw" Molecules 23, no. 8: 1877. https://doi.org/10.3390/molecules23081877