Supercritical CO2 Extraction of Nannochloropsis sp.: A Lipidomic Study on the Influence of Pretreatment on Yield and Composition
Abstract
:1. Introduction
2. Results and Discussion
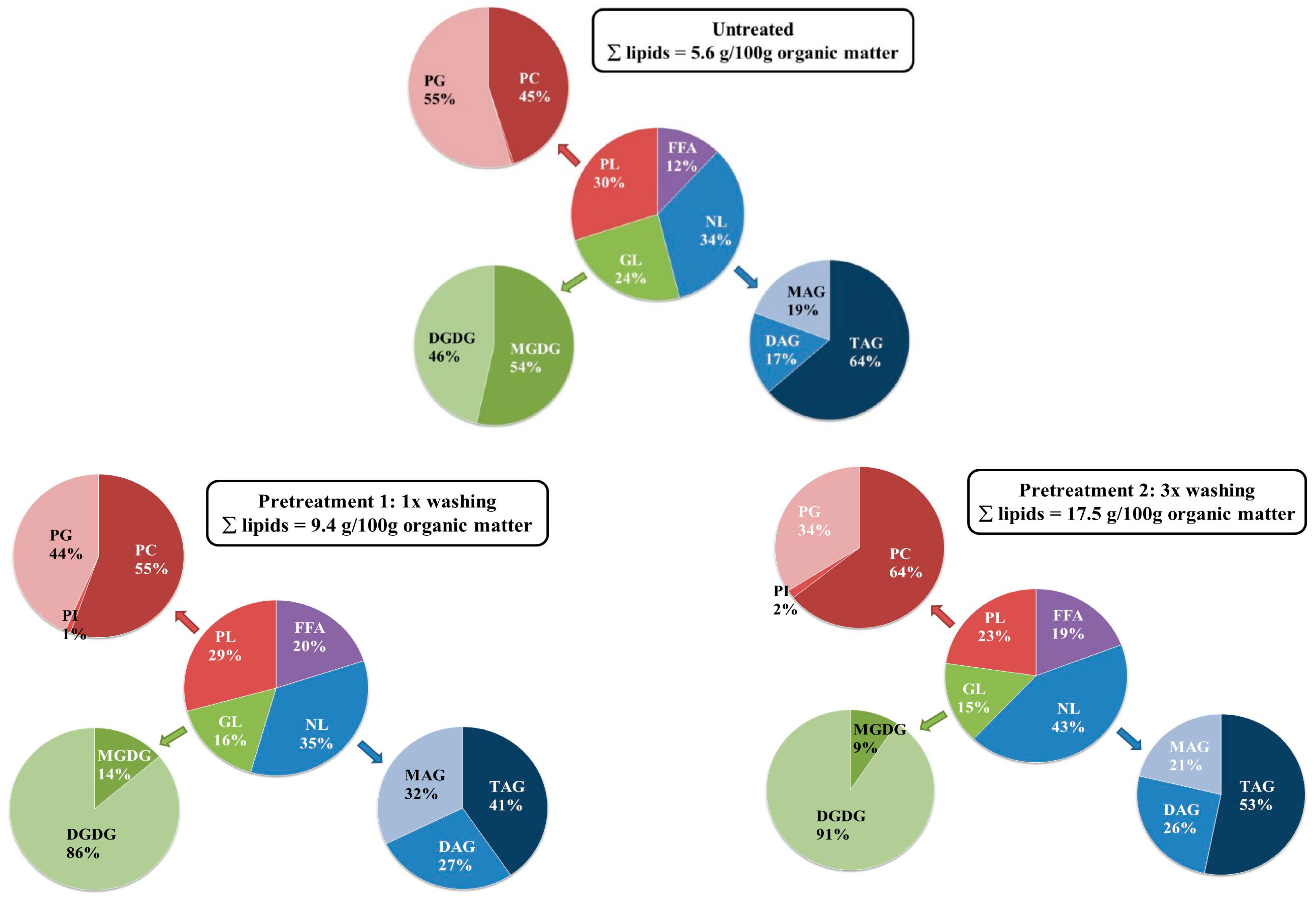
2.1. Influence of the Washing and Freeze-Drying Procedure on the Algal Biomass
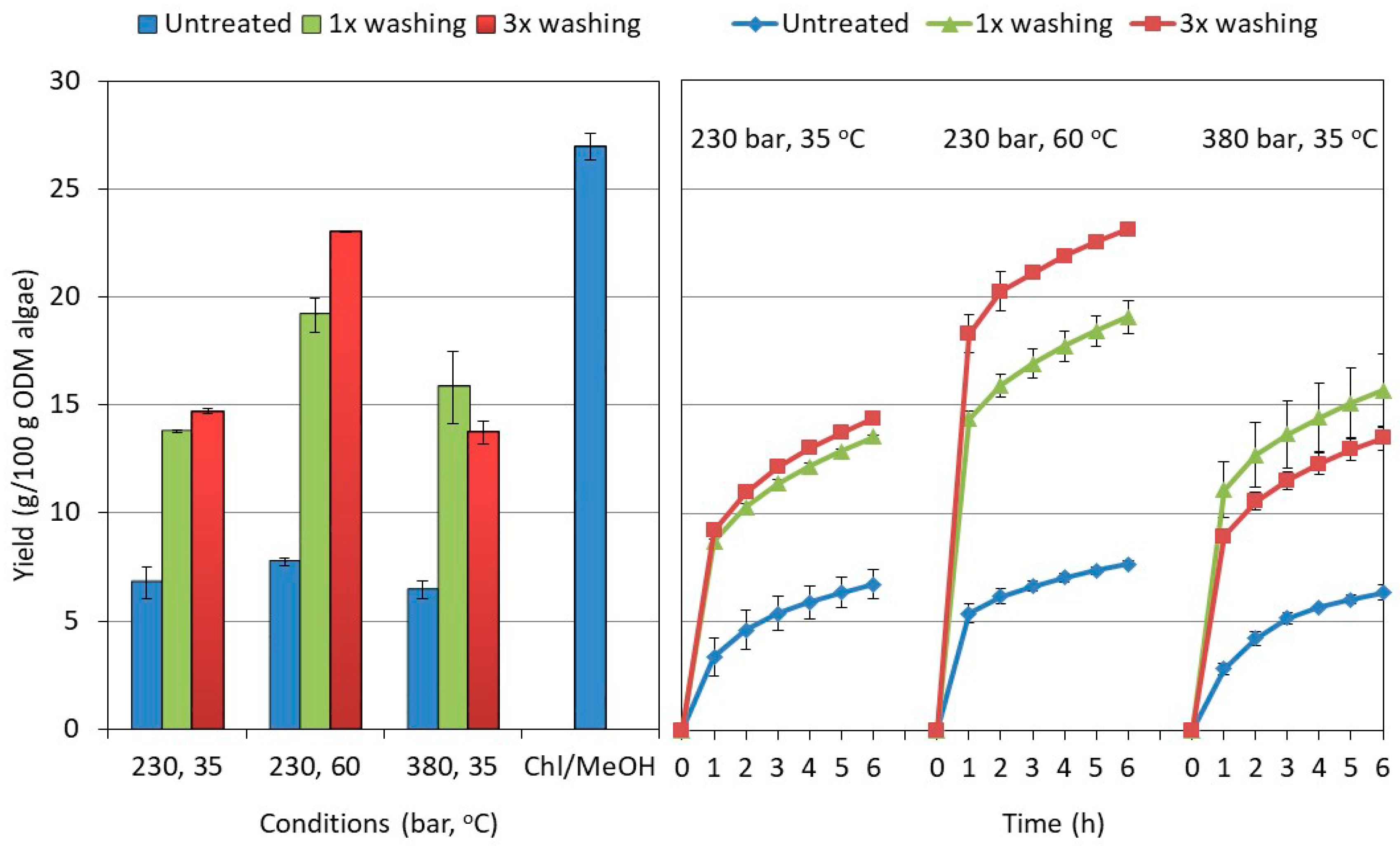
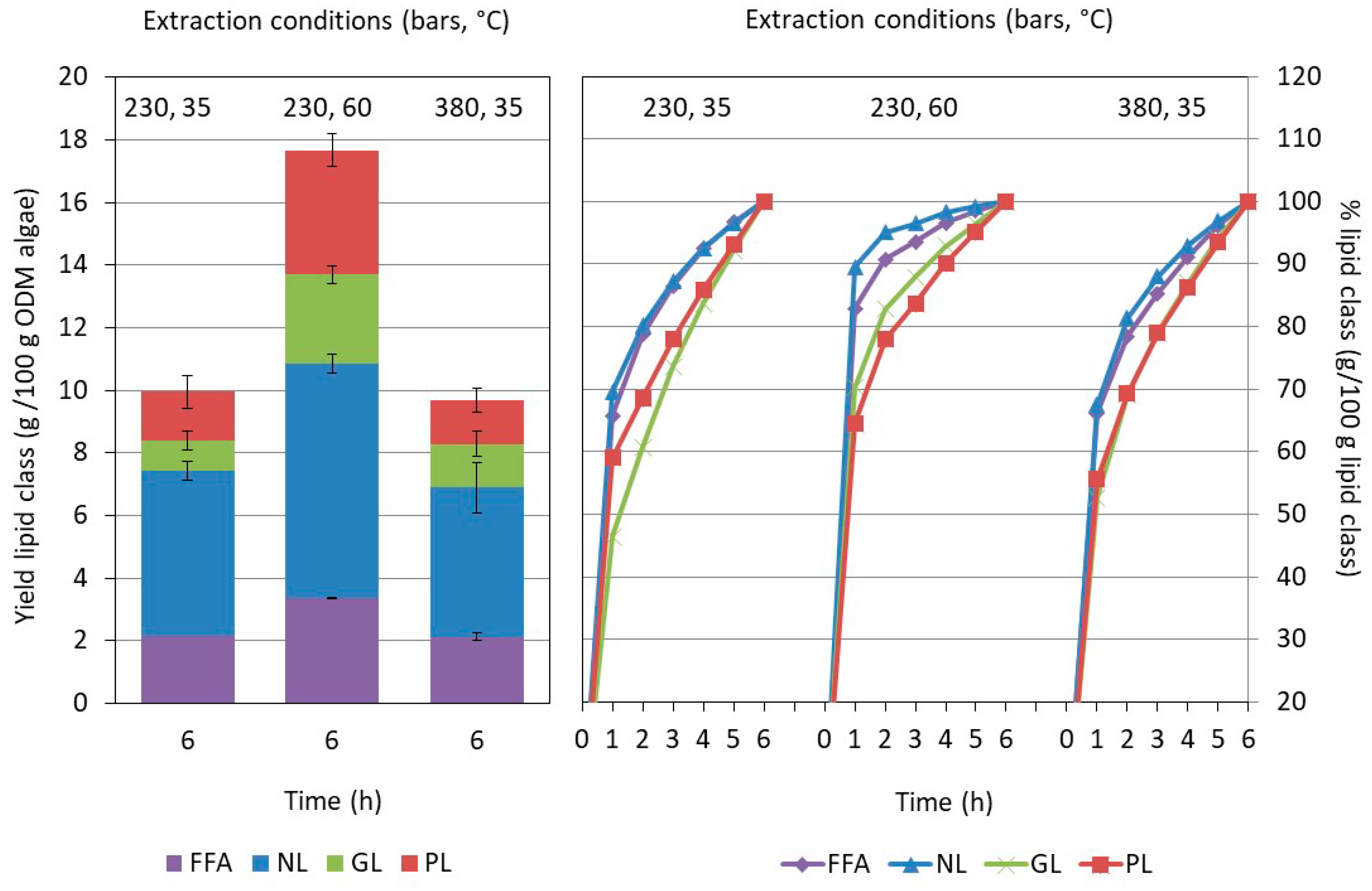
2.2. Influence of the Washing and Freeze-Drying Procedure on the Supercritical CO2 Extraction
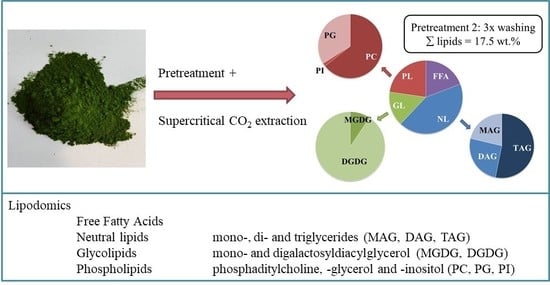
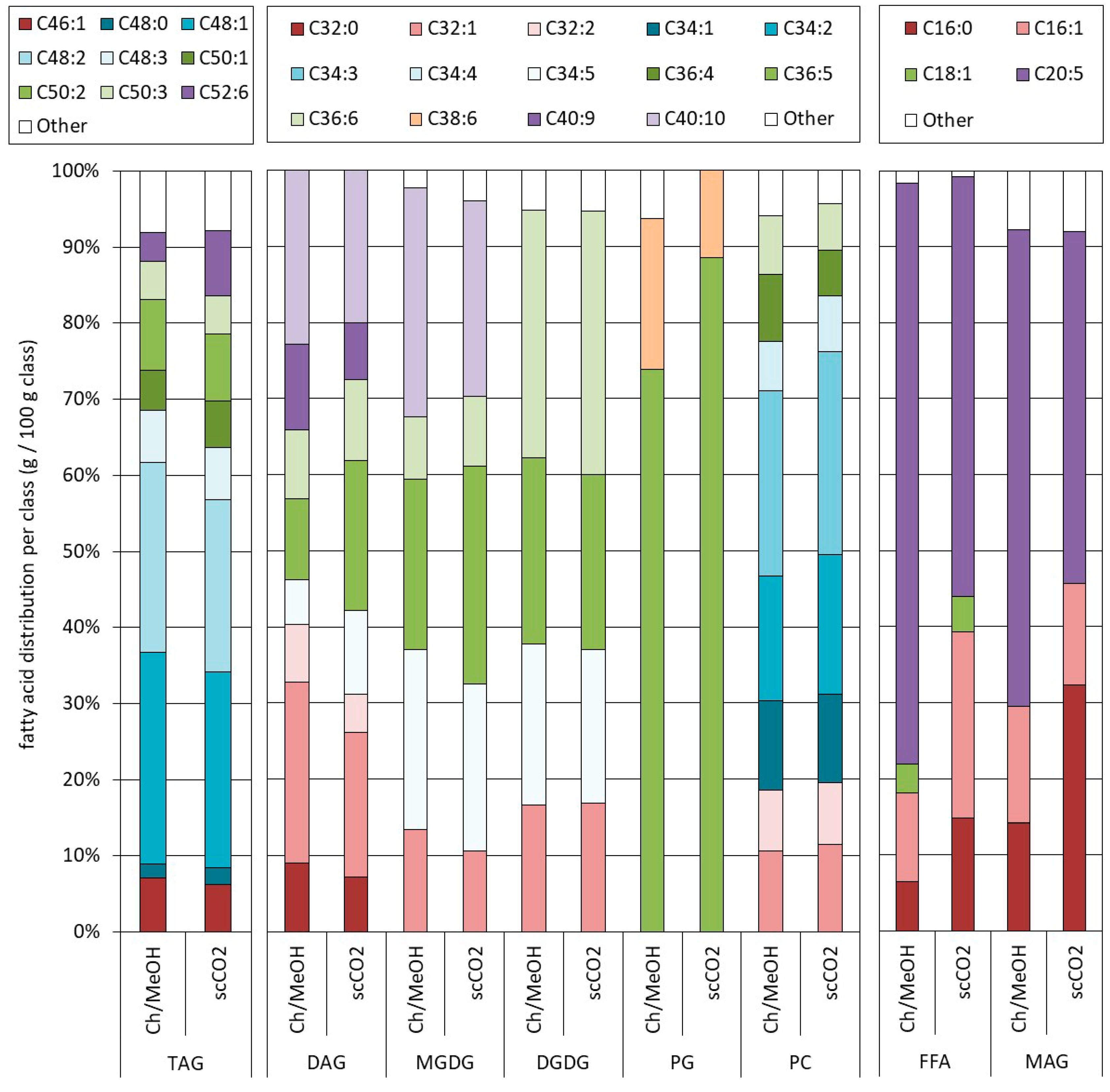
2.3. Influence of the Pretreatment and Extraction Conditions on the Lipid Composition of the Extract
2.3.1. Influence of Pretreatment on the Composition at Optimal Extraction Conditions
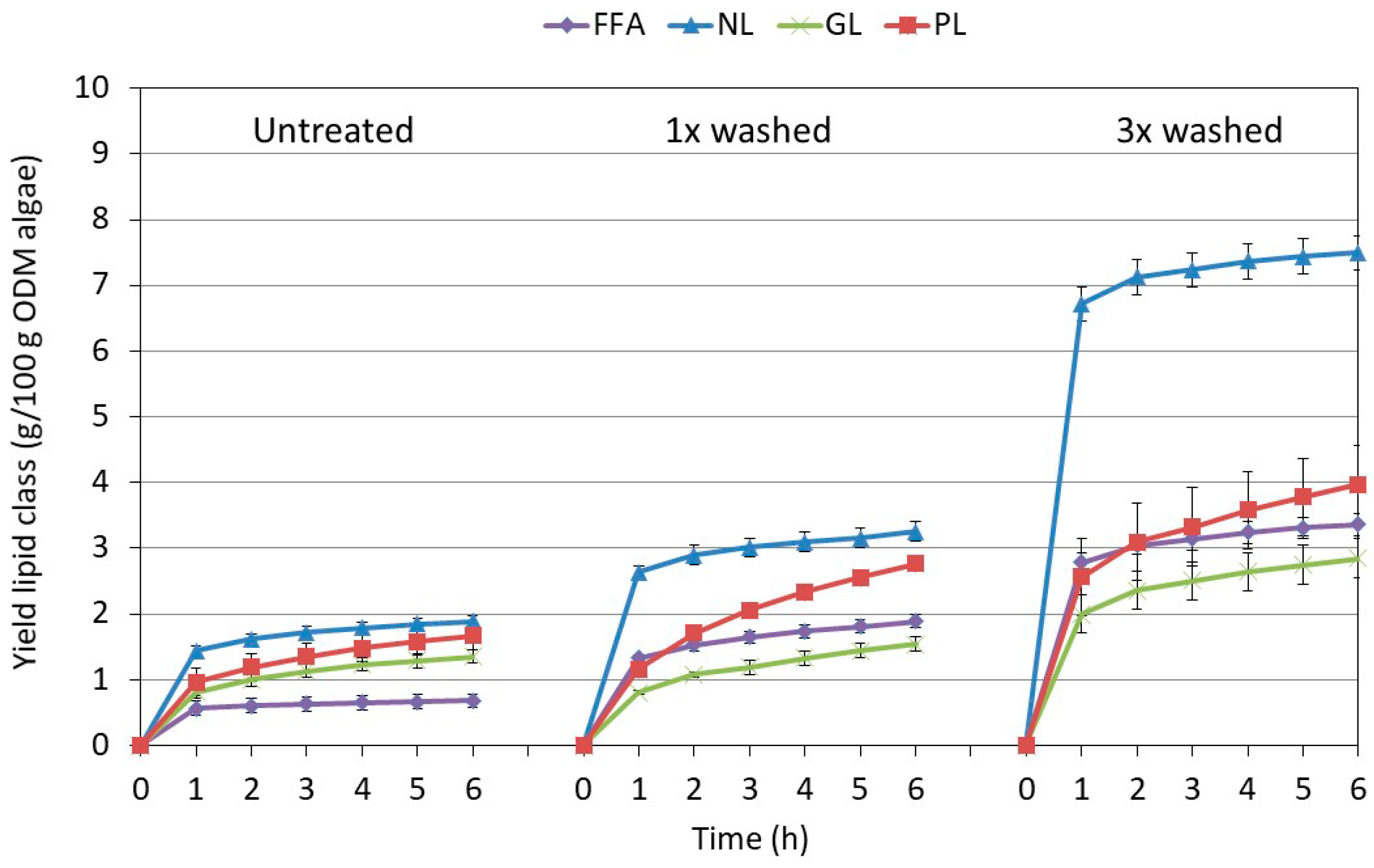
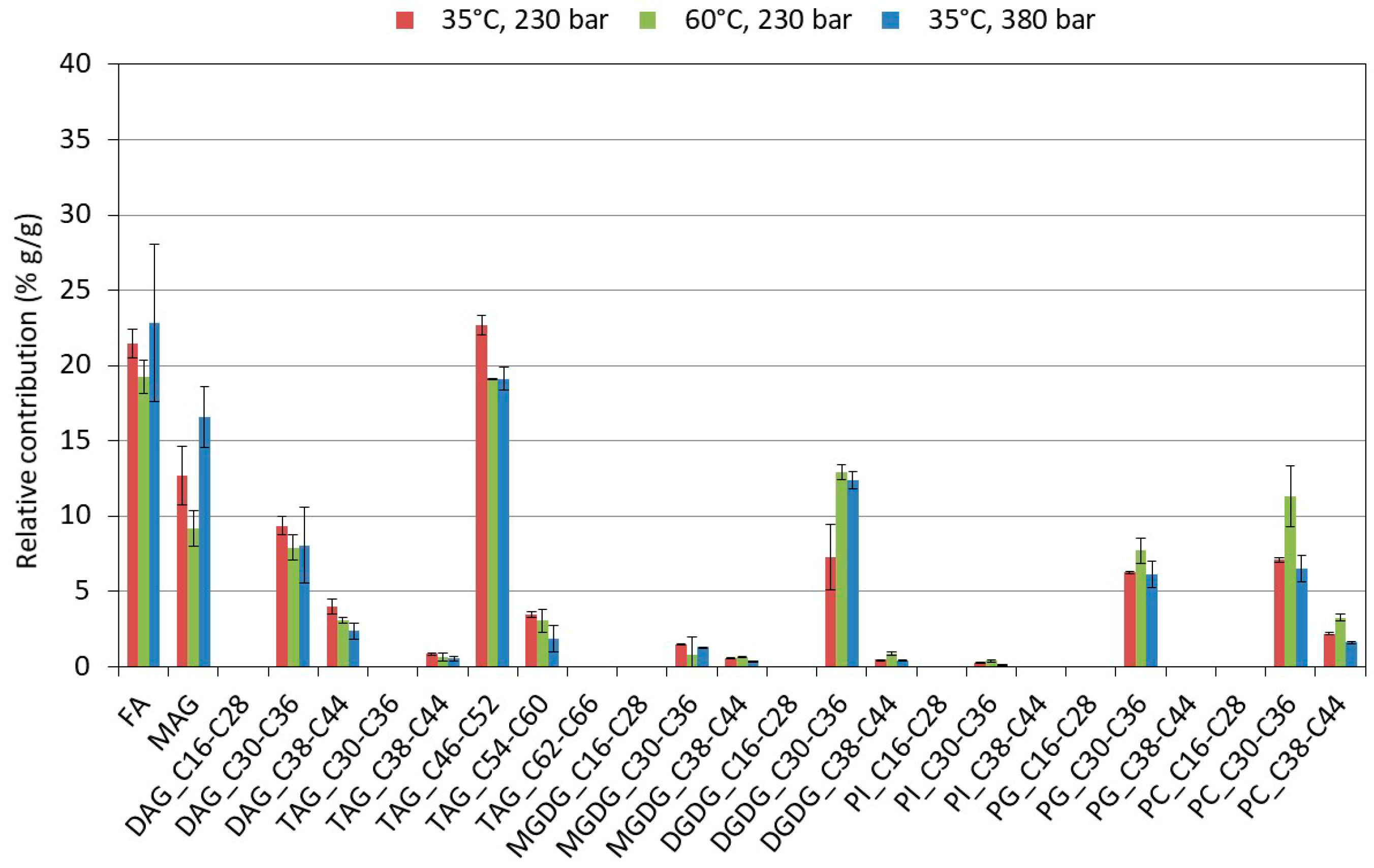
2.3.2. Influence of the Extraction Conditions on the Composition for Algae Pretreated Three Times
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Pretreatment of Nannochloropsis sp.
3.3. Reference Method: Extraction with 1:1 Chloroform/Methanol (v/v)
3.4. Supercritical CO2 Extraction
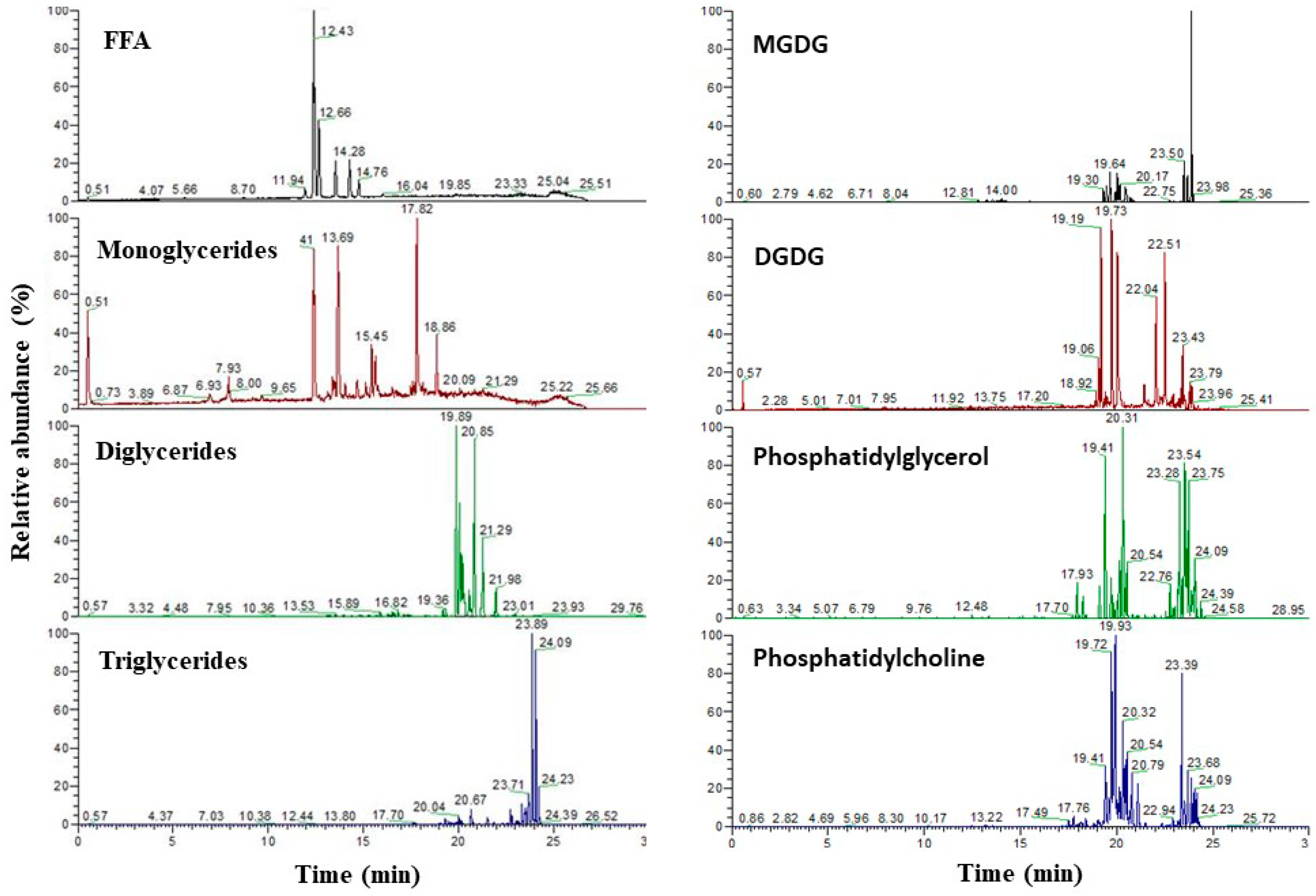
3.5. Characterization of the Lipids
3.6. Calculations and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DGDG | Digalactosyldiglyceride |
| FFAs | Free Fatty Acids |
| GLs | Glycolipids |
| HESI | Heated-electrospray ionization |
| MDGD | Monogalactosyldiglyceride |
| NLs | Neutral lipids |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| ODM | Organic dry matter |
| PC | Phosphatidylcholine |
| PG | Phosphatidylglycerol |
| PI | Phosphatidylinositol |
| PLs | Phospholipids |
| scCO2 | Supercritical Carbon dioxide |
| SFE | Supercritical Fluid Extraction |
| UHPLC-PDA-amMS | Ultra-High Performance Liquid Chromatography-Photodiode Array-Accurate mass spectrometry |
| UHPLC-PDA-HR-MS | Ultra-High Performance Liquid Chromatography-Photodiode Array-High resolution-mass spectrometry |
References
- Ruocco, N.; Costantini, S.; Guariniello, S.; Costantini, M. Polysaccharides from the marine environment with pharmacological, cosmeceutical and nutraceutical potential. Molecules 2016, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, R.H.; Barbosa, M.J. An outlook on microalgal biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pohnert, G.; Wei, D. Extracellular metabolites from industrial microalgae and their biotechnological potential. Mar. Drugs 2016, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.M.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, L.; Van Roy, S.; Thomassen, G.; Elst, K. Biorefinery of Algae: Technical and Economic Considerations. In Microalgae-Based biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Gonzales-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2018; ISBN 9780081010273. [Google Scholar]
- Ma, X.N.; Chen, T.P.; Yang, B.; Liu, J.; Chen, F. Lipid production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Akito, N.; Nobutoshi, M.; Sakakibara, J.; Harukuni, T.; Hoyoku, N.; Akio, I. Anti-tumour-promoting glyceroglycolipids from the green alga, Chlorella vulgaris. Phytochemistry 1995, 40, 1433–1437. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Muylaert, K.; Foubert, I. Optimization of an analytical procedure for extraction of lipids from microalgae. J. Am. Oil Chem. Soc. 2012, 89, 189–198. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Jeevan Kumar, S.P.; Vijay Kumar, G.; Dash, A.; Scholz, P.; Banerjee, R. Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Res. 2017, 21, 138–147. [Google Scholar] [CrossRef]
- Halim, R.; Gladman, B.; Danquah, M.K.; Webley, P.A. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Solana, M.; Rizza, C.S.; Bertucco, A. Exploiting microalgae as a source of essential fatty acids by supercritical fluid extraction of lipids: Comparison between Scenedesmus obliquus, Chlorella protothecoides and Nannochloropsis salina. J. Supercrit. Fluids 2014, 92, 311–318. [Google Scholar] [CrossRef]
- Cheng, C.H.; Du, T.B.; Pi, H.C.; Jang, S.M.; Lin, Y.H.; Lee, H.T. Comparative study of lipid extraction from microalgae by organic solvent and supercritical CO2. Bioresour. Technol. 2011, 102, 10151–10153. [Google Scholar] [CrossRef] [PubMed]
- Mouahid, A.; Crampon, C.; Toudji, S.A.A.; Badens, E. Supercritical CO2 extraction of neutral lipids from microalgae: Experiments and modelling. J. Supercrit. Fluids 2013, 77, 7–16. [Google Scholar] [CrossRef]
- Servaes, K.; Maesen, M.; Prandi, B.; Sforza, S.; Elst, K. Polar Lipid Profile of Nannochloropsis oculata Determined Using a Variety of Lipid Extraction Procedures. J. Agric. Food Chem. 2015, 63, 3931–3941. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.L.; Reis, A.D.; Palavra, A.F. Supercritical CO2extraction of γ-linolenic acid and other lipids from Arthrospira (Spirulina)maxima: Comparison with organic solvent extraction. Food Chem. 2006, 99, 57–63. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Crampon, C.; Mouahid, A.; Toudji, S.A.A.; Leṕine, O.; Badens, E. Influence of pretreatment on supercritical CO2extraction from Nannochloropsis oculata. J. Supercrit. Fluids 2013, 79, 337–344. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.; Elst, K.; Wijffels, R. Flow cytometry to estimate the cell disruption yield and biomass release of Chlorella sp. during bead milling. Algal Res. 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Ranjith Kumar, R.; Hanumantha Rao, P.; Arumugam, M. Lipid Extraction Methods from Microalgae: A Comprehensive Review. Front. Energy Res. 2015, 2, 1–9. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M.; Tariq, S. Supercritical carbon dioxide extraction of microalgae lipid: Process optimization and laboratory scale-up. J. Supercrit. Fluids 2014, 86, 57–66. [Google Scholar] [CrossRef]
- Viguera, M.; Marti, A.; Masca, F.; Prieto, C.; Calvo, L. The process parameters and solid conditions that affect the supercritical CO2extraction of the lipids produced by microalgae. J. Supercrit. Fluids 2016, 113, 16–22. [Google Scholar] [CrossRef]
- Sovová, H.; Nobre, B.P.; Palavra, A. Modelling of the kinetics of supercritical fluid extraction of lipids from microalgae with emphasis on extract desorption. Materials 2016, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Sovová, H. Mathematical model for supercritical fluid extraction of natural products and extraction curve evaluation. J. Supercrit. Fluids 2005, 33, 35–52. [Google Scholar] [CrossRef]
- Siaut, M.; Cuiné, S.; Cagnon, C.; Fessler, B.; Nguyen, M.; Carrier, P.; Beyly, A.; Beisson, F.; Triantaphylidès, C.; Li-Beisson, Y.; et al. Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.A. Lipids and membrane function in green algae. Biochim. Biophys. Acta-Lipids Lipid Metab. 1996, 1302, 17–45. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Bermúdez, S.P.C.; Termote-Verhalle, R.; Bruneel, C.; Muylaert, K.; Parra-Saldivar, R.; Foubert, I. Influence of extraction solvent system on the extractability of lipid components from the biomass of Nannochloropsis gaditana. J. Appl. Phycol. 2014, 26, 1501–1510. [Google Scholar] [CrossRef]
- Andrich, G.; Nesti, U.; Venturi, F.; Zinnai, A.; Fiorentini, R. Supercritical fluid extraction of bioactive lipids from the microalga Nannochloropsis sp. Eur. J. Lipid Sci. Technol. 2005, 107, 381–386. [Google Scholar] [CrossRef]
- Yao, L.; Gerde, J.A.; Lee, S.L.; Wang, T.; Harrata, K.A. Microalgae lipid characterization. J. Agric. Food Chem. 2015, 63, 1773–1787. [Google Scholar] [CrossRef] [PubMed]
- Fret, J.; Roef, L.; Blust, R.; Diels, L.; Tavernier, S.; Vyverman, W.; Michiels, M. Reuse of rejuvenated media during laboratory and pilot scale cultivation of Nannochloropsis sp. Algal Res. 2017, 27, 265–273. [Google Scholar] [CrossRef]
- Dos Santos, R.R.; Moreira, D.M.; Kunigami, C.N.; Aranda, D.A.G.; Teixeira, C.M.L.L. Comparison between several methods of total lipid extraction from Chlorella vulgaris biomass. Ultrason. Sonochem. 2015, 22, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Bijttebier, S.; D’Hondt, E.; Noten, B.; Hermans, N.; Apers, S.; Voorspoels, S. Ultra high performance liquid chromatography versus high performance liquid chromatography: Stationary phase selectivity for generic carotenoid screening. J. Chromatogr. A 2014, 1332. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the extracts produced are not available from the authors. |
| Process | Algae Product | ||||||
|---|---|---|---|---|---|---|---|
| Wash Water 1/2/3 | Algae End | ||||||
| Conductivity (ms/cm) | DM (g/100 g) | Yield (g/100 g) | DM (g/100 g) | Ash (g/100 g) | ODM (g/100 g) | Disr% (%) | |
| Untreated | - | - | - | 96.4 ± 0.1 | 7.9 ± 1.0 | 88.57 ± 0.04 | 0 |
| Pretreatment 1 | 9.0 | 1.7 | 82.5 | 92.7 ± 0.1 | 4.1 ± 0.3 | 88.6 ± 0.1 | 66 1 |
| Pretreatment 2 | 8.5/2.2/0.6 | 0.2/0.5/4.5 | 73.5 | 85.5 ± 1.1 | 2.9 ± 0.9 | 82.6 ± 1.1 | nd 2/59 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elst, K.; Maesen, M.; Jacobs, G.; Bastiaens, L.; Voorspoels, S.; Servaes, K. Supercritical CO2 Extraction of Nannochloropsis sp.: A Lipidomic Study on the Influence of Pretreatment on Yield and Composition. Molecules 2018, 23, 1854. https://doi.org/10.3390/molecules23081854
Elst K, Maesen M, Jacobs G, Bastiaens L, Voorspoels S, Servaes K. Supercritical CO2 Extraction of Nannochloropsis sp.: A Lipidomic Study on the Influence of Pretreatment on Yield and Composition. Molecules. 2018; 23(8):1854. https://doi.org/10.3390/molecules23081854
Chicago/Turabian StyleElst, Kathy, Miranda Maesen, Griet Jacobs, Leen Bastiaens, Stefan Voorspoels, and Kelly Servaes. 2018. "Supercritical CO2 Extraction of Nannochloropsis sp.: A Lipidomic Study on the Influence of Pretreatment on Yield and Composition" Molecules 23, no. 8: 1854. https://doi.org/10.3390/molecules23081854