The Keap1/Nrf2-ARE Pathway as a Pharmacological Target for Chalcones
Abstract
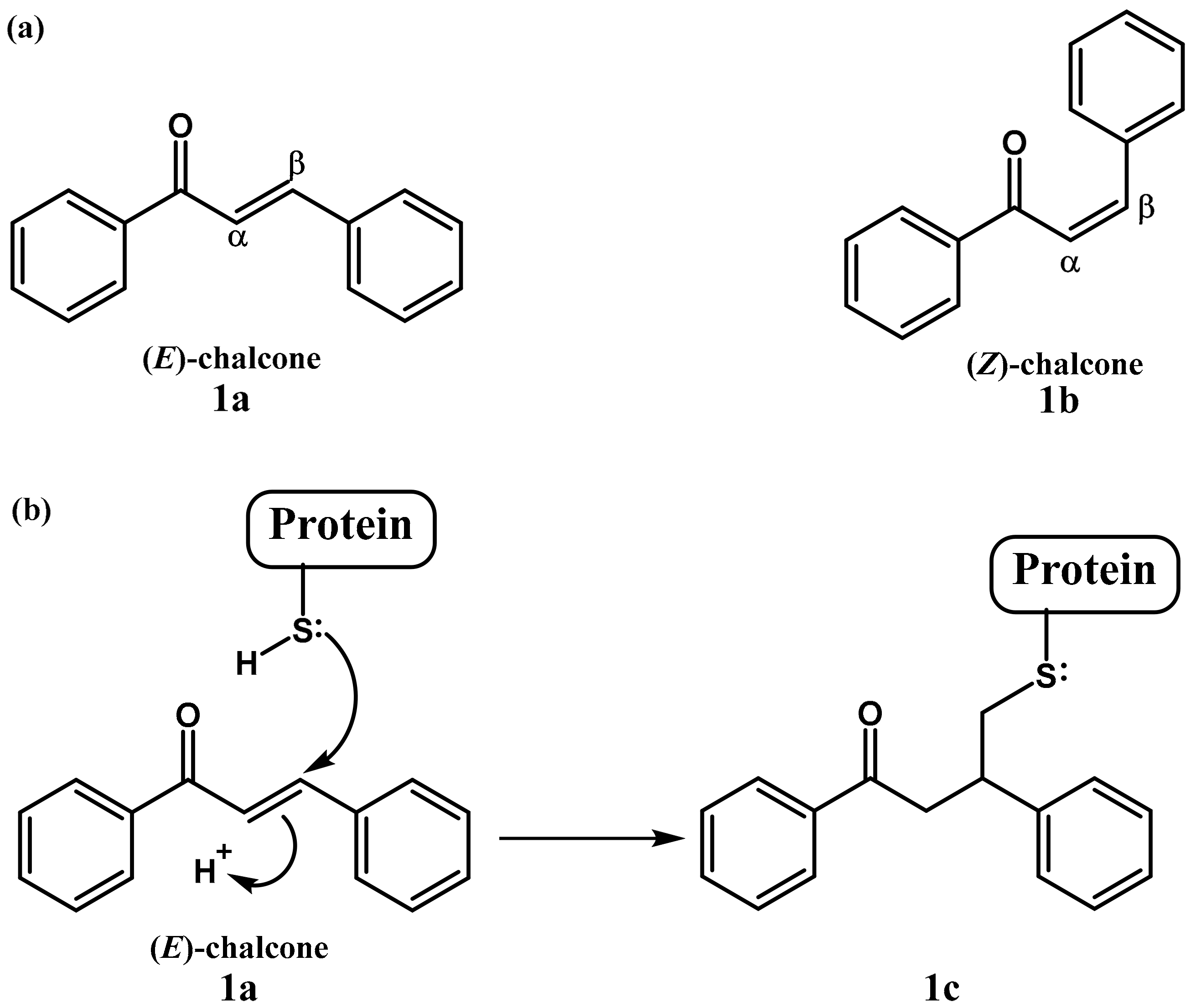
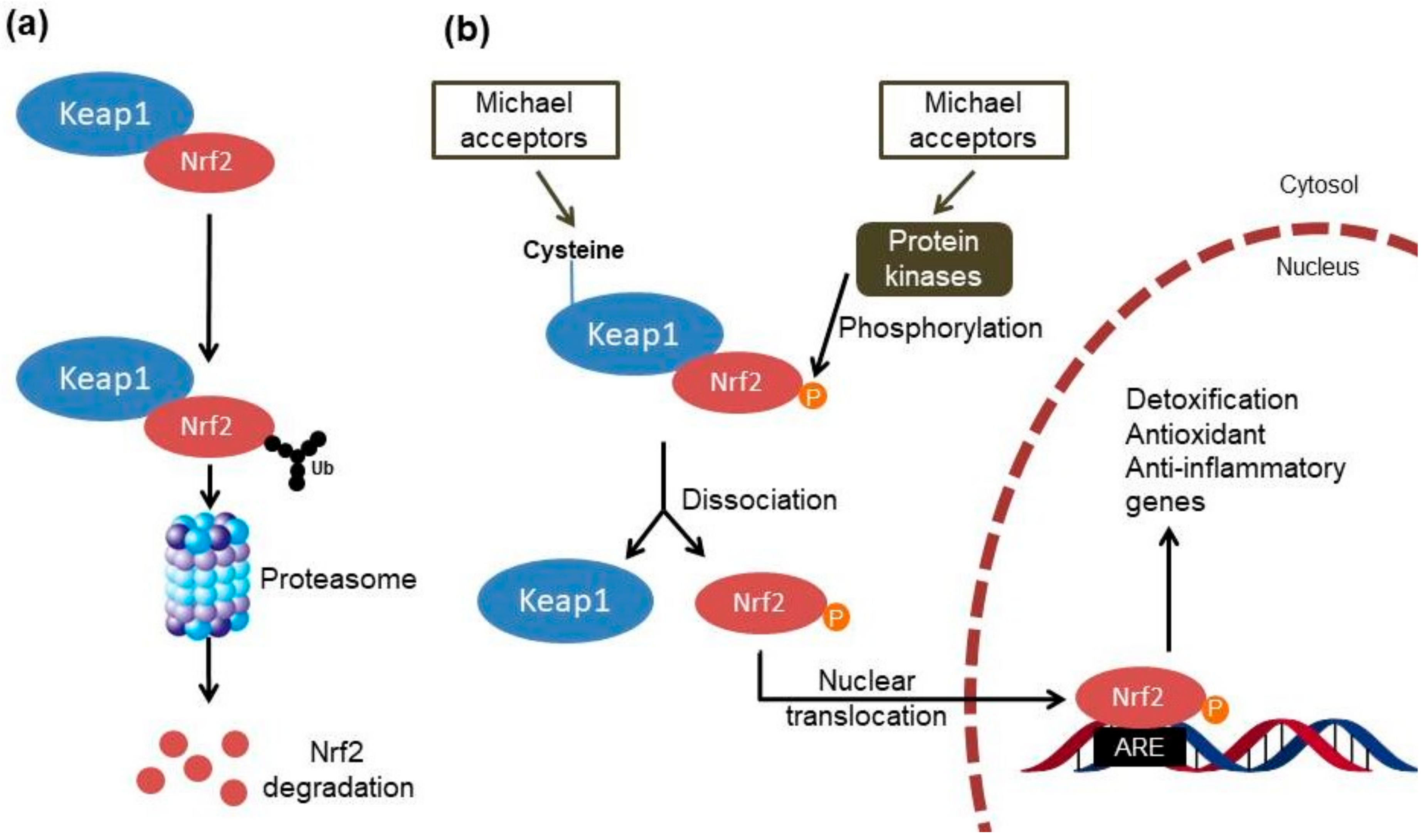
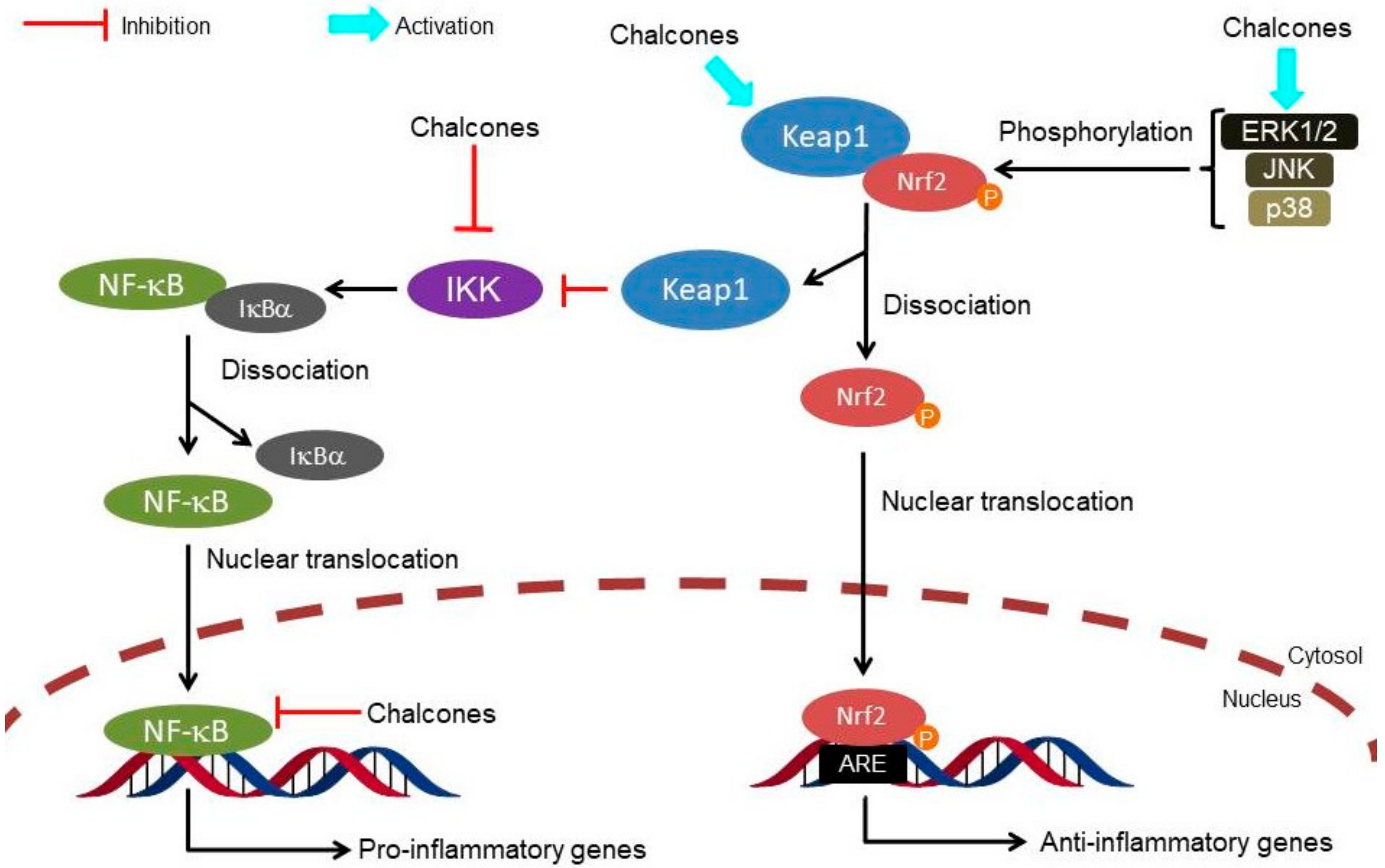
:1. Introduction
2. Targeting the Nrf2 Pathway by Natural Chalcones
3. Targeting the Nrf2 Pathway by Synthetic Chalcones
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone derivatives: Promising starting points for drug design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xing, C. Diverse molecular targets for chalcones with varied bioactivities. Med. Chem. (Los. Angeles). 2015, 5, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Maydt, D.; De Spirt, S.; Muschelknautz, C.; Stahl, W.; Müller, T.J.J. Chemical reactivity and biological activity of chalcones and other α,β-unsaturated carbonyl compounds. Xenobiotica 2013, 43, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Massiah, M.A.; Bozak, R.E.; Hicks, R.J.; Talalay, P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc. Natl. Acad. Sci. USA 2001, 98, 3404–3409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, X.M.; Kensler, T.W.; Motohashi, H. The Keap1-Nrf2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; Fishbein, J.C. Chemistry of the cysteine sensors in Kelch-Like ECH-Associated Protein 1. Antioxid. Redox Signal. 2010, 13, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Lipton, S. Recent advances in understanding NRF2 as a druggable target: Development of pro-electrophilic and non-covalent NRF2 activators to overcome systemic side effects of electrophilic drugs like dimethyl fumarate. F1000Research 2017, 6, 2138. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kazantsev, A.G. Activation of Nrf2 signaling as a common treatment of neurodegenerative diseases. Neurodegener. Dis. Manag. 2017, 7, 97–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBean, G.J.; López, M.G.; Wallner, F.K. Redox-based therapeutics in neurodegenerative disease. Br. J. Pharmacol. 2017, 174, 1750–1770. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Rojo, A.I.; Velasco, D.; De Sagarra, R.M.; Cuadrado, A. Glycogen synthase kinase-3β inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006, 281, 14841–14851. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.; Ponsford, A.; Sanderson, C. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription factor Nrf2 as a therapeutic target for chronic diseases: A systems medicine approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Sandur, S.K.; Sung, B.; Sethi, G.; Kunnumakkara, A.B.; Aggarwal, B.B. Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-κB and NF-κB-regulated gene expression through direct inhibition of IκBα kinase β on cysteine 179 residue. J. Biol. Chem. 2007, 282, 17340–17350. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.; Alvarez, R.; Ortiz, M.A.; Alvarez, S.; Piedrafita, F.J.; de Lera, A.R. Inhibition of IκB kinase-β and anticancer activities of novel chalcone adamantyl arotinoids. J. Med. Chem. 2008, 51, 5431–5440. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi-Tago, M.; Tanabe, S.; Tago, K.; Itoh, H.; Mashino, T.; Sonoda, Y.; Kasahara, T. Licochalcone a potently inhibits Tumor Necrosis Factor α-Induced Nuclear Factor-κB activation through the direct inhibition of IκB Kinase complex activation. Mol. Pharmacol. 2009, 76, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-H.; Chang, J.-K.; Hsu, Y.-L.; Kuo, P.-L. Chalcone arrests cell cycle progression and induces apoptosis through induction of mitochondrial pathway and inhibition of Nuclear Factor Kappa B signalling in human bladder cancer cells. Basic Clin. Pharmacol. Toxicol. 2007, 101, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-C.; Ji, J.-A.; Jiang, Z.-Y.; You, Q.-D. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: An update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, J.; Lin, H.; Gu, K.; Feng, F. Recent progress in the development of small molecule Nrf2 modulators: A patent review (2012–2016). Expert Opin. Ther. Pat. 2017, 27, 763–785. [Google Scholar] [CrossRef] [PubMed]
- Abed, D.A.; Goldstein, M.; Albanyan, H.; Jin, H.; Hu, L. Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents. Acta Pharm. Sin. B 2015, 5, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.; Yamamoto, M. The rise of antioxidant signaling-The evolution and hormetic actions of Nrf2. Toxicol. Appl. Pharmacol. 2010, 244, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Hsieh, C.W.; Wu, C.C.; Wung, B.S. Chalcone inhibits the activation of NF-κB and STAT3 in endothelial cells via endogenous electrophile. Life Sci. 2007, 80, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Vale, D.L.; Steffen, V.S.; Vicentini, F.T.M.C.; Vignoli, J.A.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Trans-chalcone added in topical formulation inhibits skin inflammation and oxidative stress in a model of ultraviolet B radiation skin damage in hairless mice. J. Photochem. Photobiol. B Biol. 2017, 171, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Lim, J.; Gal, J.; Kang, J.C.; Kim, H.J.; Kang, B.Y.; Choi, H.J. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem. Int. 2011, 58, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, B.; Ge, C.; Peng, S.; Fang, J. Xanthohumol, a polyphenol chalcone present in hops, activating nrf2 enzymes to confer protection against oxidative damage in pc12 cells. J. Agric. Food Chem. 2015, 63, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Brodziak-Jarosz, L.; Fujikawa, Y.; Pastor-Flores, D.; Kasikci, S.; Jirásek, P.; Pitzl, S.; Owen, R.W.; Klika, K.D.; Gerhäuser, C.; Amslinger, S.; et al. A click chemistry approach identifies target proteins of xanthohumol. Mol. Nutr. Food Res. 2016, 60, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Lim, J.; Bang, Y.; Gal, J.; Lee, S.U.; Cho, Y.C.; Yoon, G.; Kang, B.Y.; Cheon, S.H.; Choi, H.J. Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: Therapeutic relevance to neurodegenerative disease. J. Nutr. Biochem. 2012, 23, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Zhang, Y.; Wang, Y.F.; Li, X.C.; Xiang, M.X.; Bian, C.; Chen, P. Upregulation of heme oxygenase-1 expression by hydroxysafflor yellow A conferring protection from anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int. J. Cardiol. 2012, 160, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, M.; Yang, Q.; Wang, M.; Wang, Z.; Zhu, Y.; Zhang, Y.; Wang, C.; Jia, Y.; Li, Y.; et al. Antioxidant effects of hydroxysafflor yellow A and acetyl-11-keto-boswellic acid in combination on isoproterenol-induced myocardial injury in rats. Int. J. Mol. Med. 2016, 37, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wei, G.; Xi, M.; Yan, J.; Wu, X.; Wang, Y.; Zhu, Y.; Wang, C.; Wen, A. Synergistic cardioprotective effects of Danshensu and hydroxysafflor yellow A against myocardial ischemia-reperfusion injury are mediated through the Akt/Nrf2/HO-1 pathway. Int. J. Mol. Med. 2016, 38, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Li, B.; Im, N.K.; Kim, Y.C.; Jeong, G.S. 4,2′,5′-Trihydroxy-4′-methoxychalcone from dalbergia odorifera exhibits anti-inflammatory properties by inducing heme oxygenase-1 in murine macrophages. Int. Immunopharmacol. 2013, 16, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Yakubu, M.T.; Oladiji, A.T. Electrophilic and reactive oxygen species detoxification potentials of chalcone dimers is mediated by redox transcription factor Nrf-2. J. Biochem. Mol. Toxicol. 2014, 28, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, C.Y.; Bai, L.P.; Pan, H.D.; Shu, L.M.; Kong, A.N.T.; Leung, E.L.H.; Liu, L.; Li, T. Flavonoids derived from liquorice suppress murine macrophage activation by up-regulating heme oxygenase-1 independent of Nrf2 activation. Int. Immunopharmacol. 2015, 28, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Chen, Y.; Ding, R.; Feng, L.; Fu, Z.; Yang, S.; Deng, X.; Xie, Z.; Zheng, S. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-ΚB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J. Neuroinfl. 2017, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Cho, S.S.; Yang, J.H.; Kim, K.M.; Jang, C.H.; Park, D.E.; Bang, J.S.; Jung, Y.S.; Ki, S.H. The chalcone compound isosalipurposide (ISPP) exerts a cytoprotective effect against oxidative injury via Nrf2 activation. Toxicol. Appl. Pharmacol. 2015, 287, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, C.; Qiao, Y.; Lu, C.; Li, J.; Song, W.; Sun, J.; Zhai, X.; Niu, J.; Ren, Q.; et al. Safflower yellow B suppresses HepG2 cell injury induced by oxidative stress through the AKT/Nrf2 pathway. Int. J. Mol. Med. 2016, 37, 603–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinner, K.D.; Wales, C.T.K.; Gristock, R.A.; Vo, H.T.; So, N.; Jacobs, A.T. Flavokawains A and B from kava (Piper methysticum) activate heat shock and antioxidant responses and protect against hydrogen peroxide-induced cell death in HepG2 hepatocytes. Pharm. Biol. 2016, 54, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Pala, D.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A.; Casagrande, R. Topical formulation containing hesperidin methyl chalcone inhibits skin oxidative stress and inflammation induced by ultraviolet B irradiation. Photochem. Photobiol. Sci. 2016, 15, 554–563. [Google Scholar] [CrossRef] [PubMed]
- De Spirt, S.; Eckers, A.; Wehrend, C.; Micoogullari, M.; Sies, H.; Stahl, W.; Steinbrenner, H. Interplay between the chalcone cardamonin and selenium in the biosynthesis of Nrf2-regulated antioxidant enzymes in intestinal Caco-2 cells. Free Radic. Biol. Med. 2016, 91, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Hou, Y.; Yao, J.; Fang, J. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ka, S.O.; Lee, Y.; Park, B.H.; Bae, E.J. Butein induction of HO-1 by p38 MAPK/Nrf2 pathway in adipocytes attenuates high-fat diet induced adipose hypertrophy in mice. Eur. J. Pharmacol. 2017, 799, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Vicente, A.M.; Araico, A.; Dominguez, J.N.; Terencio, M.C.; Ferrándiz, M.L. Role of nuclear factor-κB and heme oxygenase-1 in the mechanism of action of an anti-inflammatory chalcone derivative in RAW 264.7 cells. Br. J. Pharmacol. 2004, 142, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Sohn, D.H.; Jin, X.Y.; Kim, S.W.; Choi, S.C.; Seo, G.S. 2′,4′,6′-Tris(methoxymethoxy) chalcone protects against trinitrobenzene sulfonic acid-induced colitis and blocks tumor necrosis factor-α-induced intestinal epithelial inflammation via heme oxygenase 1-dependent and independent pathways. Biochem. Pharmacol. 2007, 74, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Park, P.H.; Kim, H.S.; Jin, X.Y.; Jin, F.; Hur, J.; Ko, G.; Sohn, D.H. KB-34, a newly synthesized chalcone derivative, inhibits lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages via heme oxygenase-1 induction and blockade of activator protein-1. Eur. J. Pharmacol. 2009, 606, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Park, P.H.; Kim, H.S.; Hur, J.; Jin, X.Y.; Jin, Y.L.; Sohn, D.H. YL-I-108, a synthetic chalcone derivative, inhibits lipopolysaccharide- stimulated nitric oxide production in RAW 264.7 murine macrophages: Involvement of heme oxygenase-1 induction and blockade of activator protein-1. Arch. Pharm. Res. 2009, 32, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Y.; Lee, S.H.; Park, P.H.; Hur, J.; Kim, S.A.; Kim, H.S.; Sohn, D.H. 2′-Methoxy-4′6′-Bis(Methoxymethoxy)chalcone inhibits nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 macrophages. Basic Clin. Pharmacol. Toxicol. 2010, 106, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, A.; Onda, K.; Kawahara, H.; Uchiyama, Y.; Nakayama, H.; Omi, T.; Nagaoka, M.; Matsui, H.; Hirano, T. Sofalcone, a gastric mucosa protective agent, increases vascular endothelial growth factor via the Nrf2-heme-oxygenase-1 dependent pathway in gastric epithelial cells. Biochem. Biophys. Res. Commun. 2010, 398, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Kachadourian, R.; Pugazhenthi, S.; Velmurugan, K.; Backos, D.S.; Franklin, C.C.; McCord, J.M.; Day, B.J. 2′,5′-Dihydroxychalcone-induced glutathione is mediated by oxidative stress and kinase signaling pathways. Free Radic. Biol. Med. 2011, 51, 1146–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Kumar, S.; Hassan, M.; Wu, H.; Thimmulappa, R.K.; Kumar, A.; Sharma, S.K.; Parmar, V.S.; Biswal, S.; Malhotra, S. V Novel chalcone derivatives as potent Nrf2 activators in mice and human lung epithelial cells. J. Med. Chem. 2011, 54, 4147–4159. [Google Scholar] [CrossRef] [PubMed]
- Sussan, T.E.; Gajghate, S.; Chatterjee, S.; Mandke, P.; McCormick, S.; Sudini, K.; Kumar, S.; Breysse, P.N.; Diette, G.B.; Sidhaye, V.K.; et al. Nrf2 reduces allergic asthma in mice through enhanced airway epithelial cytoprotective function. Am. J. Physiol. Cell. Mol. Physiol. 2015, 309, L27–L36. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhu, H.; Lord, G.; Bhattachayya, M.; Jones, B.M.; Allaway, G.; Biswal, S.S.; Korman, B.; Marangoni, R.G.; Tourtellotte, W.G.; et al. Nrf2 exerts cell-autonomous antifibrotic effects: Compromised function in systemic sclerosis and therapeutic rescue with a novel heterocyclic chalcone derivative. Transl. Res. 2017, 183, 71–86.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Cheng, C.C.; Shen, L.L.; Wang, Z.K.; Wu, S.B.; Li, W.L.; Chen, S.H.; Zhou, R.P.; Qiu, P.H. Synthetic chalcones with potent antioxidant ability on H2O2-induced apoptosis in PC12 cells. Int. J. Mol. Sci. 2014, 15, 18525–18539. [Google Scholar] [CrossRef] [PubMed]
- Rücker, H.; Al-Rifai, N.; Rascle, A.; Gottfried, E.; Brodziak-Jarosz, L.; Gerhäuser, C.; Dick, T.P.; Amslinger, S. Enhancing the anti-inflammatory activity of chalcones by tuning the Michael acceptor site. Org. Biomol. Chem. 2015, 13, 3040–3047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobst, B.; Weigl, J.; Michl, C.; Vivarelli, F.; Pinz, S.; Amslinger, S.; Rascle, A. Inhibition of interleukin-3- and interferon- α-induced JAK/STAT signaling by the synthetic α-X-2′,3,4,4′-tetramethoxychalcones α-Br-TMC and α-CF3-TMC. Biol. Chem. 2016, 397, 1187–1204. [Google Scholar] [CrossRef] [PubMed]
- Lounsbury, N.; Mateo, G.; Jones, B.; Papaiahgari, S.; Thimmulappa, R.K.; Teijaro, C.; Gordon, J.; Korzekwa, K.; Ye, M.; Allaway, G.; et al. Heterocyclic chalcone activators of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) with improved in vivo efficacy. Bioorg. Med. Chem. 2015, 23, 5352–5359. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, K.B.; Al-Rifai, N.; Ulbrich, F.; Schallner, N.; Rücker, H.; Enzinger, M.; Petkes, H.; Pitzl, S.; Goebel, U.; Amslinger, S. The Cytoprotective Effects of E-α-(4-Methoxyphenyl)-2′,3,4,4′-Tetramethoxychalcone (E-α-p-OMe-C6H4-TMC)—A Novel and Non-Cytotoxic HO-1 Inducer. PLoS ONE 2015, 10, e0142932. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, K.B.; Gothwal, M.; Schallner, N.; Ulbrich, F.; Rücker, H.; Amslinger, S.; Goebel, U. The anti-inflammatory effects of E-α-(p-methoxyphenyl)-2′,3,4,4′-tetramethoxychalcone are mediated via HO-1 induction. Int. Immunopharmacol. 2016, 35, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Zhong, P.; Wu, L.; Qian, Y.; Fang, Q.; Liang, D.; Wang, J.; Zeng, C.; Wang, Y.; Liang, G. Blockage of ROS and NF-κB-mediated inflammation by a new chalcone L6H9 protects cardiomyocytes from hyperglycemia-induced injuries. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Rampa, A.; Montanari, S.; Pruccoli, L.; Bartolini, M.; Falchi, F.; Feoli, A.; Cavalli, A.; Belluti, F.; Gobbi, S.; Tarozzi, A.; et al. Chalcone-based carbamates for Alzheimer’s disease treatment. Future Med. Chem. 2017, 9, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Son, H.J.; Choi, J.W.; Kim, J.; Han, S.H.; Shin, N.; Kim, J.H.; Kim, S.J.; Heo, J.Y.; Kim, D.J.; et al. Activation of the Nrf2 signaling pathway and neuroprotection of nigral dopaminergic neurons by a novel synthetic compound KMS99220. Neurochem. Int. 2018, 112, 96–107. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Qian, J.; Sun, C.; Zhang, H.; Ye, S.; Chen, T.; Xu, Z.; Wang, J.; Huang, W.; Liang, G. An Aza resveratrol–chalcone derivative 6b protects mice against diabetic cardiomyopathy by alleviating inflammation and oxidative stress. J. Cell. Mol. Med. 2018, 22, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ge, X.; Xu, F.; Zhang, Y.; Liu, Z.; Pan, J.; Song, J.; Dai, Y.; Zhou, J.; Feng, J.; et al. Design, synthesis and biological evaluation of paralleled Aza resveratrol-chalcone compounds as potential anti-inflammatory agents for the treatment of acute lung injury. Bioorganic Med. Chem. Lett. 2015, 25, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Sihvola, V.; Levonen, A.L. Keap1 as the redox sensor of the antioxidant response. Arch. Biochem. Biophys. 2017, 617, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, D.; Engel, C.K.; Glombik, H. The Keap1–Nrf2 protein–protein interaction: A suitable target for small molecules. Drug Discov. Today Technol. 2017, 24, 11–17. [Google Scholar] [CrossRef] [PubMed]
| Compound | Active Concentration Against Nrf2 | Target Disease | Study Model | Activity | Ref. |
|---|---|---|---|---|---|
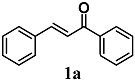 (E)-chalcone (trans-chalcone) | 10–50 μM | Atherogenesis | Aortic endothelial cells | Antioxidant and anti-inflammatory | [23] |
| 1% w/w a | UVB skin damage | Hairless mice | Antioxidant and anti-inflammatory | [24] | |
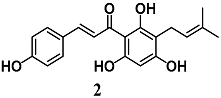 (E)-3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxy-3-(3-methylbut-2-en-1-yl)phenyl)prop-2-en-1-one (Xanthohumol) | 5 μg/mL | Neuroinflammation | Microglial BV2 cells | Anti-inflammatory | [25] |
| 0.5 μM | Parkinson’s disease | Neuronal PC12 cells | Antioxidant and neuroprotective | [26] | |
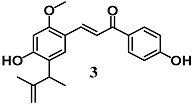 (E)-3-(4-hydroxy-2-methoxy-5-(3-methylbut-3-en-2-yl)phenyl)-1-(4-hydroxyphenyl)prop-2-en-1-one (Licochalcone E) | 5 μM | Parkinson’s disease | Microglial BV2 and neuronal SH-SY5Y cells | Antioxidant and neuroprotective | [27,28] |
 3,4,5-trihydroxy-2-((E)-3-(4-hydroxyphenyl)acryloyl)-4-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)-6-((2S,3R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)-tetrahydro-2H-pyran-2-yl)cyclohexa-2,5-dien-1-one (Safflor yellow A) | 20 μM | Myocardial infarction | Cardiomyocyte H9c2 cells | Cytoprotective | [29] |
| 10 μM 100 mg/kg b | Myocardial infarction | Cardiomyocyte H9C2 cells Rats | Antioxidant and cytoprotective | [30] | |
| 80 μM | Myocardial infarction | Cardiomyocyte H9c2 cells | Cytoprotective | [31] | |
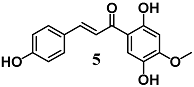 (E)-1-(2,5-dihydroxy-4-methoxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one | 40 μM | Inflammatory disease | Peritoneal macrophages | Anti-inflammatory | [32] |
 (2S,3R)-3-(5-((E)-3-(2,4-dihydroxyphenyl)-3-oxoprop-1-en-1-yl)-2-hydroxyphenyl)-6-hydroxy-2-(4-hydroxyphenyl)chroman-4-one (Lophirones B)  (E)-3-((3S)-3-(2,4-dihydroxybenzoyl)-2-(4-hydroxyphenyl)-2,3-dihydrobenzofuran-5-yl)-1-(2,4-dihydroxyphenyl)prop-2-en-1-one (Lophirones C) | 20 mg/Kg b | Liver diseases | Rats | Antioxidant and detoxification | [33] |
 (E)-1-(2,4-dihydroxyphenyl)-3-(4-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)phenyl)prop-2-en-1-one (Isoliquiritin) 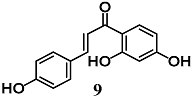 (E)-1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one (Isoliquiritigenin) | 25–50 μM | Inflammatory disease | Macrophage RAW 264.7 and Hepatic HepG2-C8 cells | Anti-inflammatory | [34] |
| 20 mg/Kg c | Intracerebral hemorrhage | Rats | Antioxidant and anti-inflammatory | [35] | |
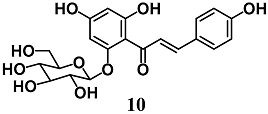 (E)-1-(2,4-dihydroxy-6-(((2S,3R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)phenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one (isosalipurposide) | 10–100 μM | Oxidative stress | Hepatic HepG2 cells | Antioxidant and cytoprotective | [36] |
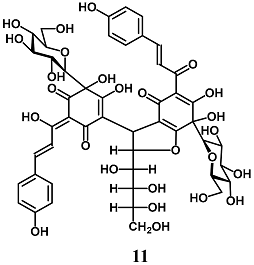 (Z)-4-(6,7-dihydroxy-5-((E)-3-(4-hydroxyphenyl)acryloyl)-4-oxo-2-((1S,2R,3R)-1,2,3,4-tetrahydroxybutyl)-7-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)-2,3,4,7-tetrahydrobenzofuran-3-yl)-5,6-dihydroxy-2-((E)-1-hydroxy-3-(4-hydroxyphenyl)allylidene)-6-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)cyclohex-4-ene-1,3-dione (Safflor yellow B) | 100–150 nM | Oxidative stress | Hepatic HepG2 cells | Antioxidant | [37] |
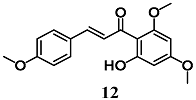 (E)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (Flavokawains A) 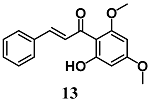 (E)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-phenylprop-2-en-1-one (Flavokawains B) | 20–100 μM | Oxidative stress | Hepatic HepG2 cells | Antioxidant and cytoprotective | [38] |
| 10–20 μM | |||||
 (E)-3-(3-hydroxy-4-methoxyphenyl)-1-(2-hydroxy-6-methoxy-4-(((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-((((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)methyl)tetrahydro-2H-pyran-2-yl)oxy)phenyl)prop-2-en-1-one Hesperidin methyl chalcone (HMC) | 1% w/w a | UVB skin damage | Hairless mice | Antioxidant and cytoprotective | [39] |
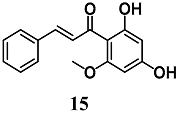 (E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenylprop-2-en-1-one (Cardamonin) | 50 μM d | Oxidative stress | Intestinal Caco-2 cells | Antioxidant | [40] |
| 10 μM | Parkinson’s disease | Neuronal PC12 cells | Neuroprotective | [41] | |
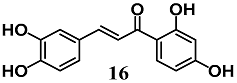 (E)-1-(2,4-dihydroxyphenyl)-3-(3,4-dihydroxyphenyl)prop-2-en-1-one (Butein) | 30 μM | Obesity and Type 2 diabetes | Adipocyte 3T3-L1 cells | Antioxidant | [42] |
| Compound | Active Concentration Against Nrf2 | Target Disease | Study Model | Activity | Ref. |
|---|---|---|---|---|---|
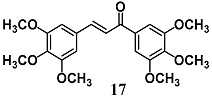 (E)-1,3-bis(3,4,5-trimethoxyphenyl)prop-2-en-1-one | 30 μM | Inflammatory disease | Macrophage RAW 264.7 cells | Anti-inflammatory | [43] |
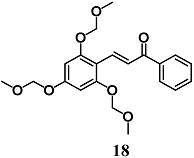 (E)-1-phenyl-3-(2,4,6-tris(methoxymethoxy)phenyl)prop-2-en-1-one | 20 μM | Inflammatory intestinal disease | Intestinal HT-29 cells | Anti-inflammatory | [44] |
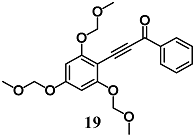 1-phenyl-3-(2,4,6-tris(methoxymethoxy)phenyl)prop-2-yn-1-one | 2 μM | Inflammatory disease | Macrophage RAW 264.7 cells | Anti-inflammatory | [45] |
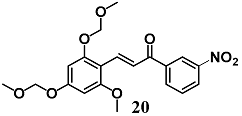 (E)-3-(2-methoxy-4,6-bis(methoxymethoxy)phenyl)-1-(3-nitrophenyl)prop-2-en-1-one | 2 μM | Inflammatory disease | Macrophage RAW 264.7 cells | Anti-inflammatory | [46] |
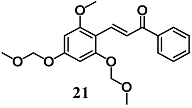 (E)-3-(2-methoxy-4,6-bis(methoxymethoxy)phenyl)-1-phenylprop-2-en-1-one (MBMC) | 2 μM | Inflammatory disease | Macrophage RAW 264.7 cells | Anti-inflammatory | [47] |
 (E)-2-(5-((2-methylprop-1-en-1-yl)oxy)-2-(3-(4-((2-methylprop-1-en-1-yl)oxy)phenyl)acryloyl)phenoxy)-acetic acid (Sofalcone) | 20–50 μM | Gastric ulcer | Gastric epithelial RGM-1 cells | Antiulcer | [48] |
 (E)-1-(2,5-dihydroxyphenyl)-3-phenylprop-2-en-1-one | 10–20 μM | Oxidative stress | MCF-7/AREc32 | Antioxidant | [49] |
 (E)-1-(2-methoxyphenyl)-3-(2-(trifluoromethyl)phenyl)prop-2-en-1-one | 10–20 μM a 50 mg/Kg a,b | Inflammatory disease | Bronchial epithelial Beas-2B cells Mice | Anti-inflammatory | [50] |
| 400 mg/Kg b | Allergic asthma | Mice | Antioxidant and anti-inflammatory | [51] | |
| 5 μM 400 mg/Kg b | Systemic sclerosis | Fibroblasts Mice | Antioxidant | [52] | |
 (E)-2-methoxy-4-(3-(4-methoxyphenyl)-3-oxoprop-1-en-1-yl)phenyl acrylate | 10 μM a | Oxidative stress | Neuronal PC12 cells | Antioxidant | [53] |
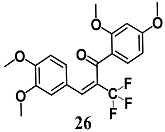 (E)-1-(2,4-dimethoxyphenyl)-3-(3,4-dimethoxyphenyl)-2-(trifluoromethyl)prop-2-en-1-one 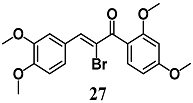 (Z)-2-bromo-1-(2,4-dimethoxyphenyl)-3-(3,4-dimethoxyphenyl)prop-2-en-1-one 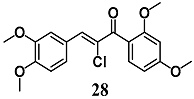 (Z)-2-chloro-1-(2,4-dimethoxyphenyl)-3-(3,4-dimethoxyphenyl)prop-2-en-1-one | 3.13 μM | Inflammatory disease | MCF-7/AREc32 cells | Anti-inflammatory | [54,55] |
| 6.25 μM | |||||
| 6.25 μM | |||||
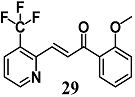 (E)-1-(2-methoxyphenyl)-3-(3-(trifluoromethyl)pyridin-2-yl)prop-2-en-1-one | 5 μM a 400 mg/Kg a,b | Oxidative stress | Bronchial epithelial Beas-2B cells Mice | Antioxidant | [56] |
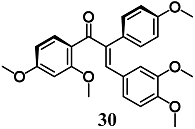 (E)-1-(2,4-dimethoxyphenyl)-3-(3,4-dimethoxyphenyl)-2-(4-methoxyphenyl)prop-2-en-1-one | 30 μM | Inflammatory disease | Macrophage RAW264.7 cells | Antioxidant, cytoprotective and anti-inflammatory | [57,58] |
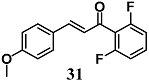 (E)-1-(2,6-difluorophenyl)-3-(4-methoxyphenyl)prop-2-en-1-one | 10 μM 20 mg/kg b | Diabetic cardiomyopathy | Cardiomyocyte H9c2 cells Mice | Anti-inflammatory and cytoprotective | [59] |
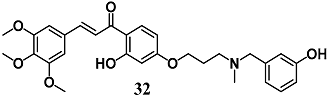 (E)-1-(2-hydroxy-4-(3-((3-hydroxybenzyl)(methyl)amino)propoxy)phenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one | 10 μM a | Alzheimer’s disease | Neuronal SH-SY5Y cells | Neuroprotective activity | [60] |
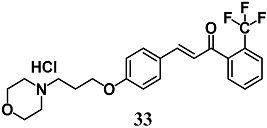 (E)-3-(4-(3-morpholinopropoxy)-phenyl)-1-(2-(trifluoromethyl)phenyl)-prop-2-en-1-one hydrochloride | 3 μM 30 mg/kg a | Parkinson’s disease | Dopaminergic CATH.a cells Mice | Neuroprotective | [61] |
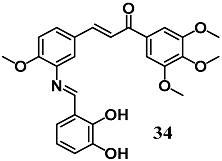 (E)-3-(3-(((E)-2,3-dihydroxybenzylidene)amino)-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one | 5–10 μM 5–20 mg/kg b | Diabetic cardiomyopathy | Cardiomyocyte H9c2 cells Mice | Antioxidant and anti-inflammatory | [62] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Freitas Silva, M.; Pruccoli, L.; Morroni, F.; Sita, G.; Seghetti, F.; Viegas Jr, C.; Tarozzi, A. The Keap1/Nrf2-ARE Pathway as a Pharmacological Target for Chalcones. Molecules 2018, 23, 1803. https://doi.org/10.3390/molecules23071803
De Freitas Silva M, Pruccoli L, Morroni F, Sita G, Seghetti F, Viegas Jr C, Tarozzi A. The Keap1/Nrf2-ARE Pathway as a Pharmacological Target for Chalcones. Molecules. 2018; 23(7):1803. https://doi.org/10.3390/molecules23071803
Chicago/Turabian StyleDe Freitas Silva, Matheus, Letizia Pruccoli, Fabiana Morroni, Giulia Sita, Francesca Seghetti, Claudio Viegas Jr, and Andrea Tarozzi. 2018. "The Keap1/Nrf2-ARE Pathway as a Pharmacological Target for Chalcones" Molecules 23, no. 7: 1803. https://doi.org/10.3390/molecules23071803






