Improved Enantioselectivity for Atenolol Employing Pivot Based Molecular Imprinting
Abstract
:1. Introduction
2. Results
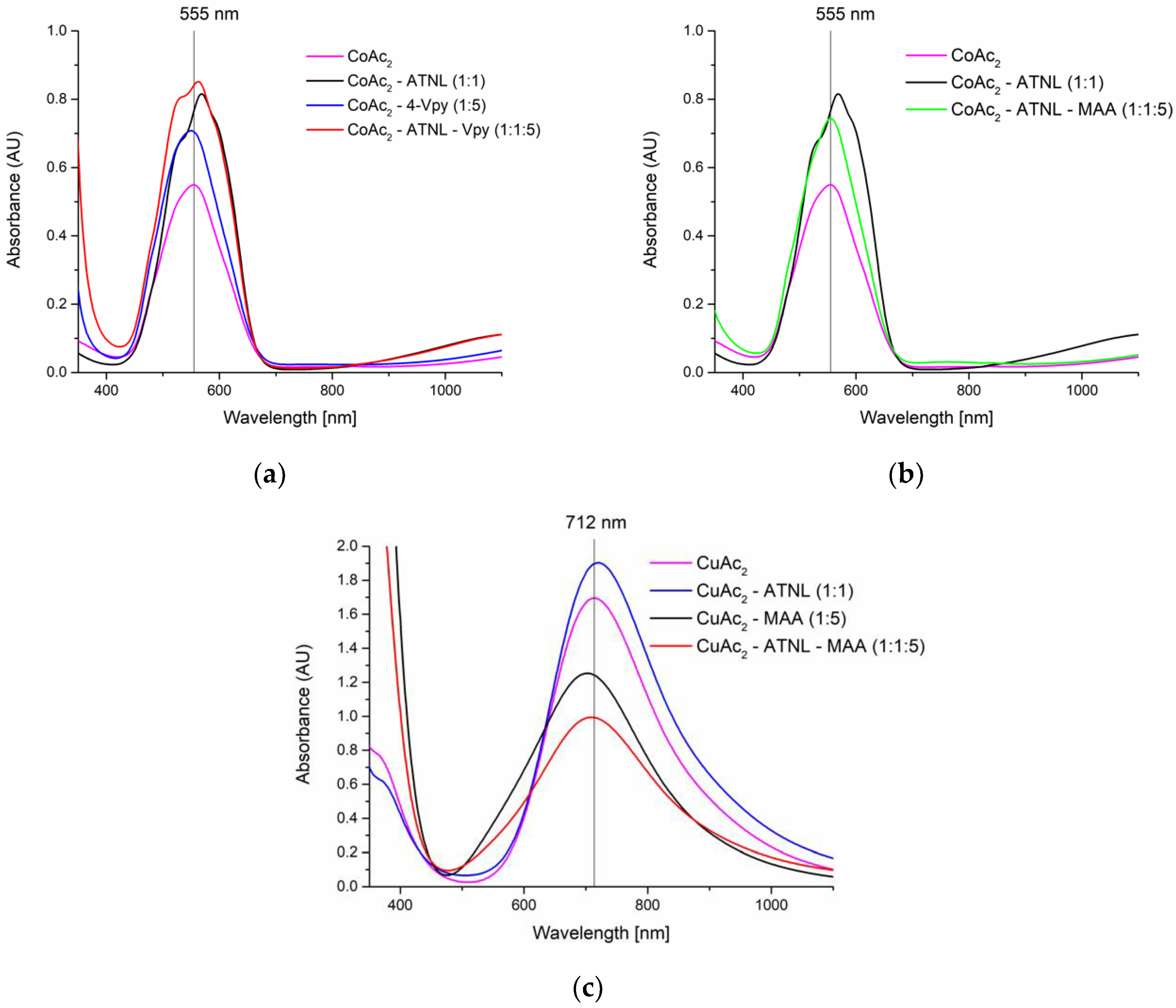
2.1. Ternary Metal Complexes of ATNL
2.2. Preparation of MIPs
2.2.1. Non-Covalent Molecular Imprinting
2.2.2. One-Monomer Molecularly Imprinted Polymer (OMNiMIP)
2.2.3. Metal Ion Mediated Molecular Imprinting
2.2.4. Bulk Imprinting vs. MIP Monolith
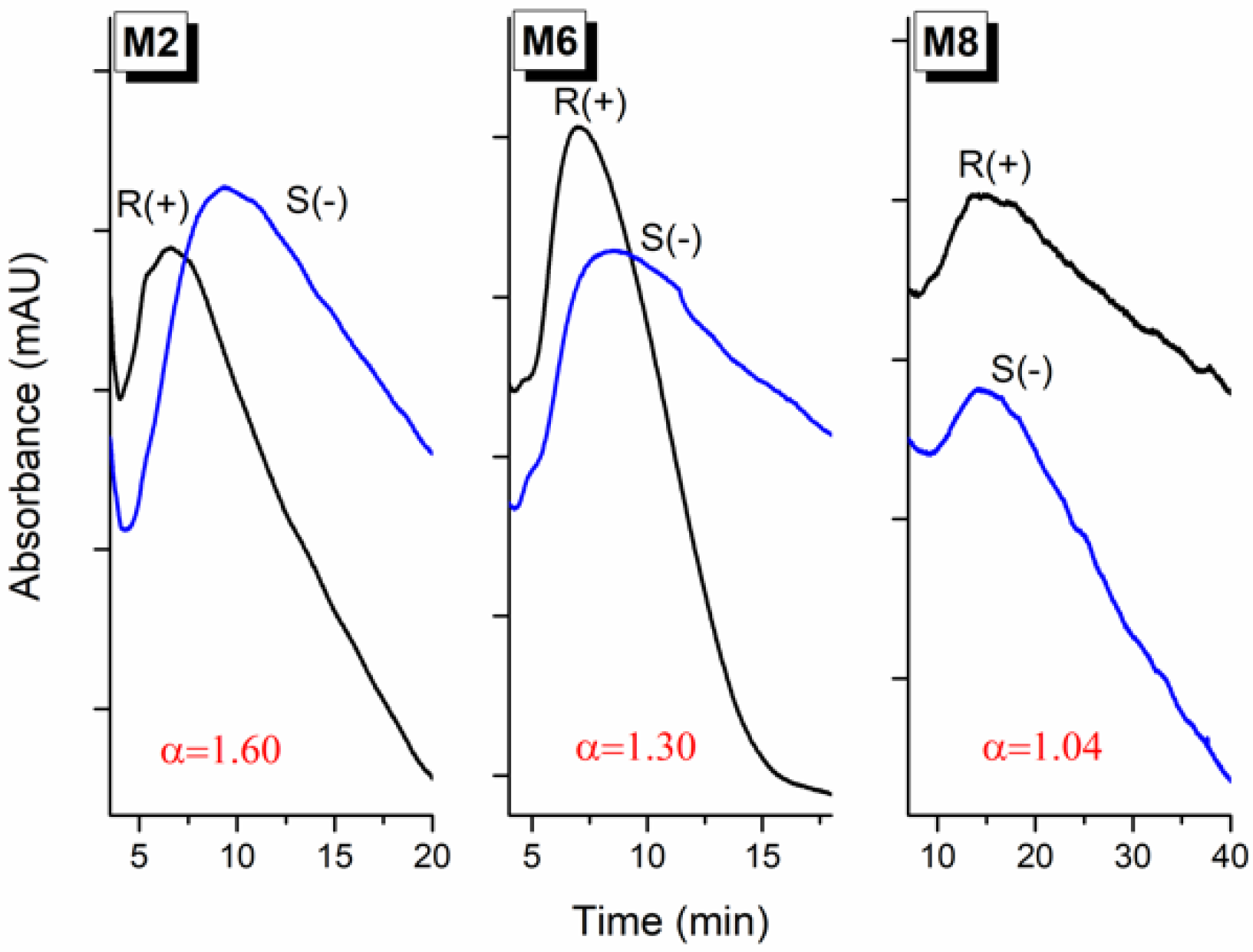
2.3. Chromatographic Evaluation of the MIPs
3. Discussion
3.1. Ternary Metal Complexes of ATNL
3.2. Preparation of MIPs
3.2.1. Non-Covalent Molecular Imprinting
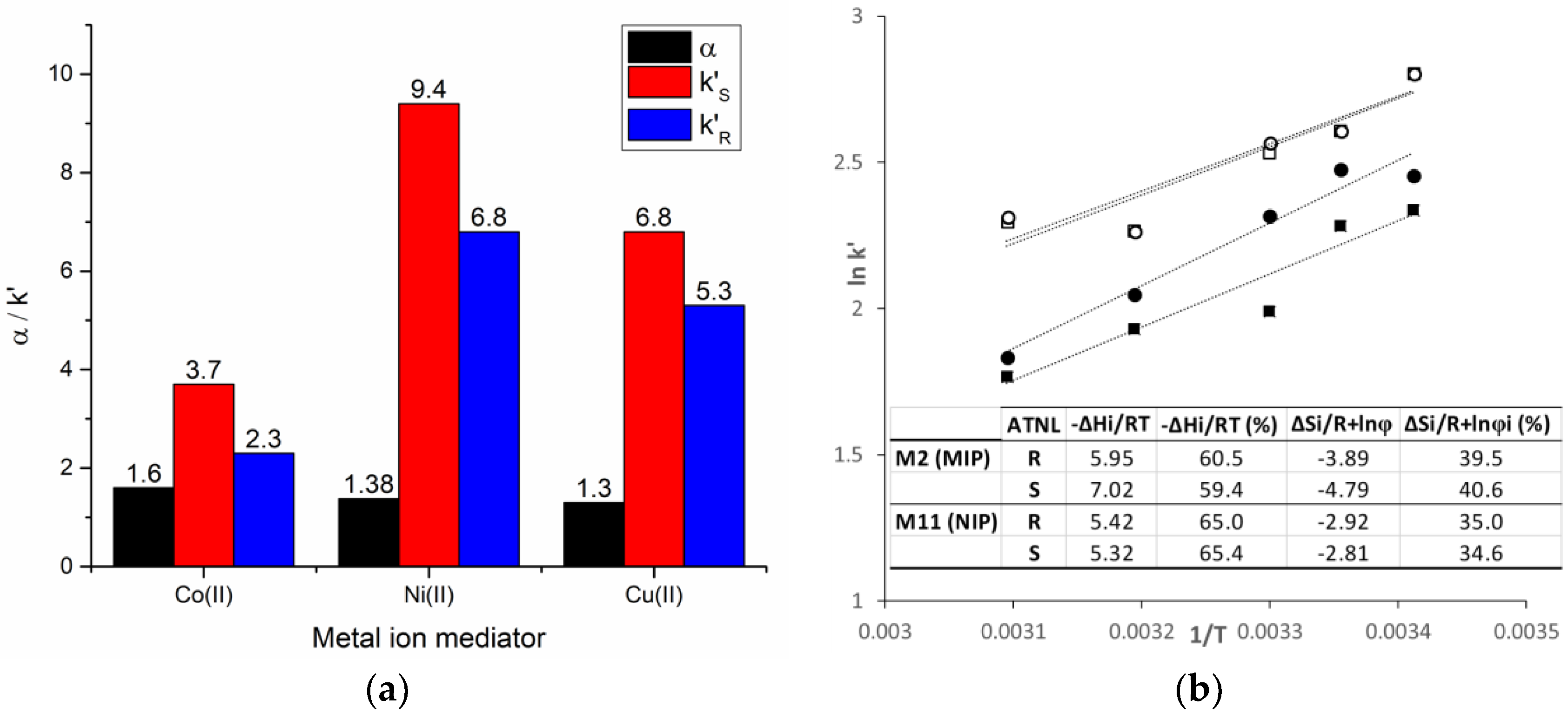
3.2.2. Metal Ion-Mediated Molecular Imprinting
Metal Ion and Functional Monomer (Secondary Ligand)
Functional Co-Monomers
Cross-Linker
Ionic Liquid
3.2.3. Bulk Imprinting vs. MIP Monolith
3.3. Chromatographic Retention Mechanism
4. Materials and Methods
4.1. Reagents
4.2. Apparatus
4.3. MIP Preparation
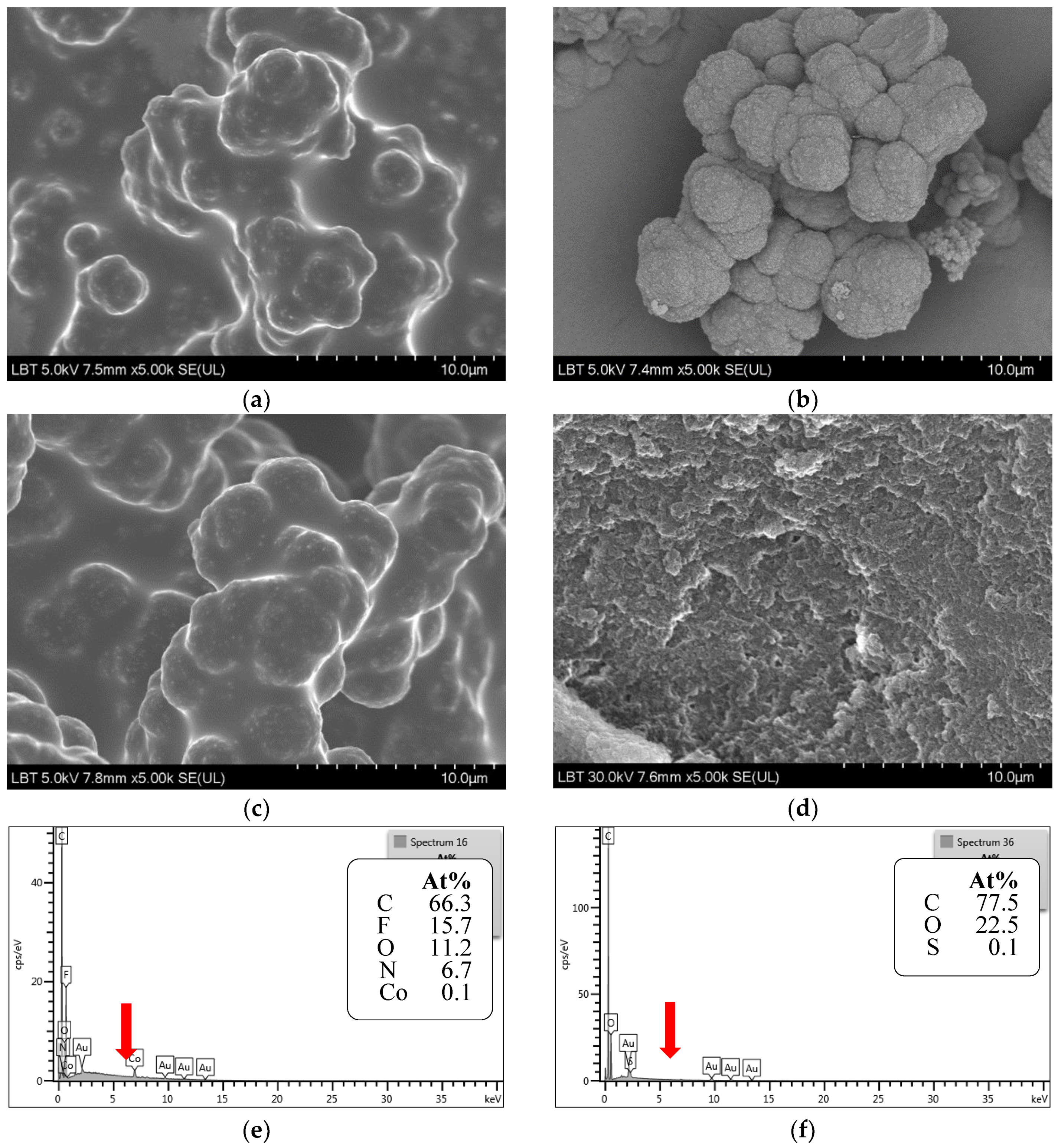
4.4. Microscopic Characterization of MIPs
4.5. Chromatographic Evaluation of The Imprinted Polymers
4.5.1. Bulk MIP
4.5.2. MIP Monolith
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Săndulescu, R.; Cristea, C.; Bodoki, E.; Oprean, R. Chapter 3: Recent Advances in the Analysis of Bioactive Compounds based on Molecular Recognition. In Frontiers in Bioactive Compounds, Natural Sources, Physicochemical Characterization Applications; Apetrei, C., Ed.; Bentham Science Publisher: Sharjah, UAE, 2016; Volume 1, pp. 69–126. [Google Scholar]
- Iacob, B.-C.; Bodoki, E.; Oprean, R. Recent advances in capillary electrochromatography using molecularly imprinted polymers. Electrophoresis 2014, 35, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.; Ali, F.; Choi, J.H.; Lee, J.O.; Sung, K.Y. Recent applications of molecular imprinted polymers for enantio-selective recognition. Talanta 2013, 106, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.J.; Ali, F.; Kim, Y.S.; Lee, J.W. Comprehensive overview of recent preparation and application trends of various open tubular capillary columns in separation science. J. Chromatogr. A 2013, 1308, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, W.; Ma, Y.; Pan, G. Molecularly imprinted polymers as receptor mimics for selective cell recognition. Chem. Soc. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, Y.; Pan, J.; Meng, Z.; Pan, G.; Sellergren, B. Molecularly Imprinted Polymers with Stimuli-Responsive Affinity: Progress and Perspectives. Polymers 2015, 7, 1689–1715. [Google Scholar] [CrossRef] [Green Version]
- Iacob, B.-C.; Bodoki, E.; Oprean, R. Chiral Electrochemical Sensors Based on Molecularly Imprinted Polymers with Pharmaceutical Applications. In Handbook of Sustainable Polymers; Pan Stanford Publishing, CRC Press: Boca Raton, FL, USA, 2016; pp. 587–614. [Google Scholar]
- Prasad, B.B.; Tiwari, M.P. Molecularly Imprinted Nanomaterial-Based Highly Sensitive and Selective Medical Devices. In Biomedical Materials and Diagnostic Devices; John Wiley & Sons, Inc.: Hoboken, NJ, USA; Scrivener Publishing LLC: Salem, MA, USA, 2012; pp. 339–391. [Google Scholar]
- Iacob, B.-C.; Bodoki, E.; Florea, A.; Bodoki, A.E.; Oprean, R. Simultaneous enantiospecific recognition of several β-blocker enantiomers using molecularly imprinted polymer-based electrochemical sensor. Anal. Chem. 2015, 87, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Iacob, B.-C.; Bodoki, E.; Farcau, C.; Barbu-Tudoran, L.; Oprean, R. Study of the Molecular Recognition Mechanism of an Ultrathin MIP Film-Based Chiral Electrochemical Sensor. Electrochim. Acta 2016, 217, 195–202. [Google Scholar] [CrossRef]
- Kupai, J.; Rojik, E.; Huszthy, P.; Szekely, G. Role of Chirality and Macroring in Imprinted Polymers with Enantiodiscriminative Power. ACS Appl. Mater. Interfaces 2015, 7, 9516–9525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-L.; Zhou, J.; Zhang, L.-S.; Huang, Y.-P.; Liu, Z.-S. Coatings of molecularly imprinted polymers based on polyhedral oligomeric silsesquioxane for open tubular capillary electrochromatography. Talanta 2016, 152, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, Y.; Jiang, Y.; Li, S.; Liu, W. Molecularly Imprinted Polymers for the Identification and Separation of Chiral Drugs and Biomolecules. Polymers 2016, 8, 216. [Google Scholar] [CrossRef]
- Didaskalou, C.; Buyuktiryaki, S.; Kecili, R.; Fonte, C.P.; Szekely, G. Valorisation of agricultural waste with an adsorption/nanofiltration hybrid process: from materials to sustainable process design. Green Chem. 2017, 19, 3116–3125. [Google Scholar] [CrossRef] [Green Version]
- Bakkour, R.; Bolotin, J.; Sellergren, B.; Hofstetter, T.B. Molecularly Imprinted Polymers for Compound-Specific Isotope Analysis of Polar Organic Micropollutants in Aquatic Environments. Anal. Chem. 2018, 90, 7292–7301. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Ma, L.; Huang, Y.-P.; Liu, Z.-S.; Aisa, H.A. Preparation of metallic pivot-based imprinted monoliths with a hydrophilic macromonomer. RSC Adv. 2015, 5, 36753–36761. [Google Scholar] [CrossRef]
- Bai, L.H.; Chen, X.X.; Huang, Y.P.; Zhang, Q.W.; Liu, Z.S. Chiral separation of racemic mandelic acids by use of an ionic liquid-mediated imprinted monolith with a metal ion as self-assembly pivot. Anal. Bioanal. Chem. 2013, 405, 8935–8943. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.-D.; Huang, Y.-P.; Xin, X.-L.; Liu, Z.-S.; Aisa, H.A. Preparation of metallic pivot-based imprinted monolith for polar template. J. Chromatogr. B 2013, 934 (Suppl. C), 109–116. [Google Scholar] [CrossRef] [PubMed]
- Iacob, B.-C.; Bodoki, A.E.; Oprean, L.; Bodoki, E. Metal—Ligand interactions in molecular imprinting. In Ligand; Chandraleka, S., Biswas, B., Eds.; Intech Open: Rijeka, Croatia, 2018; in press. [Google Scholar]
- Zhang, J.; Li, F.; Wang, X.-H.; Xu, D.; Huang, Y.-P.; Liu, Z.-S. Preparation and characterization of dual-template molecularly imprinted monolith with metal ion as pivot. Eur. Polym. J. 2016, 80 (Suppl. C), 134–144. [Google Scholar] [CrossRef]
- Zhao, L.; Ban, L.; Zhang, Q.-W.; Huang, Y.-P.; Liu, Z.-S. Preparation and characterization of imprinted monolith with metal ion as pivot. J. Chromatogr. A 2011, 1218, 9071–9079. [Google Scholar] [CrossRef] [PubMed]
- Vidyasankar, S.; Ru, M.; Arnold, F.H. Molecularly imprinted ligand-exchange adsorbents for the chiral separation of underivatized amino acids. J. Chromatogr. A 1997, 775, 51–63. [Google Scholar] [CrossRef]
- Fischer, L.; Mueller, R.; Ekberg, B.; Mosbach, K. Direct enantioseparation of .beta.-adrenergic blockers using a chiral stationary phase prepared by molecular imprinting. J. Am. Chem. Soc. 1991, 113, 9358–9360. [Google Scholar] [CrossRef]
- Tamai, G.; Edani, M.; Imai, H. Chiral separation and determination of propranolol enantiomers in rat or mouse blood and tissue by column switching high performance liquid chromatography with ovomucoid bonded stationary phase. Biomed. Chromatogr. 1990, 4, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Enein, H.Y.; Bakr, S.A. Enantiomeric Resolution of Propranolol and Analogs on Two Cellulose (Chiralcel of and OC) AND One Amylose (Chiralpak Ad) Chiral Stationary Phases. J. Liquid Chromatogr. Relat. Technol. 1998, 21, 1137–1145. [Google Scholar] [CrossRef]
- Moldovan, R.-C.; Dascăl, G.-S.; Mirel, V.; Bodoki, E.; Oprean, R. Chiral separation of 16 beta-blockers on immobilized polysaccharide chiral stationary phases. Farmacia 2015, 63, 909–912. [Google Scholar]
- Weng, X.; Bao, Z.; Xing, H.; Zhang, Z.; Yang, Q.; Su, B.; Yang, Y.; Ren, Q. Synthesis and characterization of cellulose 3,5-dimethylphenylcarbamate silica hybrid spheres for enantioseparation of chiral β-blockers. J. Chromatogr. A 2013, 1321, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.A.; Ates, H.; Mangelings, D.; Vander Heyden, Y. A separation strategy combining three HPLC modes and polysaccharide-based chiral stationary phases. J. Pharm. Biomed. Anal. 2013, 75, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Sibrian-Vazquez, M.; Spivak, D.A. Enhanced Enantioselectivity of Molecularly Imprinted Polymers Formulated with Novel Cross-Linking Monomers. Macromolecules 2003, 36, 5105–5113. [Google Scholar] [CrossRef]
- Mehvar, R.; Brocks, D.R. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans. J. Pharm. Pharm. Sci. 2001, 4, 185–200. [Google Scholar] [PubMed]
- Zaid, A.A.; Farooqui, M.; Janrao, D.M. Potentiometric study of atenolol as hypertension drug with Co(II), Ni(II), Cu(II) and Zn(II) transition metal ions in aqueous solution. J. Saudi Chem. Soc. 2015, 19, 92–96. [Google Scholar] [CrossRef]
- Gölcü, A.; Yücesoy, C.; Serin, S. Synthesis and characterization of metal complexes of acebutolol, atenolol, and propranolol antihypertension drugs. Synth. React. Inorg. Metal-Org. Chem. 2004, 34, 1259–1275. [Google Scholar] [CrossRef]
- Bontchev, P.R.; Pantcheva, I.N.; Gochev, G.P.; Mehandjiev, D.R.; Ivanov, D.S. Complexes of copper(II) with the β-blocker atenolol. Transit. Met. Chem. 2000, 25, 196–199. [Google Scholar] [CrossRef]
- Cozar, O.; Szabó, L.; Cozar, I.B.; Leopold, N.; David, L.; Căinap, C.; Chiș, V. Spectroscopic and DFT study of atenolol and metoprolol and their copper complexes. J. Mol. Struct. 2011, 993, 357–366. [Google Scholar] [CrossRef]
- Rajbhoj, A.S.; Gaikwad, S.T.; Chondhekar, T.K.; Pirzada, S.A. Stability constants of ternary complexes of drugs and aminoacids. Int. J. Chem. Sci. 2005, 3, 241–246. [Google Scholar]
- Ali, M.; Dutta, P.; Pandey, S. Effect of Ionic Liquid on Prototropic and Solvatochromic Behavior of Fluorescein. J. Phys. Chem. B 2010, 114, 15042–15051. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.A. Comprehensive Coordination Chemistry. The Synthesis, Reactions, Properties and Applications of Coordination Compounds (7 Bände). Herausgegeben von G. Wilkinson, R. Gillard and J. A. McCleverty. Pergamon Press: Oxford 1987. Volume 4: Middle Transition Elements. Angew. Chem. 1989, 101, 809. [Google Scholar]
- Lever, A.P.B. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Reddy, L.S.; Endo, T.; Reddy, G.S. Chapter 1: Electronic (absorption) spectra of 3D transition metal complexes. In Advanced Aspects of Spectroscopy; Farrukh, M.A., Ed.; Intech Open: Rijeka, Croatia, 2012. [Google Scholar]
- Ye, L. Molecular Imprinting: Principles and Applications of Micro- and Nanostructured Polymers; Pan Stanford Publishing, CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Meng, A.C.; LeJeune, J.; Spivak, D.A. Multi-analyte imprinting capability of OMNiMIPs versus traditional molecularly imprinted polymers. J. Mol. Recognit. 2009, 22, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Cheng, C.H.; Pan, H.H.; Chung, T.H.; Hwang, C.C. Chromatographic characterization of molecularly imprinted polymers. Anal. Bioanal. Chem. 2008, 390, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Svec, F.; Frechet, J.M.J. Continuous rods of macroporous polymer as high-performance liquid chromatography separation media. Anal. Chem. 1992, 64, 820–822. [Google Scholar] [CrossRef]
- Wu, X.; Du, J.; Li, M.; Wu, L.; Han, C.; Su, F. Recent advances in green reagents for molecularly imprinted polymers. RSC Adv. 2018, 8, 311–327. [Google Scholar] [CrossRef] [Green Version]
- Greaves, T.L.; Drummond, C.J. Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem. Soc. Rev. 2013, 42, 1096–1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.-J.; Yang, J.; Zhao, Y.-X.; Liu, Z.-S.; Aisa, H.A. Preparation of ionic liquid-mediated imprinted monolith for selective capture and purification of corilagin. J. Chromatogr. B 2017, 1041–1042, 98–103. [Google Scholar] [CrossRef] [PubMed]
- LeJeune, J.; Spivak, D.A. Chiral effects of alkyl-substituted derivatives of N,O-bismethacryloyl ethanolamine on the performance of one monomer molecularly imprinted polymers (OMNiMIPs). Anal. Bioanal. Chem. 2007, 389, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Denderz, N.; Lehotay, J.; Čižmárik, J.; Cibulková, Z.; Šimon, P. Thermodynamic study of molecularly imprinted polymer used as the stationary phase in high performance liquid chromatography. J. Chromatogr. A 2012, 1235, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Denderz, N.; Lehotay, J. Application of the van’t Hoff dependences in the characterization of molecularly imprinted polymers for some phenolic acids. J. Chromatogr. A 2012, 1268, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Denderz, N.; Lehotay, J. Identification of driving forces for the recognition processes on molecularly imprinted polymers. Nova Biotechnol. Chim. 2013, 12, 63–69. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| # | T (mmol) | M(s) (mmol) | Me (mmol) | C (mmol) | Molar Ratio T:M:Me:C | A (mmol) | P (mL) | k’S/k’R | α |
|---|---|---|---|---|---|---|---|---|---|
| S1 | S-ATNL (0.3) | NOBE (7.6) | - | - | 1:25:-:- | - | DMF (4) | 33.2/31.6 | 1.05 a |
| S2 | S-ATNL (0.3) | NOBE (7.6) | Cu(II) (0.3) | - | 1:25:1:- | - | DMF (4) | 33.2/31.6 | 1.05 a |
| S3 | S-ATNL (0.3) | NOBE (7.6) | Co(II) (0.3) | - | 1:25:1:- | - | DMF (4) | 36.8/36.3 | 1.01 a |
| S4 | S-ATNL (0.3) | NOBE/MAA (7.6/1) | - | - | 1:25/3:-:- | - | ACN (2) | 4.4/3.8 | 1.17 b |
| S6 | S-ATNL (0.3) | 4-VPy (0.3) | Co(II) (0.3) | TRIM (7.3) | 1:1:1:24 | - | DMF/DMSO (2/2) | 3.6/3.5 | 1.03 a |
| S7 | S-ATNL (0.3) | 4-VPy (1.5) | Co(II) (0.3) | TRIM (6.1) | 1:5:1:20 | - | DMF/DMSO (2/2) | 8.2/7.9 | 1.04 a |
| S8 | S-ATNL (0.3) | 4-VPy (3) | Co(II) (0.3) | TRIM (4.6) | 1:10:1:15 | - | DMF/DMSO (2/2) | 8.2/7.9 | 1.04 a |
| S9 | S-ATNL (0.3) | 4-VPy/AM (0.3/1.2) | Co(II) (0.3) | TRIM (6.1) | 1:1/4:1:20 | - | DMF/DMSO (2/2) | 0.5/0.5 | 1.00 |
| S10 | S-ATNL (0.3) | 4-VPy/BAM (0.3/1.2) | Co(II) (0.3) | TRIM (6.1) | 1:1/4:1:20 | - | DMF/DMSO (2/2) | 0.6/0.5 | 1.07 a |
| S11 | S-ATNL (0.15) | 4-VPy (0.9) | Co(II) (0.15) | EDMA (3.6) | 1:6:1:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 16.2/12.3 | 1.32 a |
| # | T (mmol) | M(s) (mmol) | Me (mmol) | C (mmol) | Molar Ratio T:M:Me:C | A (mmol) | P (mL) | k’S/k’R | α |
|---|---|---|---|---|---|---|---|---|---|
| M1 | S-ATNL (0.2) | 4-VPy (1) | Co(II) (0.2) | TRIM (6) | 1:5:1:30 | IL (0.9) | DMF/DMSO (0.9/0.9) | 4.3/4.3 | 1.00 |
| M2 | S-ATNL (0.15) | 4-VPy (0.9) | Co(II) (0.15) | EDMA (3.6) | 1:6:1:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 3.7/2.3 | 1.60 a |
| M3 | S-ATNL (0.15) | 4-VPy (0.9) | Ni(II) (0.15) | EDMA (3.6) | 1:6:1:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 9.4/6.8 | 1.38 a |
| M4 | S-ATNL (0.15) | 4-VPy (0.9) | Cu(II) (0.15) | EDMA (3.6) | 1:6:1:24 | IL (1.235) | DMF/DMSO (0.48/0.6) | 6.8/5.3 | 1.30 a |
| M5 | S-ATNL (0.15) | 4-VPy (0.9) | Co(II) (0.15) | EDMA (3.6) | 1:6:1:24 | - * | DMF/DMSO (0.326/1.630) | 6.3/5.8 | 1.08 a |
| M6 | S-ATNL (0.15) | 4-VPy (0.9) | - | EDMA (3.6) | 1:6:-:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 3.4/2.6 | 1.30 a |
| M7 | S-ATNL (0.15) | 4-VPy (0.9) | Co(II) (0.15) | EDMA (3.6) | 1:6:1:24 | IL/PEGMA (1.235/0.17) | DMF/DMSO (0.12/0.6) | 4.0/4.0 | 1.00 |
| M8 | - | 4-VPy (0.9) | Co(II) (0.15) | EDMA (3.6) | -:6:1:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 2.7/2.6 | 1.04 a |
| M9 | - | 4-VPy (0.9) | - | EDMA (3.6) | -:6:-:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 10.4/10.4 | 1.00 |
| M10 | S-ATNL (0.05) | 4-VPy (0.15) | Ni(II) (0.05) | TRIM (1.15) | 1:3:1:23 | - | MeOH (2) | 0.3/0.3 | 1.00 |
| # | T (mmol) | M(s) (mmol) | Me (mmol) | C (mmol) | Molar Ratio T:M:Me:C | A (mmol) | P (mL) | k’S/k’R | α |
|---|---|---|---|---|---|---|---|---|---|
| M11 | S-ATNL (0.15) | 4-VPy/AM (0.15/0.75) | Co(II) (0.15) | EDMA (3.6) | 1:1/5:1:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 12.9/10.0 | 1.29 a |
| M12 | S-ATNL (0.15) | 4-VPy/4-PBA (0.9/0.15) | Co(II) (0.15) | EDMA (3.6) | 1:6/1:1:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 4.6/4.4 | 1.04 a |
| M13 | S-ATNL (0.15) | 4-VPy/ CVPBA (0.9/0.15) | Co(II) (0.15) | EDMA (3.6) | 1:6/1:1:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 0.4/0.4 | 1.00 |
| M14 | S-ATNL (0.15) | 1-VIM (0.9) | Co(II) (0.15) | EDMA (3.6) | 1:6:1:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 6.2/4.3 | 1.43 a |
| M15 | S-ATNL (0.15) | MAA/AM (0.15/0.75) | Cu(II) (0.15) | EDMA (3.6) | 1:1/5:1:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 2.5/2.2 | 1.14 b |
| M16 | S-ATNL (0.15) | MAA/AM (0.15/0.75) | - | EDMA (3.6) | 1:1/5:-:24 | IL (1.235) | DMF/DMSO (0.12/0.6) | 15.3/14.5 | 1.06 b |
| M17 | S-ATNL (0.13) | MAA (0.7) | - | EDMA (0.7) | 1:5:-:5 | - | ACN (2) | 1.8/1.5 | 1.15 c |
| M18 | S-ATNL (0.13) | MAA (0.7) | - | EDMA (0.7) | 1:5:-:5 | IL (1.235) | ACN (0.72) | 6.2/4.7 | 1.32 d |
| M19 | S-ATNL (0.13) | MAA (0.7) | - | PETRA(0.7) | 1:5:-:5 | IL (1.235) | ACN (0.72) | 8.7/7.8 | 1.11 e |
| M20 | S-ATNL (0.2) | VFC (0.1) | - | TRIM (1.25) | 2:1:-:12.5 | - | ACN (1%H2O) (5) | 0.3/0.3 | 1.00 |
| M21 | S-ATNL (0.2) | 1-VIM (0.6) | Ni(II) (0.2) | TRIM (1.25) | 1:3:1:6 | - | MeOH (5) | 0.9/0.9 | 1.00 |
| M22 | S-ATNL (0.04) | 1-VIM (0.04) | Ni(II) (0.04) | PETEA (0.25) | 1:1:1:6 | - | MeOH (1) | 0.5/0.5 | 1.00 |
| M23 | S-ATNL (0.02) | 1-VIM/BAM (0.02/0.08) | Cu(II) (0.02) | - | 1:1/4:1:- | - | MeOH | 2.4/2.4 | 1.00 |
| M24 | S-ATNL (0.2) | 1-VIM/MAA (0.6/0.2) | Cu(II) (0.2) | PETRA (3.6) | 1:3/1:1:18 | - | BuOH (5) | 3.6/3.5 | 1.03 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodoki, A.E.; Iacob, B.-C.; Gliga, L.E.; Oprean, S.L.; Spivak, D.A.; Gariano, N.A.; Bodoki, E. Improved Enantioselectivity for Atenolol Employing Pivot Based Molecular Imprinting. Molecules 2018, 23, 1875. https://doi.org/10.3390/molecules23081875
Bodoki AE, Iacob B-C, Gliga LE, Oprean SL, Spivak DA, Gariano NA, Bodoki E. Improved Enantioselectivity for Atenolol Employing Pivot Based Molecular Imprinting. Molecules. 2018; 23(8):1875. https://doi.org/10.3390/molecules23081875
Chicago/Turabian StyleBodoki, Andreea Elena, Bogdan-Cezar Iacob, Laura Elena Gliga, Simona Luminita Oprean, David A. Spivak, Nicholas A. Gariano, and Ede Bodoki. 2018. "Improved Enantioselectivity for Atenolol Employing Pivot Based Molecular Imprinting" Molecules 23, no. 8: 1875. https://doi.org/10.3390/molecules23081875






