Catalytic Dehydration of Fructose to 5-Hydroxymethylfurfural (HMF) in Low-Boiling Solvent Hexafluoroisopropanol (HFIP)
Abstract
:1. Introduction
2. Results and Discussion
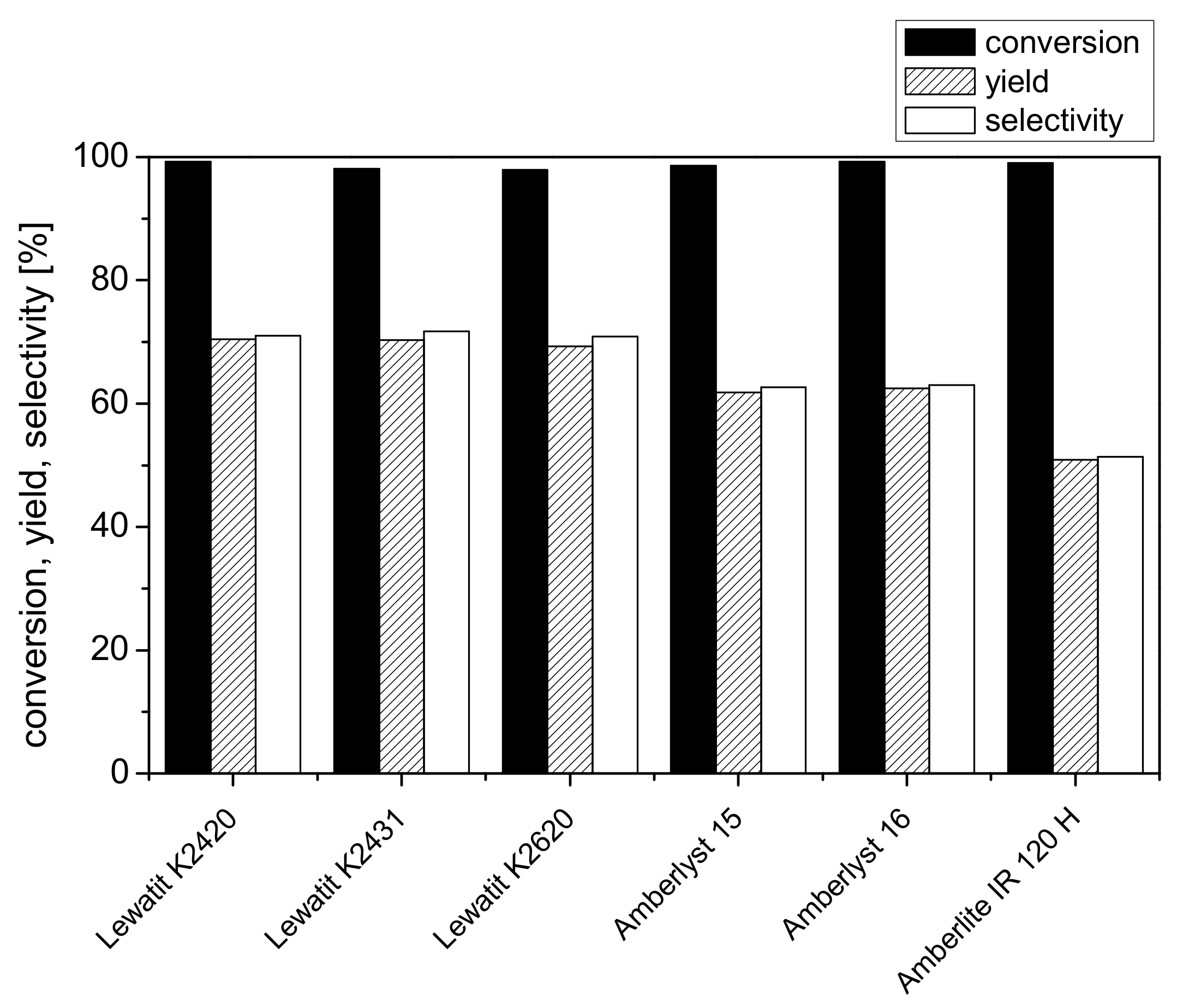
2.1. Screening of Different Ion-Exchange Resins for Dehydration of Fructose
2.2. Examination of Different Reaction Parameters
2.2.1. Effect of Initial Fructose Concentration
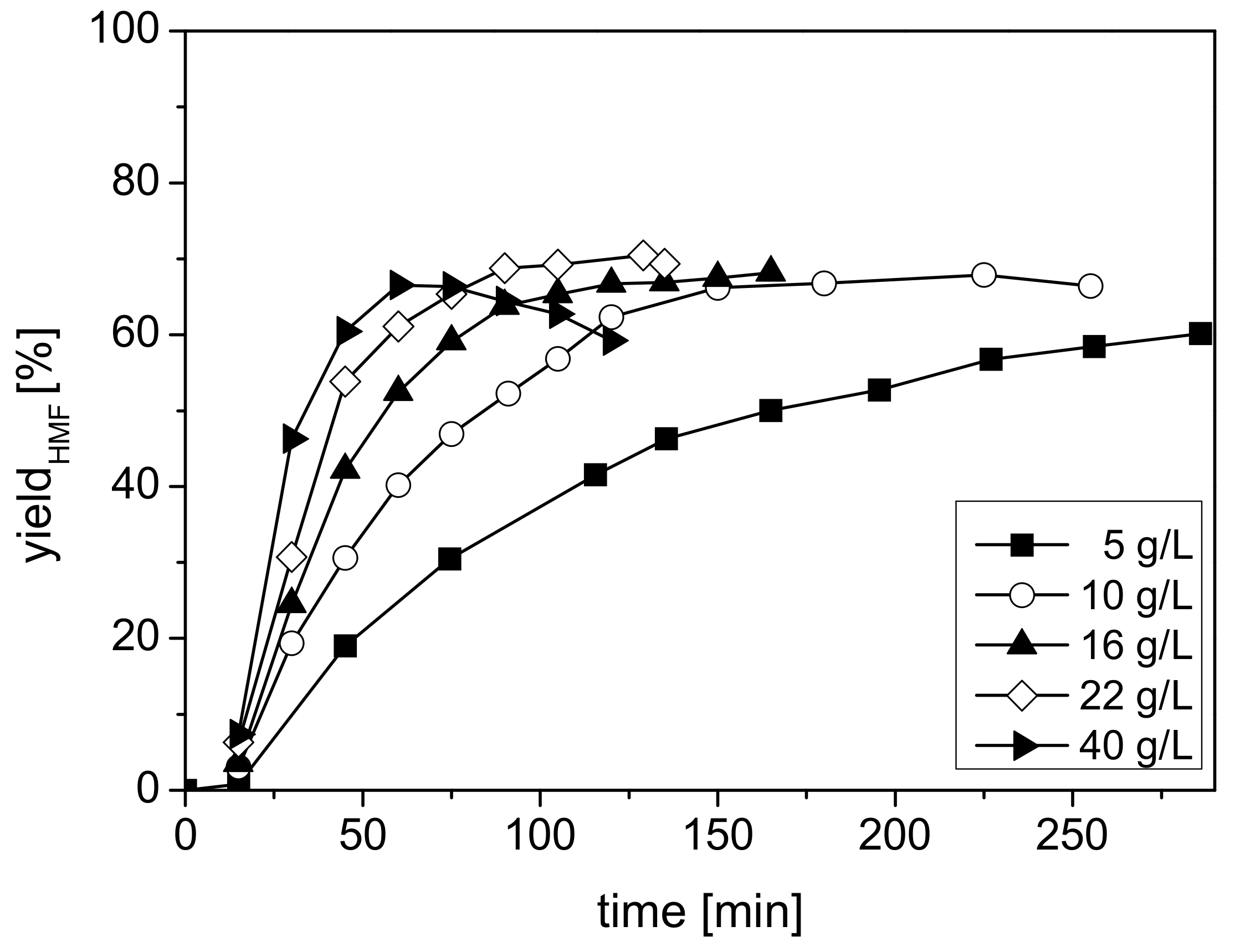
2.2.2. Effect of Catalyst Concentration
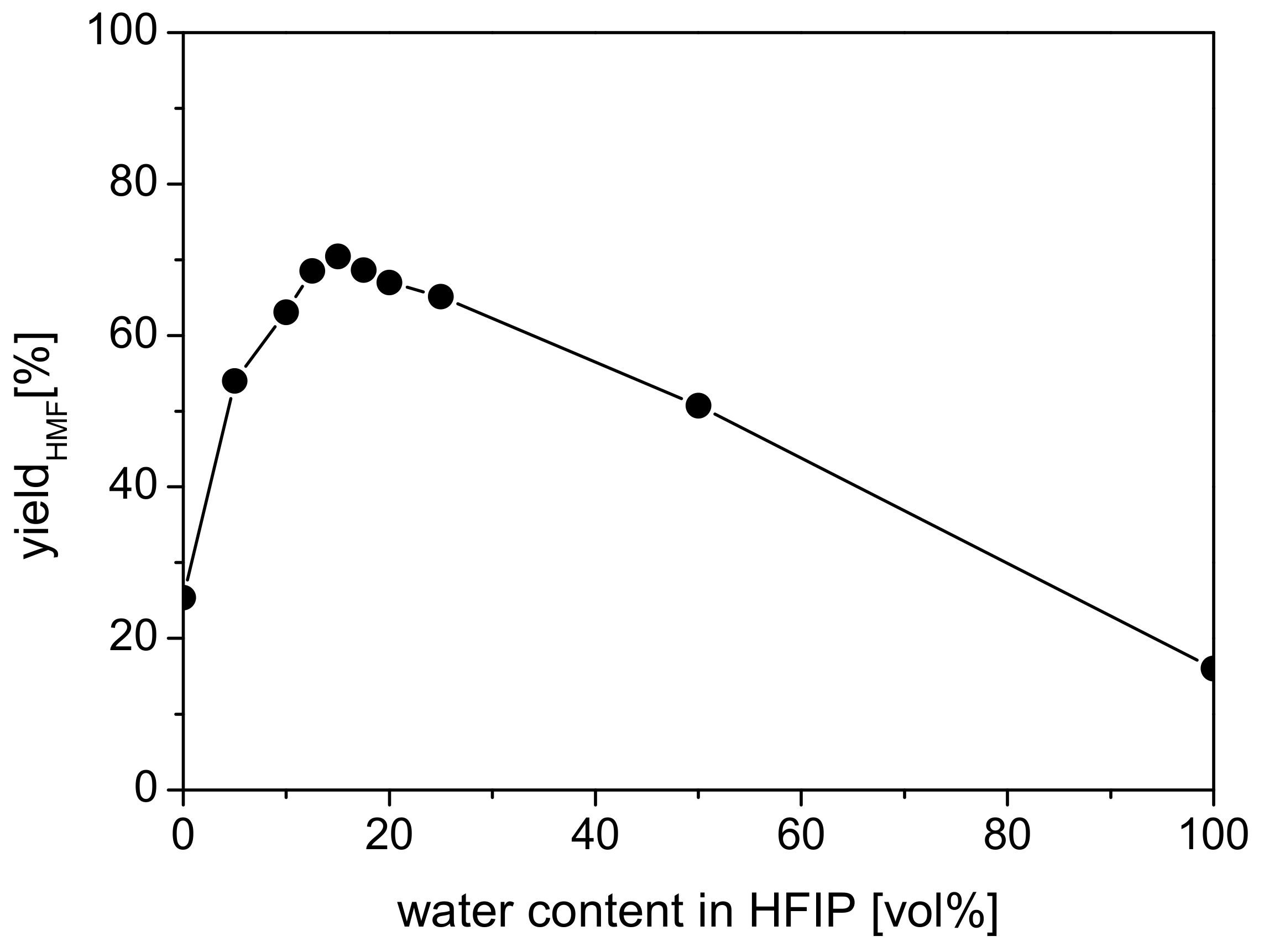
2.2.3. Effect of Water Content
2.2.4. Effect of Reaction Temperature
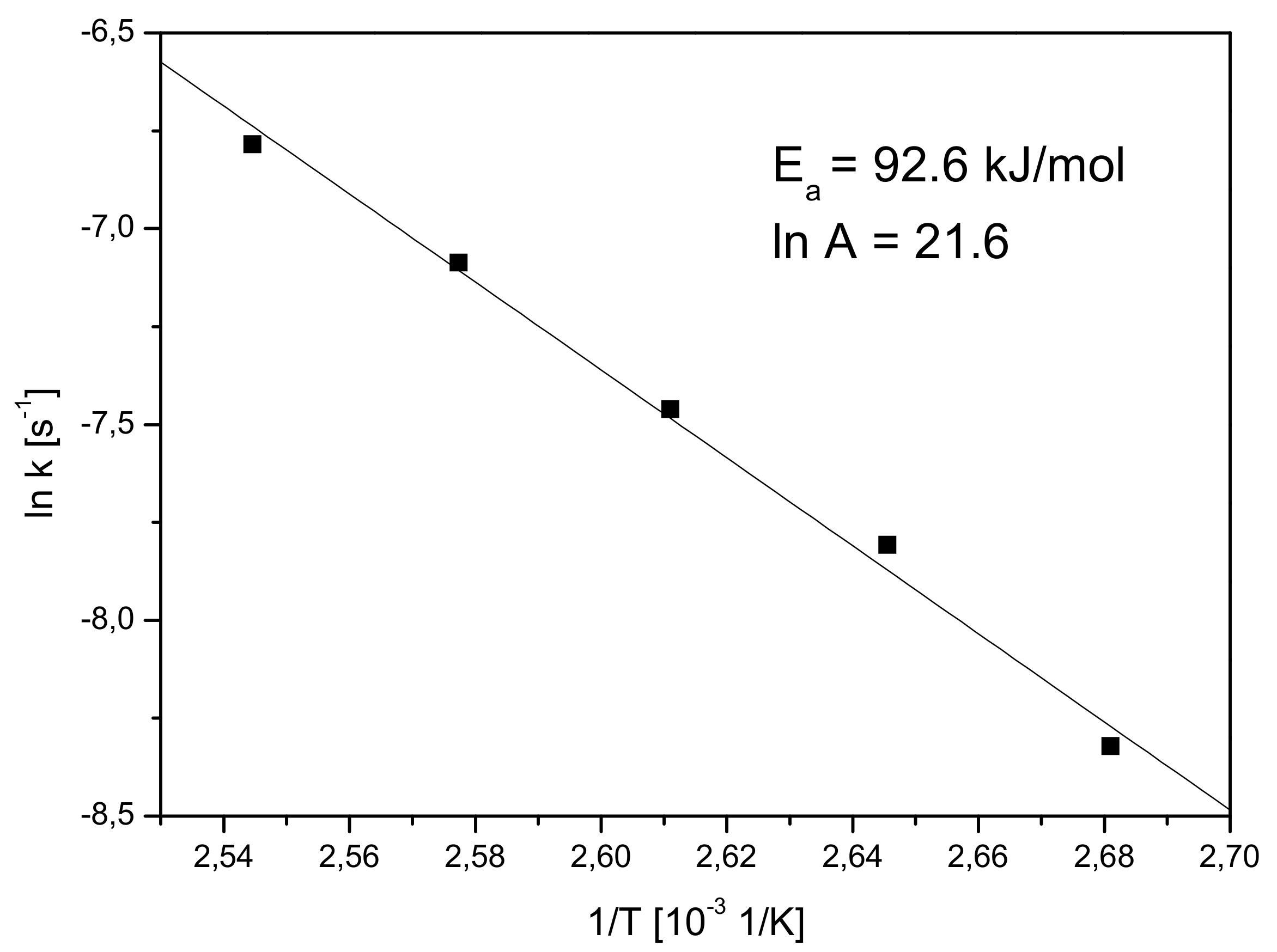
2.2.5. Kinetic Analysis
2.2.6. Effect of Catalyst Particle Size
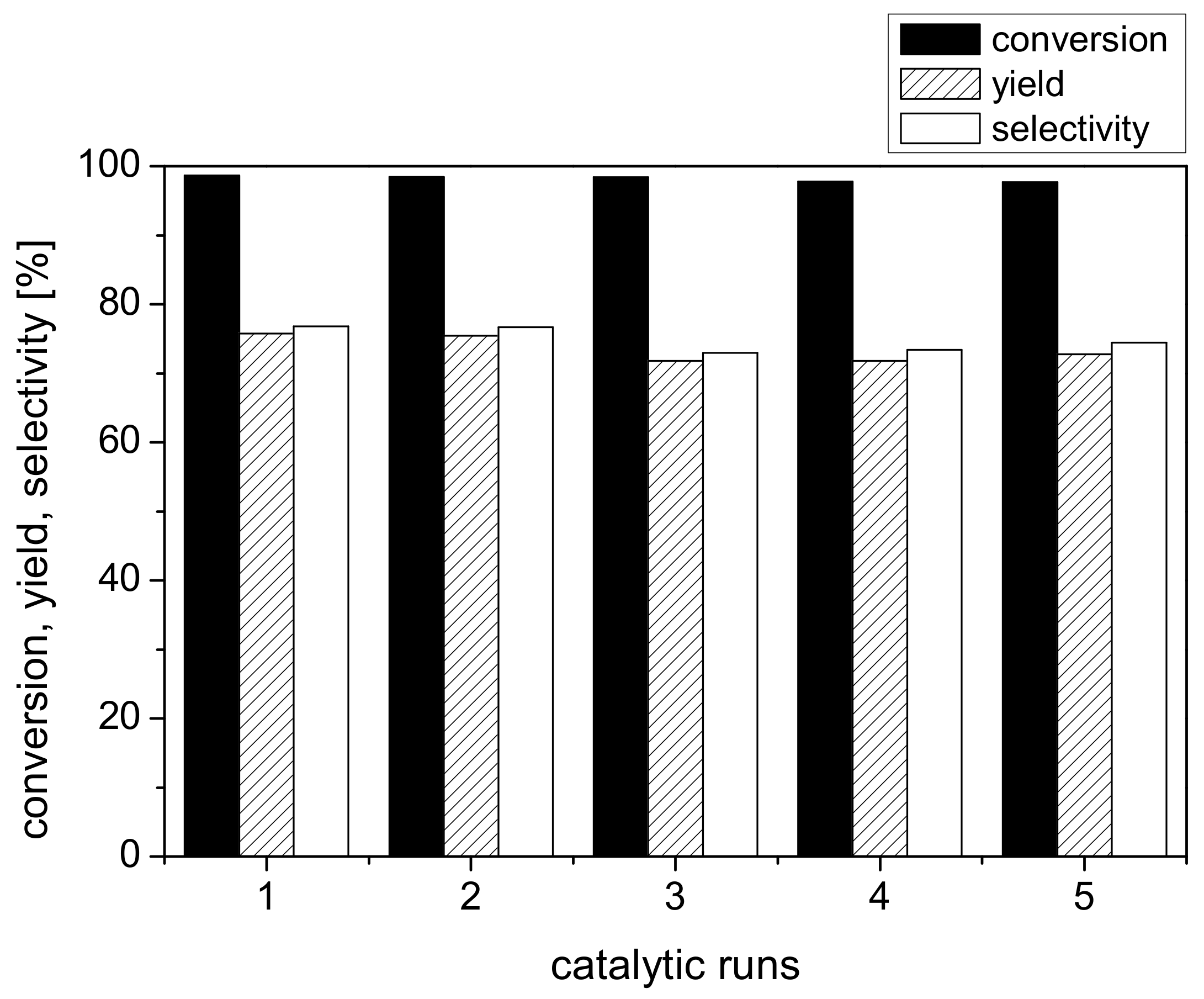
2.3. Recyclability of the Catalyst
3. Materials and Methods
3.1. Materials
3.2. Catalytic Conversion of Fructose into HMF
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Putten, R.J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Bai, X.; Du, Y. Conversion of biomass into 5-hydroxymethylfurfural using solid acid catalyst. Bioresour. Technol. 2011, 102, 3424–3429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Tong, X.; Xia, F.; Zheng, C.; Qin, L.; Jiang, X. Efficient Hydroxymethylfurfural Production over Phosphoric Carbon Solid Acids. Catal. Lett. 2018, 148, 1848–1855. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Vorlop, K.-D.; Prüße, U.; Krieg, R.; Teevs, L. Improved 5-Hydroxymethylfurfural Production Using a Multi-Fluorinated Alcohol Compound. U.S. Patent 10,005,748, 26 June 2018. [Google Scholar]
- Weingart, E.; Teevs, L.; Krieg, R.; Prüße, U. Hexafluoroisopropanol as low boiling extraction solvent for 5-hydroxymethylfurfural production. Energy Technol. 2017, 6, 432–440. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Uozumi, R.; Satsuma, A. Enhanced production of hydroxymethylfurfural from fructose with solid acid catalysts by simple water removal methods. Catal. Commun. 2009, 10, 1849–1853. [Google Scholar] [CrossRef]
- Ohara, M.; Takagaki, A.; Nishimura, S.; Ebitani, K. Syntheses of 5-hydroxymethylfurfural and levoglucosan by selective dehydration of glucose using solid acid and base catalysts. Appl. Catal. A Gen. 2010, 383, 149–155. [Google Scholar] [CrossRef]
- Wang, F.; Shi, A.-W.; Qin, X.-X.; Liu, C.-L.; Dong, W.-S. Dehydration of fructose to 5-hydroxymethylfurfural by rare earth metal trifluoromethanesulfonates in organic solvents. Carbohydr. Res. 2011, 346, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Efficient process for conversion of fructose to 5-hydroxymethylfurfural with ionic liquids. Green Chem. 2009, 11, 1327–1331. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, B.; Luo, J.; Ren, Y.; Zhang, Z. Efficient conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by the chromium-exchanged montmorillonite K-10 clay. Biomass Bioenergy 2014, 60, 171–177. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Song, C.; Gu, X.; Li, H.; Zhu, W.; Yin, S.; Han, C. The dehydration of fructose to 5-hydroxymethylfurfural efficiently catalyzed by acidic ion-exchange resin in ionic liquid. Bioresour. Technol. 2013, 133, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Floyd, A.J.; Kinsman, R.G.; Roshan-Ali, Y. Dehydration Reactions of Fructose in Non-aqueous Media. Chem. Tech. Biotechnol. 1982, 32, 920–924. [Google Scholar] [CrossRef]
- Lai, L.; Zhang, Y. The Production of 5-Hydroxymethylfurfural from Fructose in Isopropyl Alcohol: A Green and Efficient System. ChemSusChem 2011, 4, 1745–1748. [Google Scholar] [CrossRef] [PubMed]
- Bicker, M.; Hirth, J.; Vogel, H. Dehydration of fructose to 5-hydroxymethylfurfural in sub- and supercritical acetone. Green Chem. 2003, 5, 280–284. [Google Scholar] [CrossRef]
- Kuster, B.F.M.; van der Baan, H.S. The influence of pH and weak-acid anions on the dehydration of d-fructose. Carbohydr. Res. 1977, 54, 165–176. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Selective Conversion of D-Fructose to 5-Hydroxymethylfurfural by Ion-Exchange Resin in Acetone/Dimethyl sulfoxide Solvent Mixtures. Ind. Eng. Chem. Res. 2008, 47, 9234–9239. [Google Scholar] [CrossRef]
- Sampath, G.; Kannan, S. Fructose dehydration to 5-hydroxymethylfurfural: Remarkable solvent influence on recyclability of Amberlyst-15 catalyst and regeneration studies. Catal. Commun. 2013, 37, 41–44. [Google Scholar] [CrossRef]
- Antonetti, C.; Raspolli Galetti, A.M.; Fulignati, S.; Licursi, D. Amberlyst A-70: A surprisingly active catalyst for the MW-assisted dehydration of fructose and inulin to HMF in water. Catal. Commun. 2017, 97, 146–150. [Google Scholar] [CrossRef]
- Weingart, E.; Tschirner, S.; Teevs, L.; Prüße, U. Conversion of Fructose to HMF in a Continuous Fixed Bed Reactor with Outstanding Selectivity. Molecules 2018, in press. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the author. |



| Temperature (°C) | Reaction Time (min) | Fructose Conversion (%) | HMF Selectivity (%) | HMF Yield (%) |
|---|---|---|---|---|
| 90 | 405 | 95 | 67 | 64 |
| 95 | 315 | 98 | 69 | 67 |
| 100 | 225 | 97 | 72 | 70 |
| 105 | 137 | 98 | 72 | 71 |
| 110 | 129 | 99 | 71 | 70 |
| 115 | 105 | 99 | 67 | 66 |
| 120 | 90 | 99 | 68 | 67 |
| Particle Size (µm) | Reaction Time (min) | Fructose Conversion (%) | HMF Selectivity (%) | HMF Yield (%) |
|---|---|---|---|---|
| Original (>500) | 137 | 98 | 72 | 71 |
| 150–300 | 120 | 99 | 76 | 75 |
| 25–100 | 120 | 99 | 77 | 76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tschirner, S.; Weingart, E.; Teevs, L.; Prüße, U. Catalytic Dehydration of Fructose to 5-Hydroxymethylfurfural (HMF) in Low-Boiling Solvent Hexafluoroisopropanol (HFIP). Molecules 2018, 23, 1866. https://doi.org/10.3390/molecules23081866
Tschirner S, Weingart E, Teevs L, Prüße U. Catalytic Dehydration of Fructose to 5-Hydroxymethylfurfural (HMF) in Low-Boiling Solvent Hexafluoroisopropanol (HFIP). Molecules. 2018; 23(8):1866. https://doi.org/10.3390/molecules23081866
Chicago/Turabian StyleTschirner, Sarah, Eric Weingart, Linda Teevs, and Ulf Prüße. 2018. "Catalytic Dehydration of Fructose to 5-Hydroxymethylfurfural (HMF) in Low-Boiling Solvent Hexafluoroisopropanol (HFIP)" Molecules 23, no. 8: 1866. https://doi.org/10.3390/molecules23081866







