Squalene Cyclases and Cycloartenol Synthases from Polystichum polyblepharum and Six Allied Ferns
Abstract
:1. Introduction
2. Results
2.1. Isolation of cDNAs Encoding SC and OSC
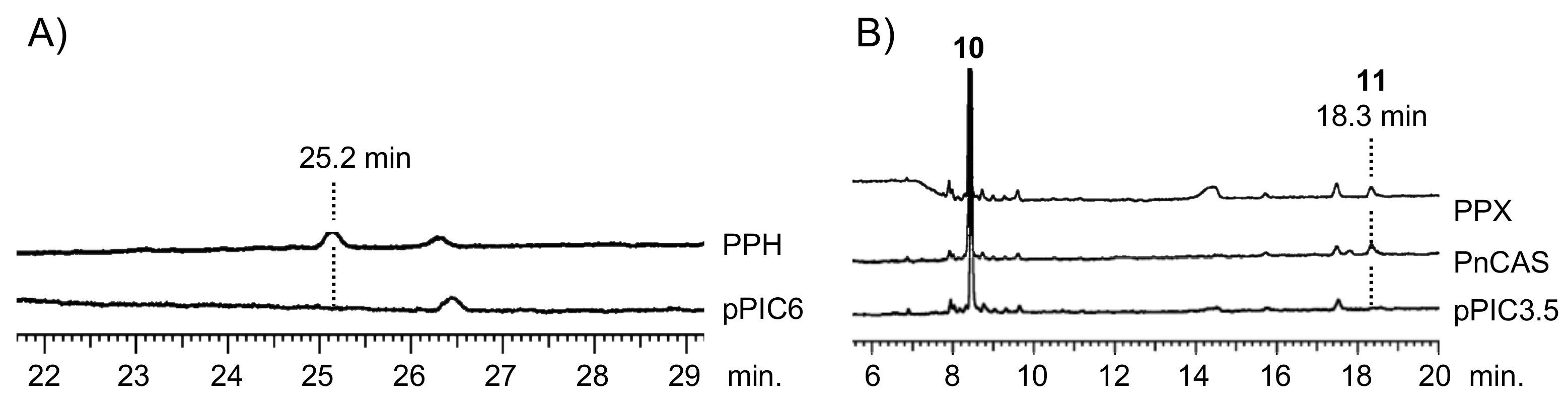
2.2. Heterologous Expression and Biochemical Characterization of PPH and PPX
2.3. Comparison of Active Site Residues between Hopene and Hydroxyhopane Synthases
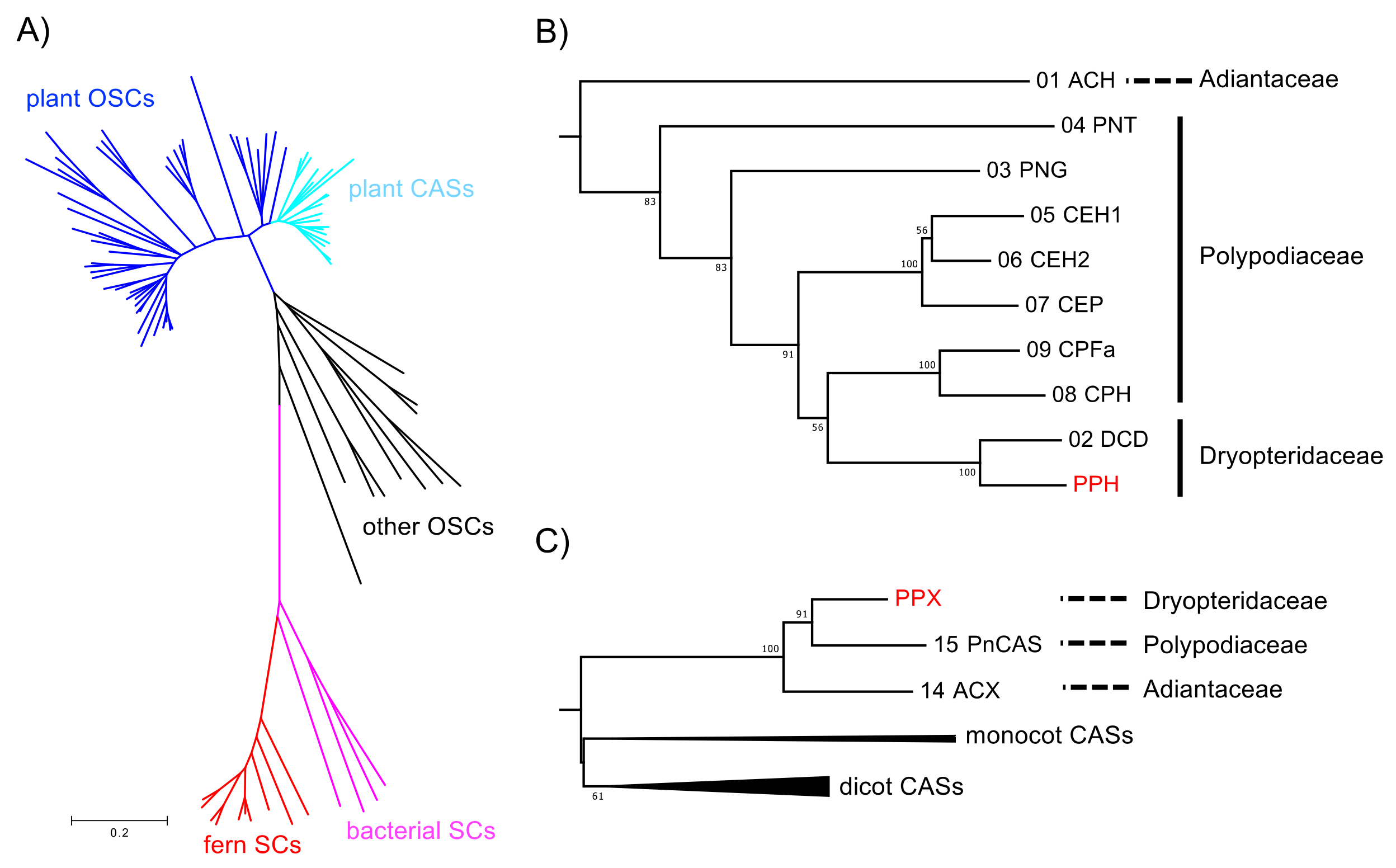
2.4. Phylogenetic Analysis of SCs and OSCs
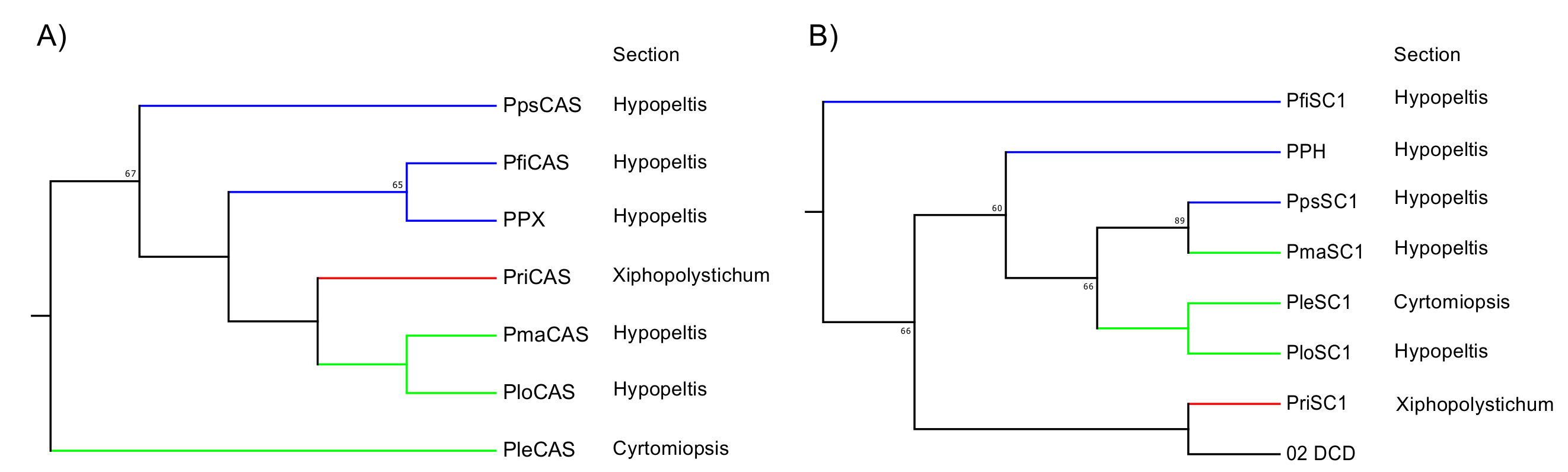
2.5. Phylogenetic Relationships between Polystichum Triterpene Synthases
3. Discussion
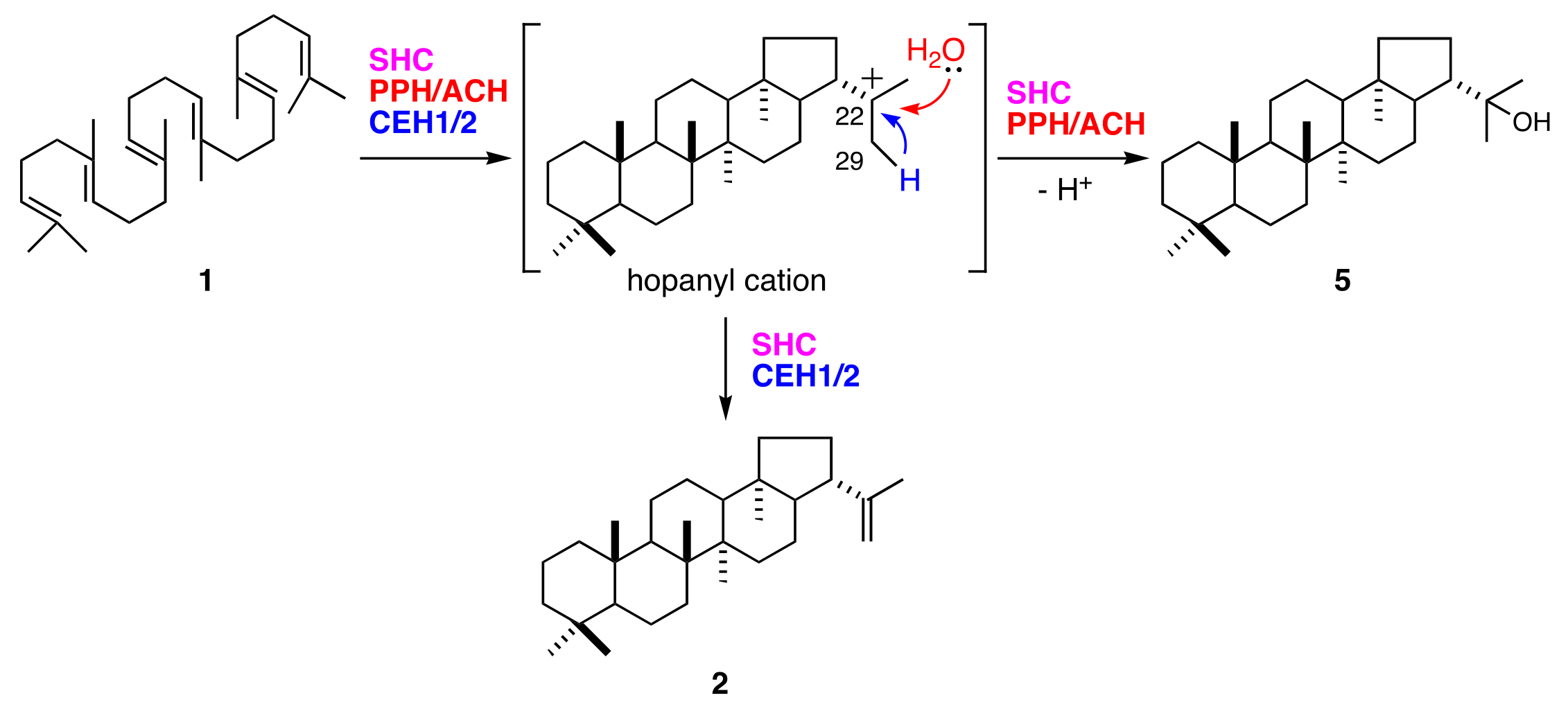
3.1. Hydroxyhopane Synthases and Hopene Synthases
3.2. Relationship between Phylogeny and Taxonomy
4. Materials and Methods
4.1. RNA Extraction and cDNA Synthesis
4.2. Cloning of Squalene Cyclase cDNAs
4.3. PPH Expression in Pichia pastoris and Product Analysis
4.4. Cloning of Oxidosqualene Cyclase cDNA
4.5. Expression of PPX in Pichia pastoris and Analysis of Product
4.6. Phylogenetic Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murakami, T.; Tanaka, N. Progress in the Chemistry of Organic Natural Products; Springer-Verlag: New York, NY, USA, 1988; Volume 54. [Google Scholar]
- Konoshima, T.; Takasaki, M.; Tokuda, H.; Masuda, K.; Arai, Y.; Shiojima, K.; Ageta, H. Antitumor-promoting activities of triterpenoids from ferns. I. Biol. Pharm. Bull. 1996, 19, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Na, M.K.; Jang, J.P.; Min, B.S.; Lee, S.J.; Lee, M.S.; Kim, B.Y.; Oh, W.K.; Ahn, J.-S. Fatty acid synthase inhibitory activity of acylphloroglucinols isolated from Dryopteris crassirhizoma. Bioorg. Med. Chem. Lett. 2006, 16, 4738–4742. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Na, M.K.; An, R.B.; Min, B.S.; Lee, H.K. Antioxidant activity of two phloroglucinol derivatives from Dryopteris crassirhizoma. Biol. Pharm. Bull. 1996, 26, 1354–1356. [Google Scholar] [CrossRef]
- Ochs, D.; Kaletta, C.; Entian, K.-D.; Beck-Sickinger, A.; Poralla, K. Cloning, expression, and sequencing of squalene-hopene cyclase, a key enzyme in triterpenoid metabolism. J. Bacteriol. 1992, 174, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Reipen, I.; Poralla, K.; Sahm, H.; Sprenger, G.A. Zymomonas mobilis squalene-hopene cyclase gene (shc): Cloning, DNA sequence analysis, and expression in Escherichia coli. Microbiology 1995, 141, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Perzl, M.; Müller, P.; Poralla, K.; Kannenberg, E.L. Squalene-hopene cyclase from Bradyrhizobium japonicum: Cloning, expression, sequence analysis and comparison to other triterpenoid cyclases. Microbiology 1997, 143, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Tippelt, A.; Jahnke, L.; Poralla, K. Squalene-hopene cyclase from Methylococcus capsulatus (Bath): A bacterium producing hopanoids and steroids. Biochem. Biophys. Acta 1998, 1391, 223–232. [Google Scholar] [CrossRef]
- Ghimire, G.P.; Oh, T.J.; Lee, H.C.; Sohng, J.K. Squalene-hopene cyclase (Spterp25) from Streptomyces peucetius: Sequence analysis, expression and functional characterization. Biotechnol. Lett. 2009, 31, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Seitz, M.; Klebensberger, J.; Siebenhaller, S.; Breuer, M.; Siedenburg, G.; Jendrossek, D.; Hauer, B. Substrate specificity of a novel squalene-hopene cyclase from Zymomonas mobilis. J. Mol. Cat. B Enzym. 2012, 84, 72–77. [Google Scholar] [CrossRef]
- Shinozaki, J.; Shibuya, M.; Masuda, K.; Ebizuka, Y. Squalene and oxidosqualene cyclase from a fern. FEBS Lett. 2008, 582, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, J.; Shibuya, M.; Masuda, K.; Ebizuka, Y. Dammaradiene synthase. A squalene cyclase from Dryopteris crassirhizoma Nakai. Phytochemistry 2008, 69, 2559–2564. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, J.; Shibuya, M.; Takahata, Y.; Masuda, K.; Ebizuka, Y. Molecular evolution of fern squalene cyclases. ChemBioChem 2010, 11, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, J.; Hiruta, M.; Okada, T.; Masuda, K. Migrated hopene synthase from Colysis pothifolia and identification of a migration switch controlling the number of 1,2-hydride and methyl shifts. ChemBioChem 2016, 17, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Little, D.P.; Barrington, D.S. Major evolutionary events in the origin and diversification of the fern genus Polystichum (Dryopteridaceae). Am. J. Bot. 2003, 90, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, K. Dryopteridaceae. Flora of Japan; Iwatsuki, K., Yamazaki, T., Boufford, D.E., Ohba, H., Eds.; Kodansha: Tokyo, Japan, 1995; Volume 1, pp. 120–173. ISBN 9784061546035. [Google Scholar]
- Shiojima, K.; Arai, Y.; Masuda, K.; Kamada, T.; Ageta, H. Fern constituents: Polypodatetraenes, novel bicyclic triterpenoids, isolated from Polypodiaceous and Aspidiaceous plants. Tetrahedron Lett. 1983, 24, 5733–5736. [Google Scholar] [CrossRef]
- Ageta, H.; Shiojima, K.; Arai, Y.; Kasama, T.; Kajii, K. Fern constituents: Dryocrassol and dryocrassyl acetate isolated from the leaves of aspidiaceous fern. Tetrahedron Lett. 1975, 16, 3297–3298. [Google Scholar] [CrossRef]
- Shiojima, K.; Arai, Y.; Masuda, K.; Kamada, T.; Suzuki, M.; Ageta, H. Chemotaxonomy of fern plants (V). Japanese Polystichum species. Nat. Med. 1997, 51, 523–527. [Google Scholar]
- Kushiro, T.; Shibuya, M.; Ebizuka, Y. Amyrin synthase. Cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur. J. Biochem. 1998, 256, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Shibuya, M.; Lee, M.-S.; Sankawa, U.; Ebizuka, Y. Molecular cloning of pea cDNA encoding cycloartenol synthase and its functional expression in yeast. Biol. Pharm. Bull. 1997, 20, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Ageta, H.; Shiojima, K.; Suzuki, H.; Nakamura, S. NMR spectra of triterpenoids. I. Conformation of the side chain of hopane and isohopane, and their derivatives. Chem. Pharm. Bull. 1993, 41, 1939–1943. [Google Scholar] [CrossRef]
- Seckler, B.; Poralla, K. Characterization and partial purification of squalene-hopene cyclase from Bacillus acidocaldarius. Biochem. Biophys. Acta 1986, 881, 356–363. [Google Scholar] [CrossRef]
- Kleemann, G.; Kellner, R.; Poralla, K. Purification and properties of the squalene-hopene cyclase from Rhodopseudomonas palustris, a purple non-sulfur bacterium producing hopanoids and tetrahymanol. Biochem. Biophys. Acta 1990, 1210, 317–320. [Google Scholar] [CrossRef]
- Sato, T.; Hoshino, T. Catalytic function of the residues of phenylalanine and tyrosine conserved in squalene-hopene cyclases. Biosci. Biotechnol. Biochem. 2001, 65, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kouda, M.; Hoshino, T. Site-directed mutagenesis experiments on the putative deprotonation site of squalene-hopene cyclase from Alicyclobacillus acidocaldarius. Biosci. Biotechnol. Biochem. 2004, 68, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-B.; Barrington, D.S. Flora of China; Wu, Z.-Y., Raven, P.H., Hong, D.-Y., Eds.; Science Press: St. Louis, MO, USA, 2013; Volume 2–3, pp. 629–713. [Google Scholar]
- Le Péchon, T.; He, H.; Zhang, L.; Zhou, X.-M.; Gao, X.-F.; Zhang, L.-B. Using a multilocus phylogeny to test morphology-based classifications of Polystichum (Dryopteridaceae), one of the largest fern genera. BMC Evol. Biol. 2016, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, J.; Watanabe, R.; Kanai, Y.; Ono, T. Molecular cloning and expression of rat squalene epoxidase. J. Biol. Chem. 1995, 270, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, R.G.; Hanzlik, R.P. Synthesis of labeled squalene and squalene 2,3-oxide. In Methods in Enzymology; Clayton, R.B., Ed.; Academic Press: New York, NY, USA, 1969; Volume 15, pp. 346–349. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acid Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Zuckerkandl, E.; Pauling, L. Evolutionary divergence and convergence in proteins. In Evolving Genes and Proteins; Bryson, V., Vogel, H.J., Eds.; Academic Press: New York, NY, USA, 1965; pp. 97–166. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds 22-hydroxyhopane are available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinozaki, J.; Nakene, T.; Takano, A. Squalene Cyclases and Cycloartenol Synthases from Polystichum polyblepharum and Six Allied Ferns. Molecules 2018, 23, 1843. https://doi.org/10.3390/molecules23081843
Shinozaki J, Nakene T, Takano A. Squalene Cyclases and Cycloartenol Synthases from Polystichum polyblepharum and Six Allied Ferns. Molecules. 2018; 23(8):1843. https://doi.org/10.3390/molecules23081843
Chicago/Turabian StyleShinozaki, Junichi, Takahisa Nakene, and Akihito Takano. 2018. "Squalene Cyclases and Cycloartenol Synthases from Polystichum polyblepharum and Six Allied Ferns" Molecules 23, no. 8: 1843. https://doi.org/10.3390/molecules23081843



