Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders
Abstract
:1. Introduction
2. Results
2.1. Behavioral Analysis
2.2. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Behavioral Analysis
4.3. Anxiety-Like Behavioral Test
4.4. Locomotor Activity Test
4.5. Gene Expression Analysis
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onaivi, E.S.; Ishiguro, H.; Gu, S.; Liu, Q.R. CNS effects of CB2 cannabinoid receptors: Beyond neuro-immuno-cannabinoid activity. J. Psychopharmacol. 2012, 26, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Horiuchi, Y.; Ishikawa, M.; Koga, M.; Imai, K.; Suzuki, Y.; Morikawa, M.; Inada, T.; Watanabe, Y.; Takahashi, M.; et al. Brain cannabinoid CB2 receptor in schizophrenia. Biol. Psychiatry 2010, 67, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Iwasaki, S.; Teasenfitz, L.; Higuchi, S.; Horiuchi, Y.; Saito, T.; Arinami, T.; Onaivi, E.S. Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenomics J. 2007, 7, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann. N. Y. Acad. Sci. 2008, 1139, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, H.; Onaivi, E.S. Beyond the Kraepelinian Dichotomy of Schizophrenia and Bipolar Disorder. J. Schizophr. Res. 2017, 4, 1032. [Google Scholar]
- Morena, M.; Patel, S.; Bains, J.S.; Hill, M.N. Neurobiological Interactions between Stress and the Endocannabinoid System. Neuropsychopharmacology 2016, 41, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, L.; Milaneschi, Y.; Vinkers, C.H.; van Hemert, A.M.; van Velzen, L.; Schmaal, L.; Penninx, B.W. HPA Axis Genes, and Their Interaction with Childhood Maltreatment, are Related to Cortisol Levels and Stress-Related Phenotypes. Neuropsychopharmacology 2017, 42, 2446–2455. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009, 34 (Suppl. 1), S186–S195. [Google Scholar] [CrossRef] [PubMed]
- Denny, W.B.; Valentine, D.L.; Reynolds, P.D.; Smith, D.F.; Scammell, J.G. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology 2000, 141, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Gassen, N.C.; Hartmann, J.; Schmidt, M.V.; Rein, T. FKBP5/FKBP51 enhances autophagy to synergize with antidepressant action. Autophagy 2015, 11, 578–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, C.; Hosak, L.; Mossner, R.; Giegling, I.; Mandelli, L.; Bellivier, F.; Claes, S.; Collier, D.A.; Corrales, A.; Delisi, L.E.; et al. Consensus paper of the WFSBP Task Force on Genetics: Genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J. Biol. Psychiatry 2016, 7, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Young, D.A.; Inslicht, S.S.; Metzler, T.J.; Neylan, T.C.; Ross, J.A. The effects of early trauma and the FKBP5 gene on PTSD and the HPA axis in a clinical sample of Gulf War veterans. Psychiatry Res. 2018, 2018, 32068-1. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, R.; Entringer, S.; Koper, J.W.; van Rossum, E.F.; Hellhammer, D.H.; Wust, S. Sex specific associations between common glucocorticoid receptor gene variants and hypothalamus-pituitary-adrenal axis responses to psychosocial stress. Biol. Psychiatry 2007, 62, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Palma-Gudiel, H.; Cordova-Palomera, A.; Tornador, C.; Falcon, C.; Bargallo, N.; Deco, G.; Fananas, L. Increased methylation at an unexplored glucocorticoid responsive element within exon 1D of NR3C1 gene is related to anxious-depressive disorders and decreased hippocampal connectivity. Eur. Neuropsychopharmacol. 2018, 28, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Bae, K.Y.; Kim, S.W.; Shin, I.S.; Kim, H.R.; Shin, M.G.; Yoon, J.S.; Kim, J.M. Longitudinal associations between glucocorticoid receptor methylation and late-life depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sarubin, N.; Hilbert, S.; Naumann, F.; Zill, P.; Wimmer, A.M.; Nothdurfter, C.; Rupprecht, R.; Baghai, T.C.; Buhner, M.; Schule, C. The sex-dependent role of the glucocorticoid receptor in depression: Variations in the NR3C1 gene are associated with major depressive disorder in women but not in men. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hong, J.P.; Lee, J.K.; Park, Y.M.; Park, Y.; Jeon, J.; Ahn, M.H.; Yoon, S.C. Associations between the neuron-specific glucocorticoid receptor (NR3C1) Bcl-1 polymorphisms and suicide in cancer patients within the first year of diagnosis. Behav. Brain Funct. 2016, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Galfalvy, H.; Pantazatos, S.P.; Huang, Y.Y.; Rosoklija, G.B.; Dwork, A.J.; Burke, A.; Arango, V.; Oquendo, M.A.; Mann, J.J. Glucocorticoid Receptor-Related Genes: Genotype and Brain Gene Expression Relationships to Suicide and Major Depressive Disorder. Depress. Anxiety 2016, 33, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Schatzberg, A.F.; Keller, J.; Tennakoon, L.; Lembke, A.; Williams, G.; Kraemer, F.B.; Sarginson, J.E.; Lazzeroni, L.C.; Murphy, G.M. HPA axis genetic variation, cortisol and psychosis in major depression. Mol. Psychiatry 2014, 19, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Cai, J.; Wei, T.J.; Liu, L.Y.; Zhao, H.Y.; Liu, B.H.; Jing, H.B.; Jin, Z.R.; Liu, M.; et al. Activation of CRF/CRFR1 signaling in the basolateral nucleus of the amygdala contributes to chronic forced swim-induced depressive-like behaviors in rats. Behav. Brain Res. 2018, 338, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, Y.; Kasahara, T.; Kato, M.; Sakai, S.; Deguchi, Y.; Tani, M.; Kuroda, K.; Hattori, K.; Yoshida, S.; Goto, Y.; et al. The relationship between circulating mitochondrial DNA and inflammatory cytokines in patients with major depression. J. Affect. Disord. 2017, 0327, 30661–30664. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Stanczykiewicz, B.; Kotowicz, K.; Rybakowski, J.K.; Samochowiec, J.; Frydecka, D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: A systematic review. Schizophr. Res. 2017, 9964, 30202–30205. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Chen, D.C.; Tan, Y.L.; Tan, S.P.; Xiu, M.H.; Wang, Z.R.; Yang, F.D.; Soares, J.C.; Zhang, X.Y. Altered interleukin-18 levels are associated with cognitive impairment in chronic schizophrenia. J. Psychiatr. Res. 2016, 76, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rammahi, D.A.; Al-Dujaili, A.H. IL-6, IL-18, sIL-2R, and TNFalpha proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J. Affect. Disord. 2015, 182, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Shirts, B.H.; Wood, J.; Yolken, R.H.; Nimgaonkar, V.L. Comprehensive evaluation of positional candidates in the IL-18 pathway reveals suggestive associations with schizophrenia and herpes virus seropositivity. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Xiu, M.H.; Chen, D.C.; Wang, D.; Zhang, K.; Dong, A.; Tang, W.; Zhang, F.; Liu, L.J.; Liu, J.H.; Liu, H.B.; et al. Elevated interleukin-18 serum levels in chronic schizophrenia: Association with psychopathology. J. Psychiatr. Res. 2012, 46, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Tang, W.; Xiu, M.H.; Chen, D.C.; Yang, F.D.; Tan, Y.L.; Wang, Z.R.; Zhang, F.; Liu, J.; Liu, L.; et al. Interleukin 18 and cognitive impairment in first episode and drug naive schizophrenia versus healthy controls. Brain Behav. Immun. 2013, 32, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.A.; Freitas, T.H.; Stubbs, B.; Maes, M.; Solmi, M.; Veronese, N.; de Andrade, N.Q.; Morris, G.; Fernandes, B.S.; Brunoni, A.R.; et al. Peripheral Alterations in Cytokine and Chemokine Levels after Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol. Neurobiol. 2018, 55, 4195–4206. [Google Scholar] [CrossRef] [PubMed]
- Ellul, P.; Boyer, L.; Groc, L.; Leboyer, M.; Fond, G. Interleukin-1 beta-targeted treatment strategies in inflammatory depression: Toward personalized care. Acta Psychiatr. Scand. 2016, 134, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Xu, Y.Q.; Li, Y.Y.; Lu, M.F.; Shi, S.X.; Ji, J.L.; Wang, L.W. Difference in proinflammatory cytokines produced by monocytes between patients with major depressive disorder and healthy controls. J. Affect. Disord. 2018, 234, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Norman, G.J.; Karelina, K.; Zhang, N.; Walton, J.C.; Morris, J.S.; Devries, A.C. Stress and IL-1beta contribute to the development of depressive-like behavior following peripheral nerve injury. Mol. Psychiatry 2010, 15, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Wang, H.Y.; Zhang, C.; Liu, B.P.; Peng, Z.L.; Li, Y.Y.; Liu, F.M.; Song, C. Mifepristone attenuates depression-like changes induced by chronic central administration of interleukin-1beta in rats. Behav. Brain Res. 2018, 347, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.; Kishi, T.; Atake, K.; Katsuki, A.; Iwata, N. Serum Brain-Derived Neurotrophic Factor, and Plasma Catecholamine Metabolites in People with Major Depression: Preliminary Cross-Sectional Study. Front. Psychiatry 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.P.; Poddighe, L.; Boi, M.; Sanna, F.; Piludu, M.A.; Corda, M.G.; Giorgi, O.; Quartu, M. Expression of BDNF and trkB in the hippocampus of a rat genetic model of vulnerability (Roman low-avoidance) and resistance (Roman high-avoidance) to stress-induced depression. Brain Behav. 2017, 7, e00861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Shi, R.; Wang, J.; Wang, J.F.; Li, X.M. Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport 2014, 25, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, S.; Khan, D.; Kong, E.; Berger, A.; Pollak, A.; Pollak, D.D. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 2015, 149, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Levy-Gigi, E.; Szabo, C.; Kelemen, O.; Keri, S. Association among clinical response, hippocampal volume, and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol. Psychiatry 2013, 74, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Kelemen, O.; Keri, S. Changes in FKBP5 expression and memory functions during cognitive-behavioral therapy in posttraumatic stress disorder: A preliminary study. Neurosci. Lett. 2014, 569, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Z.Z.; Zuo, W.; Zhang, S.; Chu, S.F.; Chen, N.H. Effects of chronic mild stress on behavioral and neurobiological parameters—Role of glucocorticoid. Horm. Behav. 2016, 78, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry 2014, 75, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Griffin-Thomas, L. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef] [PubMed]
- Palazuelos, J.; Aguado, T.; Egia, A.; Mechoulam, R.; Guzman, M.; Galve-Roperh, I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006, 20, 2405–2407. [Google Scholar] [CrossRef] [PubMed]
- Hollins, S.L.; Zavitsanou, K.; Walker, F.R.; Cairns, M.J. Alteration of transcriptional networks in the entorhinal cortex after maternal immune activation and adolescent cannabinoid exposure. Brain Behav. Immun. 2016, 56, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, B.; Miller, M.L.; Hurd, Y.L. Cannabis Use during Adolescent Development: Susceptibility to Psychiatric Illness. Front. Psychiatry 2013, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.V.; Rincon-Cortes, M.; Grace, A.A. Adolescence as a period of vulnerability and intervention in schizophrenia: Insights from the MAM model. Neurosci. Biobehav. Rev. 2016, 70, 260–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, P.; Wozniak, A.; Nowakowska, E. Animal models of schizophrenia: Developmental preparation in rats. Acta Neurobiol. Exp. 2013, 73, 472–484. [Google Scholar]
- Bailey, K.A.; Baker, A.L.; McElduff, P.; Kavanagh, D.J. The Influence of Parental Emotional Neglect on Assault Victims Seeking Treatment for Depressed Mood and Alcohol Misuse: A Pilot Study. J. Clin. Med. 2016, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dong, X.; Wang, Y.; Liu, M.; Sun, A.; Li, N.; Lin, Y.; Geng, Z.; Jin, Y.; Li, X. Adolescent escitalopram prevents the effects of maternal separation on depression- and anxiety-like behaviours and regulates the levels of inflammatory cytokines in adult male mice. Int. J. Dev. Neurosci. 2017, 62, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Malkesman, O.; Lavi-Avnon, Y.; Maayan, R.; Weizman, A. A cross-fostering study in a genetic animal model of depression: Maternal behavior and depression-like symptoms. Pharmacol. Biochem. Behav. 2008, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Llorente, R.; Llorente-Berzal, A.; Petrosino, S.; Marco, E.M.; Guaza, C.; Prada, C.; Lopez-Gallardo, M.; Di Marzo, V.; Viveros, M.P. Gender-dependent cellular and biochemical effects of maternal deprivation on the hippocampus of neonatal rats: A possible role for the endocannabinoid system. Dev. Neurobiol. 2008, 68, 1334–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Gallardo, M.; Llorente, R.; Llorente-Berzal, A.; Marco, E.M.; Prada, C.; Di Marzo, V.; Viveros, M.P. Neuronal and glial alterations in the cerebellar cortex of maternally deprived rats: Gender differences and modulatory effects of two inhibitors of endocannabinoid inactivation. Dev. Neurobiol. 2008, 68, 1429–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recamier-Carballo, S.; Estrada-Camarena, E.; Lopez-Rubalcava, C. Maternal separation induces long-term effects on monoamines and brain-derived neurotrophic factor levels on the frontal cortex, amygdala, and hippocampus: Differential effects after a stress challenge. Behav. Pharmacol. 2017, 28, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Van Bodegom, M.; Homberg, J.R.; Henckens, M. Modulation of the Hypothalamic-Pituitary-Adrenal Axis by Early Life Stress Exposure. Front. Cell. Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Page, G.G.; Corwin, E.J.; Dorsey, S.G.; Redeker, N.S.; McCloskey, D.J.; Austin, J.K.; Guthrie, B.J.; Moore, S.M.; Barton, D.; Kim, M.T.; et al. Biomarkers as Common Data Elements for Symptom and Self-Management Science. J. Nurs. Scholarsh. 2018, 50, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Buckley, N.E. The peripheral cannabinoid receptor knockout mice: An update. Br. J. Pharmacol. 2008, 153, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Commons, K.G.; Cholanians, A.B.; Babb, J.A.; Ehlinger, D.G. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci. 2017, 8, 955–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maejima, Y.; Rita, R.S.; Santoso, P.; Aoyama, M.; Hiraoka, Y.; Nishimori, K.; Gantulga, D.; Shimomura, K.; Yada, T. Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-Fos induction in limited brain areas. Neuroendocrinology 2015, 101, 35–44. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Behavioral Test | Treatment/Strain | Statics ANOVA | Noted by Post-Hoc Analysis |
|---|---|---|---|
| Behavior | |||
| Zero maze test | Saline/Cnr2 ko mice (no Figure) | F1,34 = 0.48, p = 0.49 | |
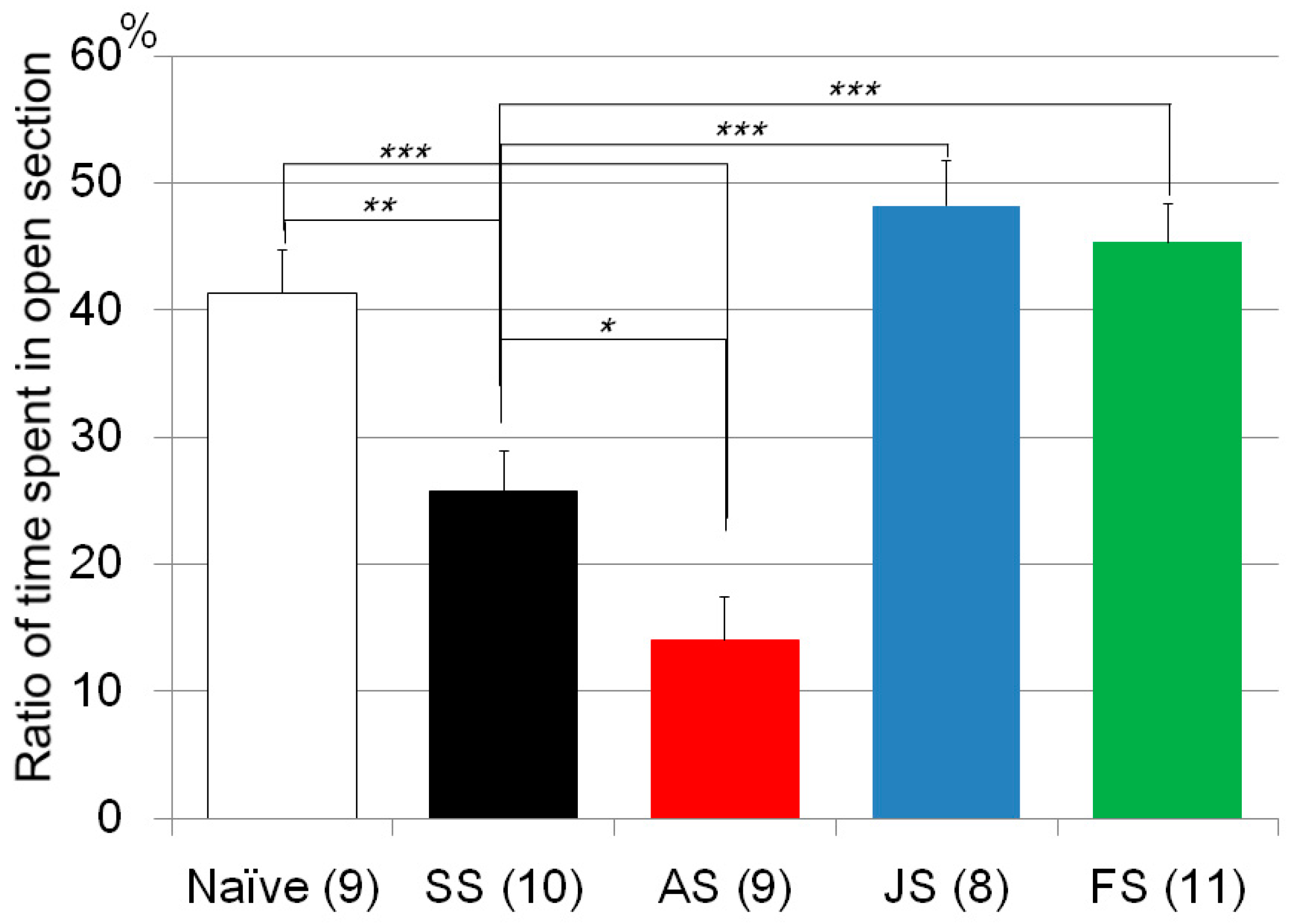
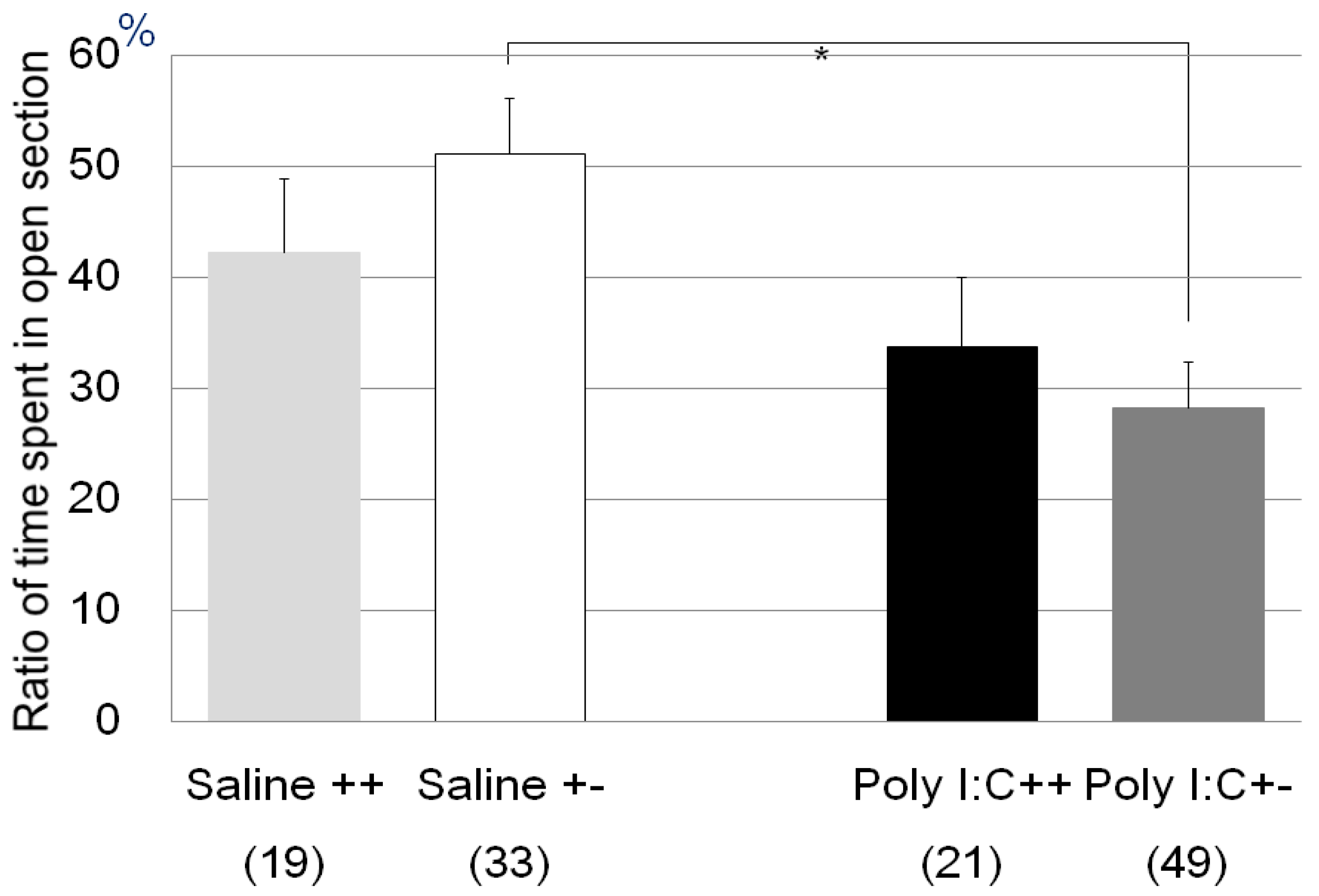
| Zero maze test | Poly I:C/Cnr2 ko mice (Figure 1) | F3,118 = 4.4, p = 0.0055 | Poly I:C vs. saline treated heterozygote mice; p = 0.0033 |
| genotype F = 0.09, p = 0.76 | |||
| treatment F = 7.7, p = 0.006 | |||
| genotype × treatment F = 1.6, p = 0.20 | |||
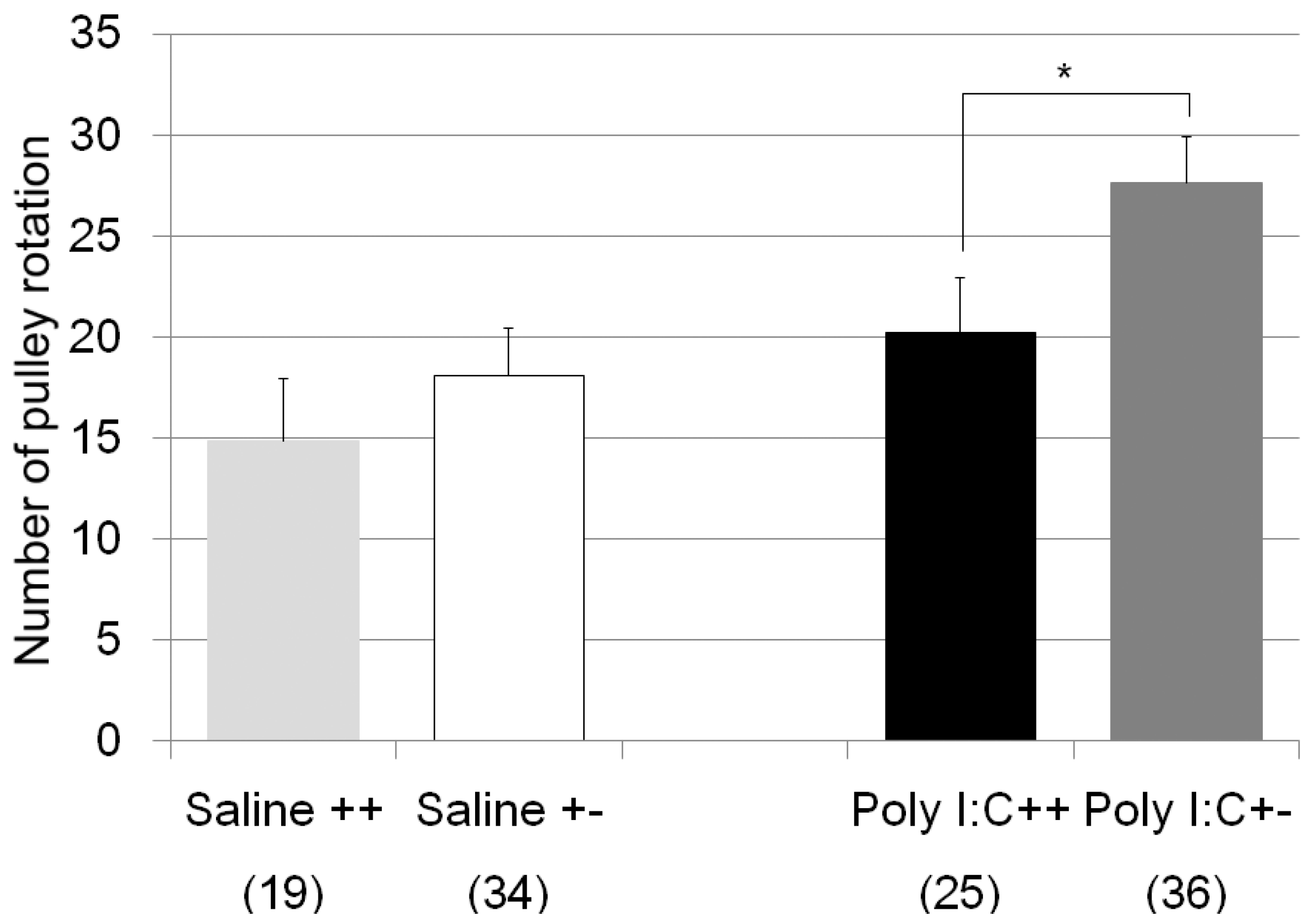
| pulley rotate test | Poly I:C/Cnr2 ko mice (Figure 2) | F3,110 = 4.8, p = 0.0034 | Poly I:C treated wildtype mice vs. heterozygote mice; p = 0.019 |
| genotype F = 4.2, p = 0.04 | |||
| treatment F = 8.2, p = 0.005 | |||
| genotype × treatment F = 0.6, p = 0.42 | |||
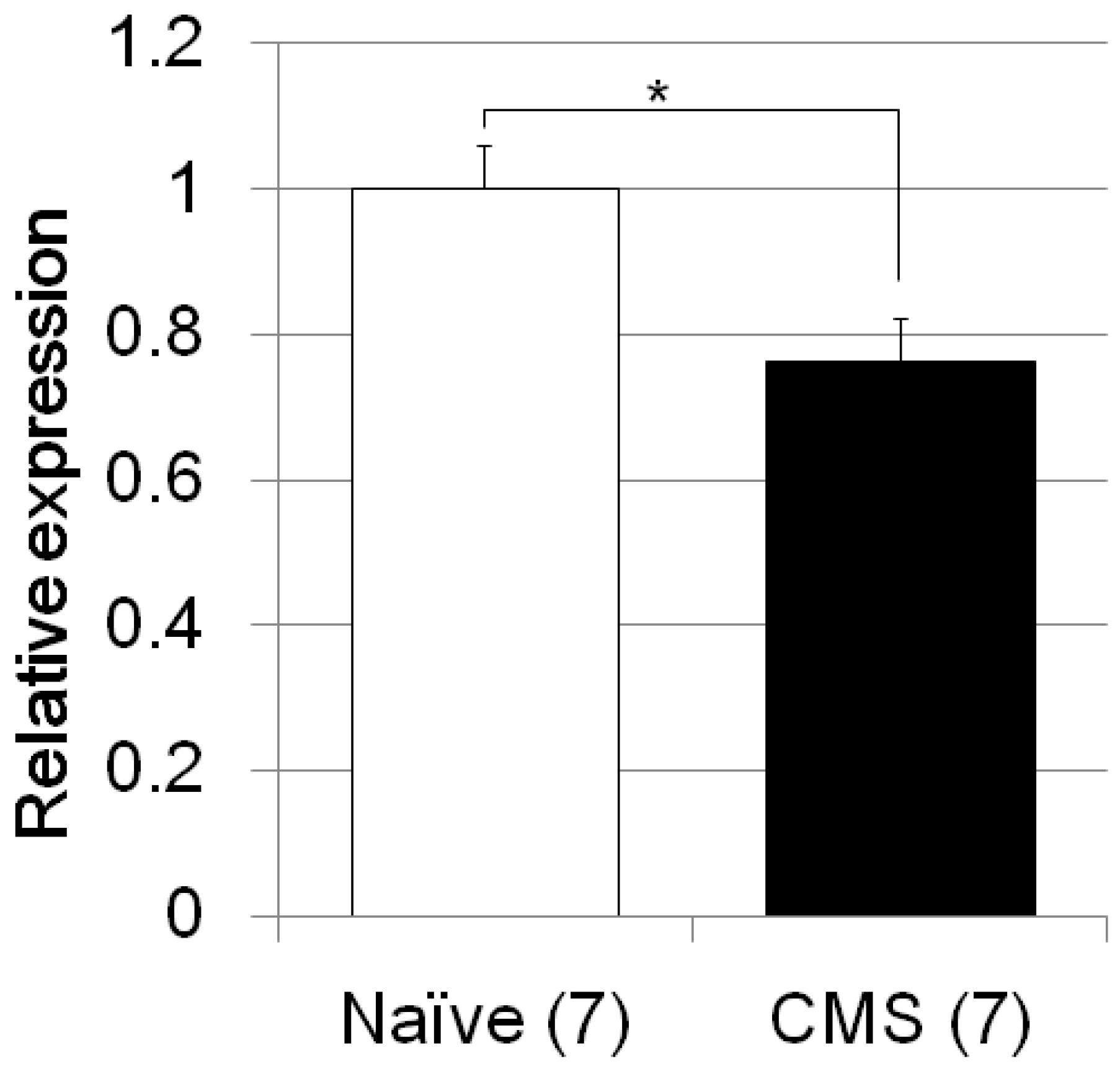
| Zero maze test | CMS/C57B/JJmsSlc mice (Figure 3) | F4, 46 = 19.0, p < 0.0001 | CMS treated vs. naïve mice; p = 0.0016 |
| AM630 vs. Saline treated mice with CMS; p = 0.015 | |||
| JWH015 vs. Saline treated mice with CMS; p < 1 × 10−3 | |||
| Fluvoxamine vs. Saline treated mice with CMS; p < 1 × 10−3 | |||
| JWH015 vs. Fluvoxamine treated mice with CMS; n.s. | |||
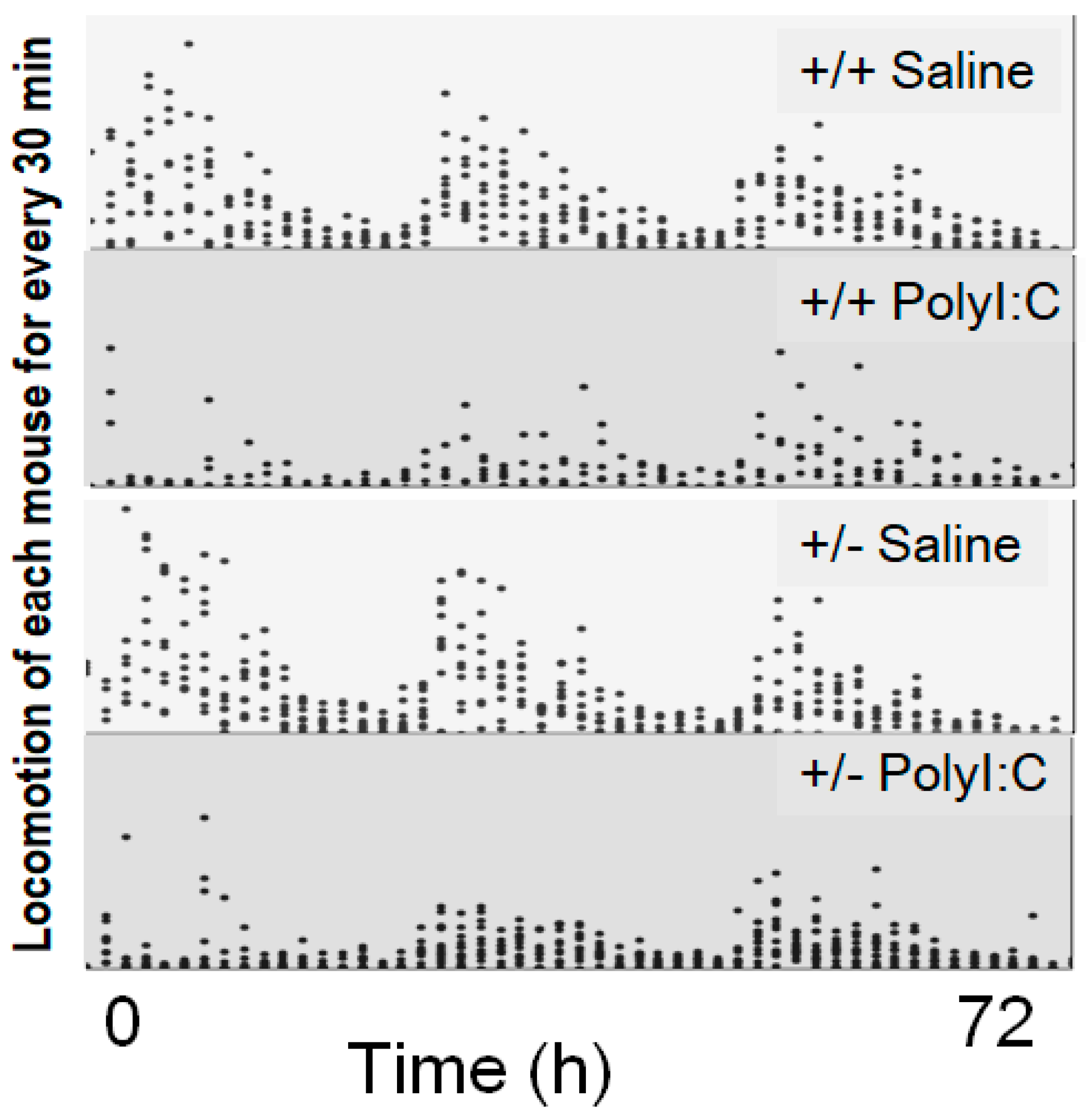
| Locomotion test | Poly I:C/Cnr2 ko mice (Figure 4) | t(1827) = 1.54, p (single sided) = 0.06 | |
| Gene expression | |||
| Fkbp5 | CMS/C57B/JJmsSlc mice (Figure 5) | F1, 12 = 8.4, p = 0.0134 | |
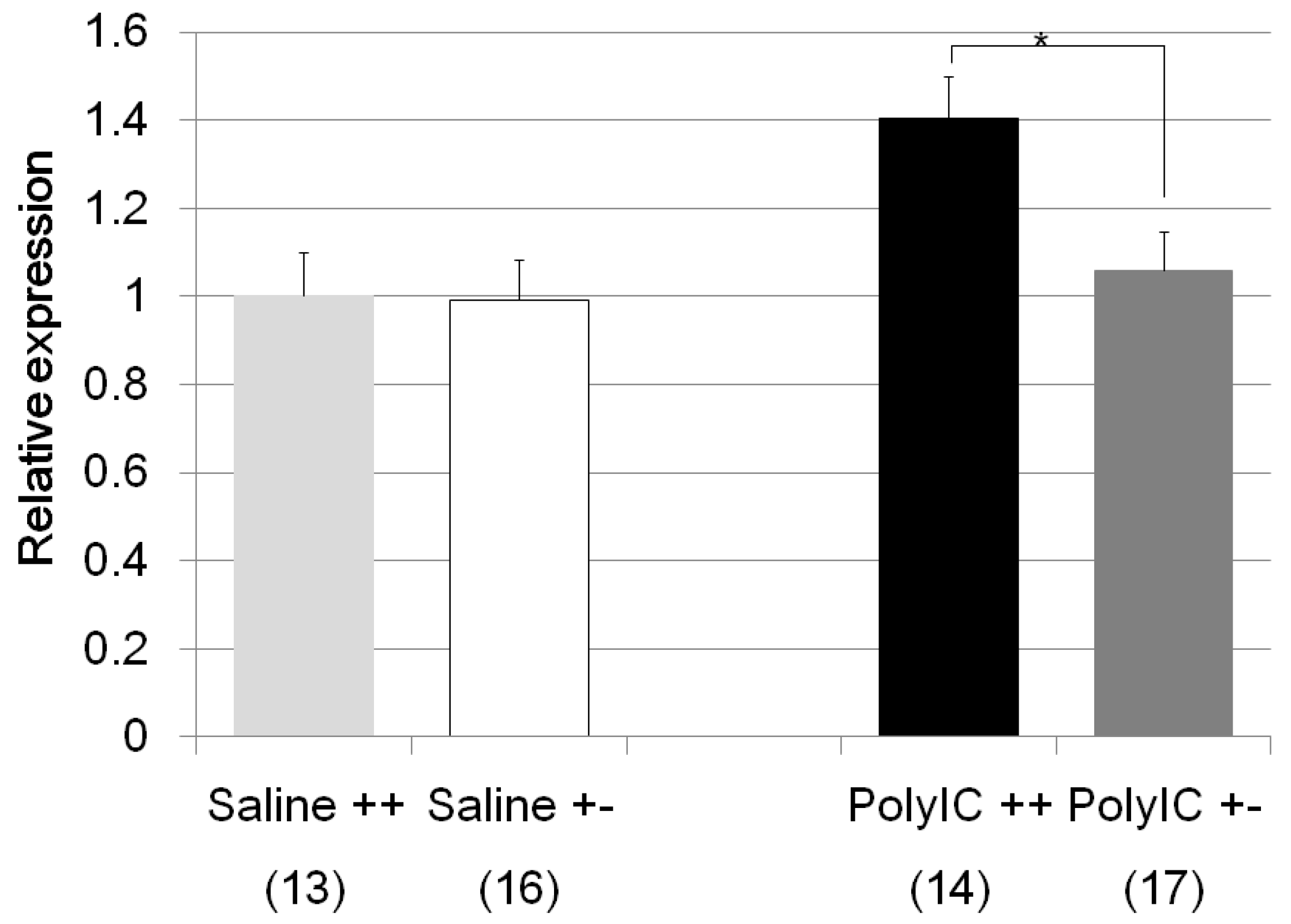
| Fkbp5 | Poly I:C/Cnr2 ko mice (Figure 6) | F3,58 = 4.6, p = 0.008 | Poly I:C treated wildtype mice vs. heterozygote mice; p = 0.048 |
| genotype F = 2.6, p = 0.01 | Poly I:C treated vs. Saline treated wildtype mice; p = 0.025 | ||
| treatment F = −1.9, p = 0.06 | Poly I:C treated wildtype mice vs. Saline treated heterozygote mice; p = 0.013 | ||
| genotype × treatment F = −1.7, p = 0.08 | |||
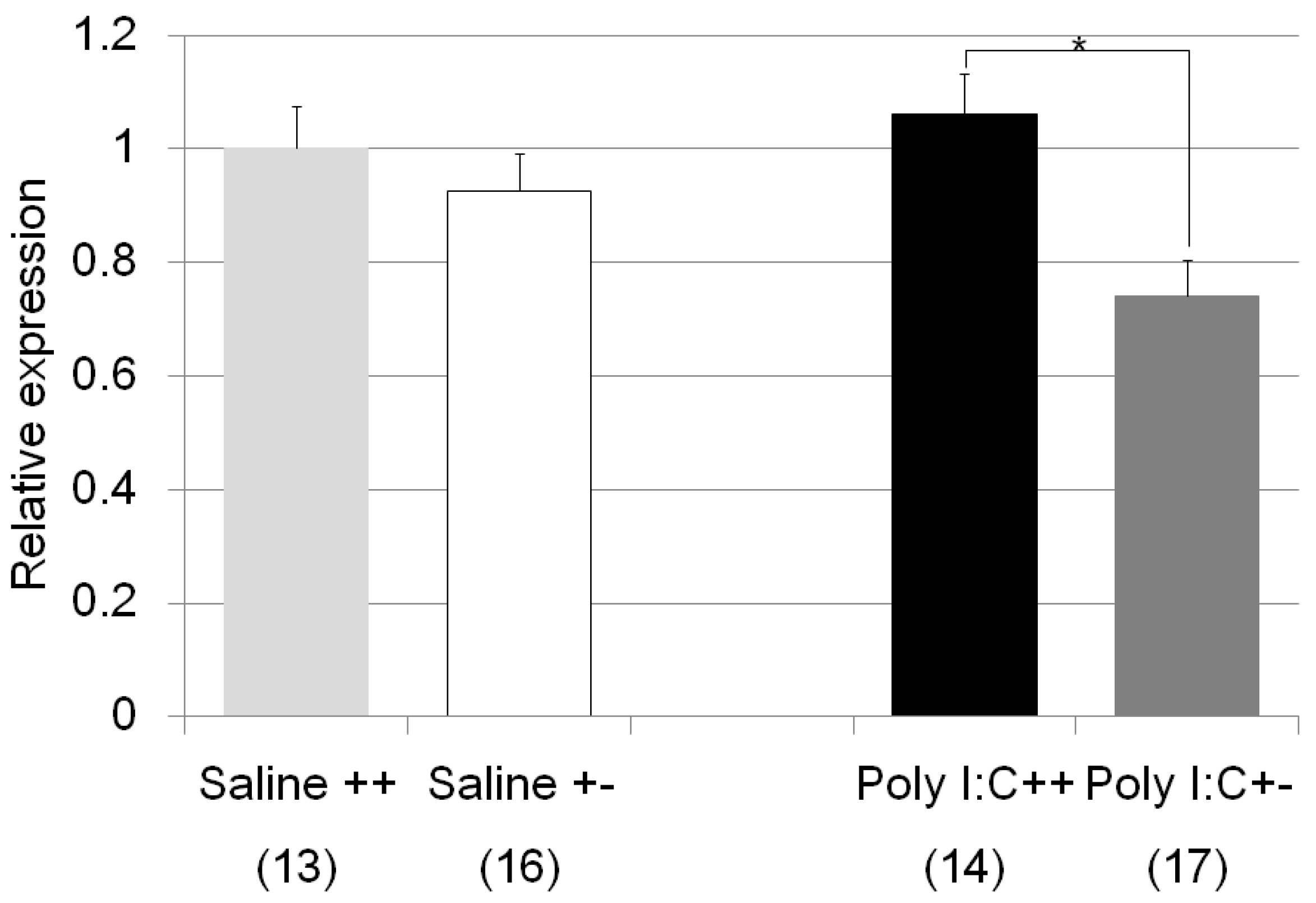
| Nr3c1 | Poly I:C/Cnr2 ko mice (Figure 7) | F3,59 = 4.4, p = 0.008 | Poly I:C treated wildtype mice vs. heterozygote mice; p = 0.007 |
| genotype F = 8.4, p = 0.005 | |||
| treatment F = 0.83, p = 0.37 | |||
| genotype x treatment F = 3.2, p = 0.078 | |||
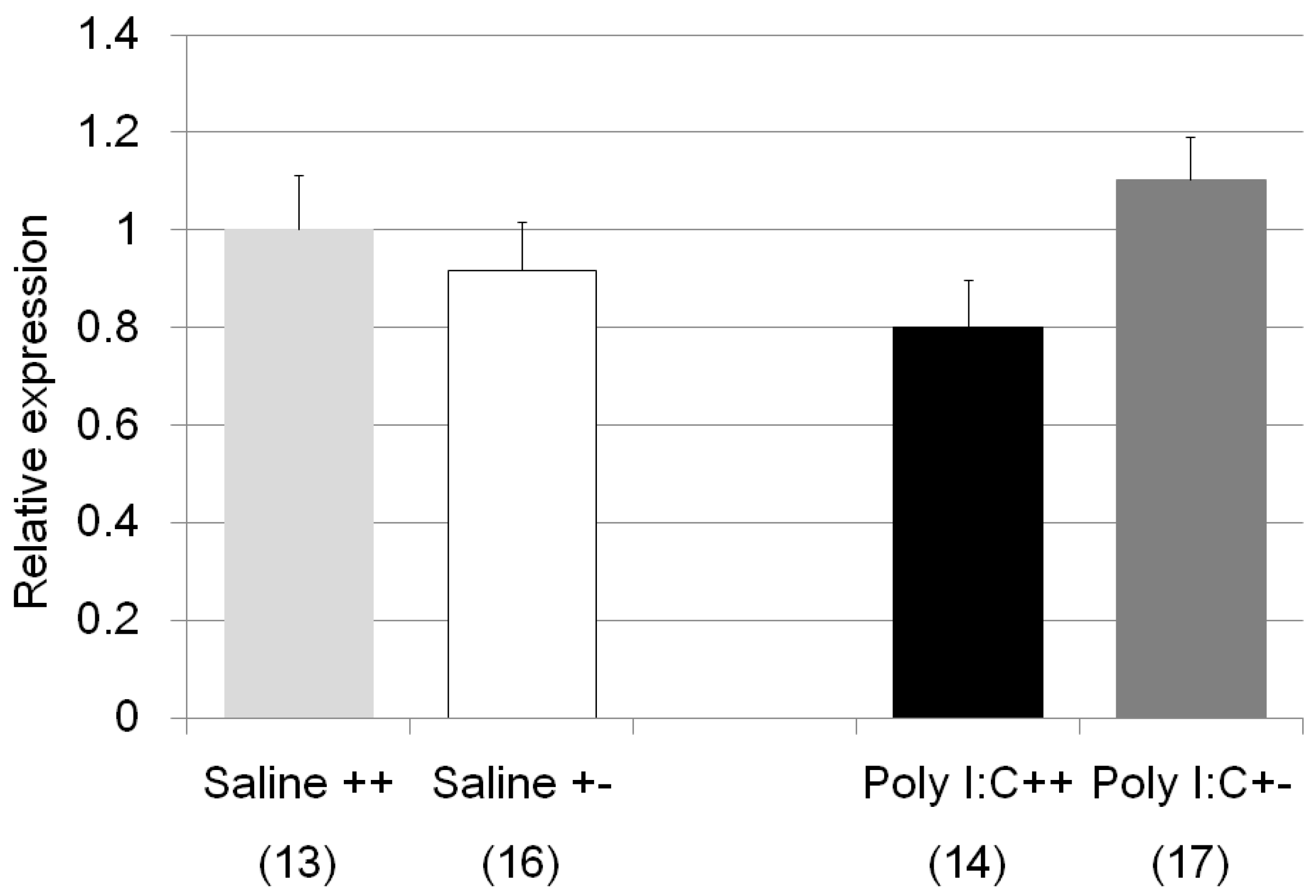
| Il1b | Poly I:C/Cnr2 ko mice (Figure 8) | F3,58 = 1.8, p = 0.17 | n.s. (Poly I:C treated wildtype mice vs. heterozygote mice; p = 0.14) |
| genotype F = 0.0004, p = 0.98 | |||
| treatment F = 0.98, p = 0.33 | |||
| genotype × treatment F = 4.05, p = 0.049 | |||
| Bdnf | Poly I:C/Cnr2 ko mice (no figure) | F3,59 = 0.15, p = 0.93 | n.s. |
| Crf | Poly I:C/Cnr2 ko mice (no figure) | F3,59 = 0.54, p = 0.66 | n.s. |
| genotype F = 0.49, p = 0.63 | |||
| treatment F = 0.54, p = 0.59 | |||
| genotype × treatment F = 0.97, p = 0.34 | |||
| Stressors | Experimental Treatment | Behavioral Tests | Gene Expression Analysis |
|---|---|---|---|
| Physical/Emotional (Chronic mild stress) | Control (10) AM630 (9) JWH015 (8) Fluvoxamine (11) Saline (10) | 1. Zero maze | Fkbp5 Control (7) vs. Saline (7) |
| Immune | Poly I:C (20) Saline (19) | 1. Locomotion in home cage | Fkbp5, Il1b, Nr3c1, Bdnf, Crf Poly I:C (31) vs. Saline (29) |
| Poly I:C (70) Saline (52) | 2. Zero maze | ||
| Poly I:C (61) Saline (53) | 3. Pulley rotating test (originally developed test) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishiguro, H.; Horiuchi, Y.; Tabata, K.; Liu, Q.-R.; Arinami, T.; Onaivi, E.S. Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders. Molecules 2018, 23, 1836. https://doi.org/10.3390/molecules23081836
Ishiguro H, Horiuchi Y, Tabata K, Liu Q-R, Arinami T, Onaivi ES. Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders. Molecules. 2018; 23(8):1836. https://doi.org/10.3390/molecules23081836
Chicago/Turabian StyleIshiguro, Hiroki, Yasue Horiuchi, Koichi Tabata, Qing-Rong Liu, Tadao Arinami, and Emmanuel S. Onaivi. 2018. "Cannabinoid CB2 Receptor Gene and Environmental Interaction in the Development of Psychiatric Disorders" Molecules 23, no. 8: 1836. https://doi.org/10.3390/molecules23081836






