Inactivation, Aggregation and Conformational Changes of Polyphenol Oxidase from Quince (Cydonia oblonga Miller) Juice Subjected to Thermal and High-Pressure Carbon Dioxide Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Quince Juice
2.2. Thermal and HPCD Treatments
2.2.1. Untreated Quince Juice
2.2.2. Thermal Processing of Juice
2.2.3. HPCD Processing of Juice
2.3. Quality Attributes of Quince Juice
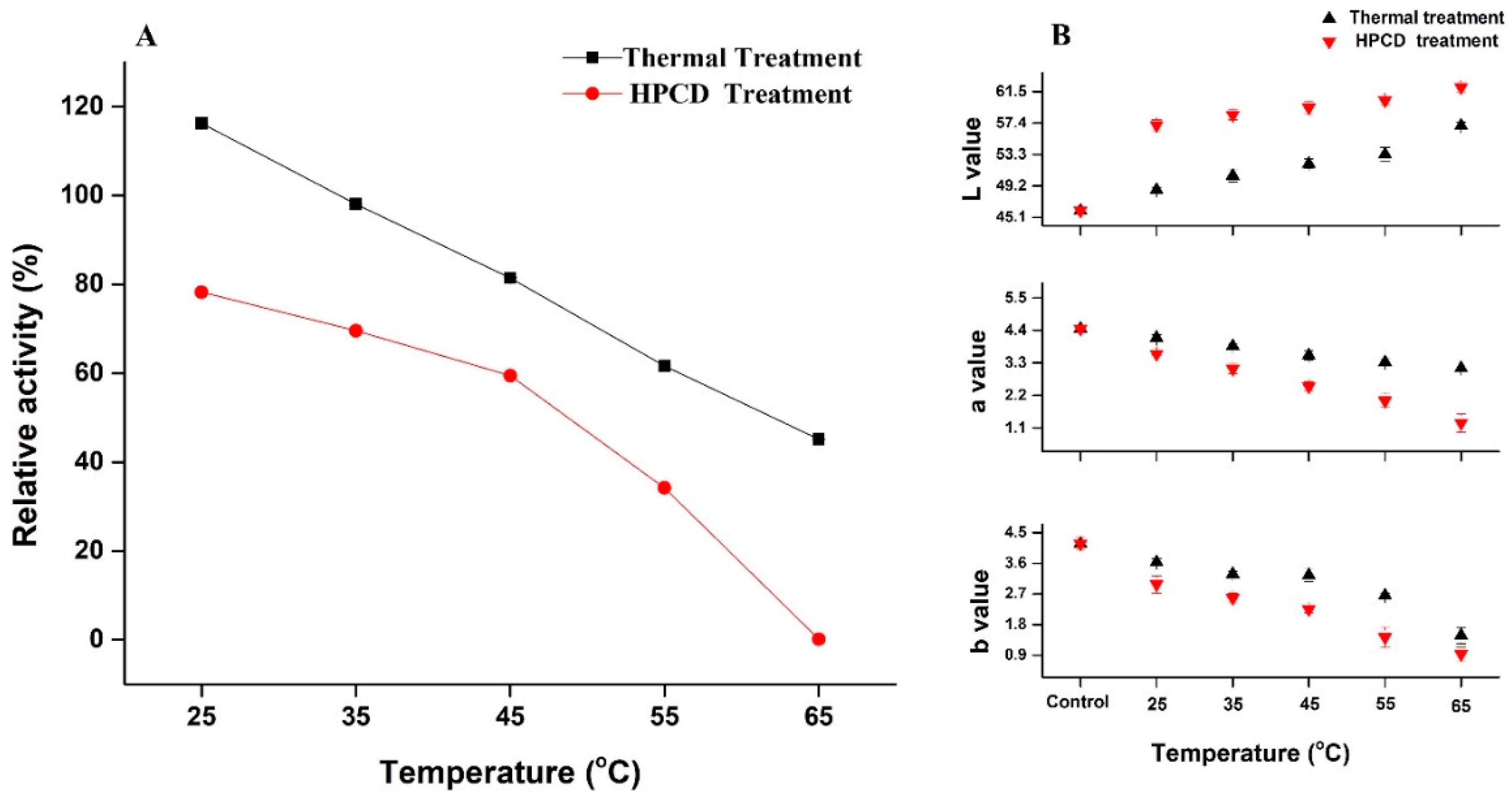
2.3.1. Colorimetric Measurement
2.3.2. Browning Determination
2.3.3. Physico-Chemical Analysis
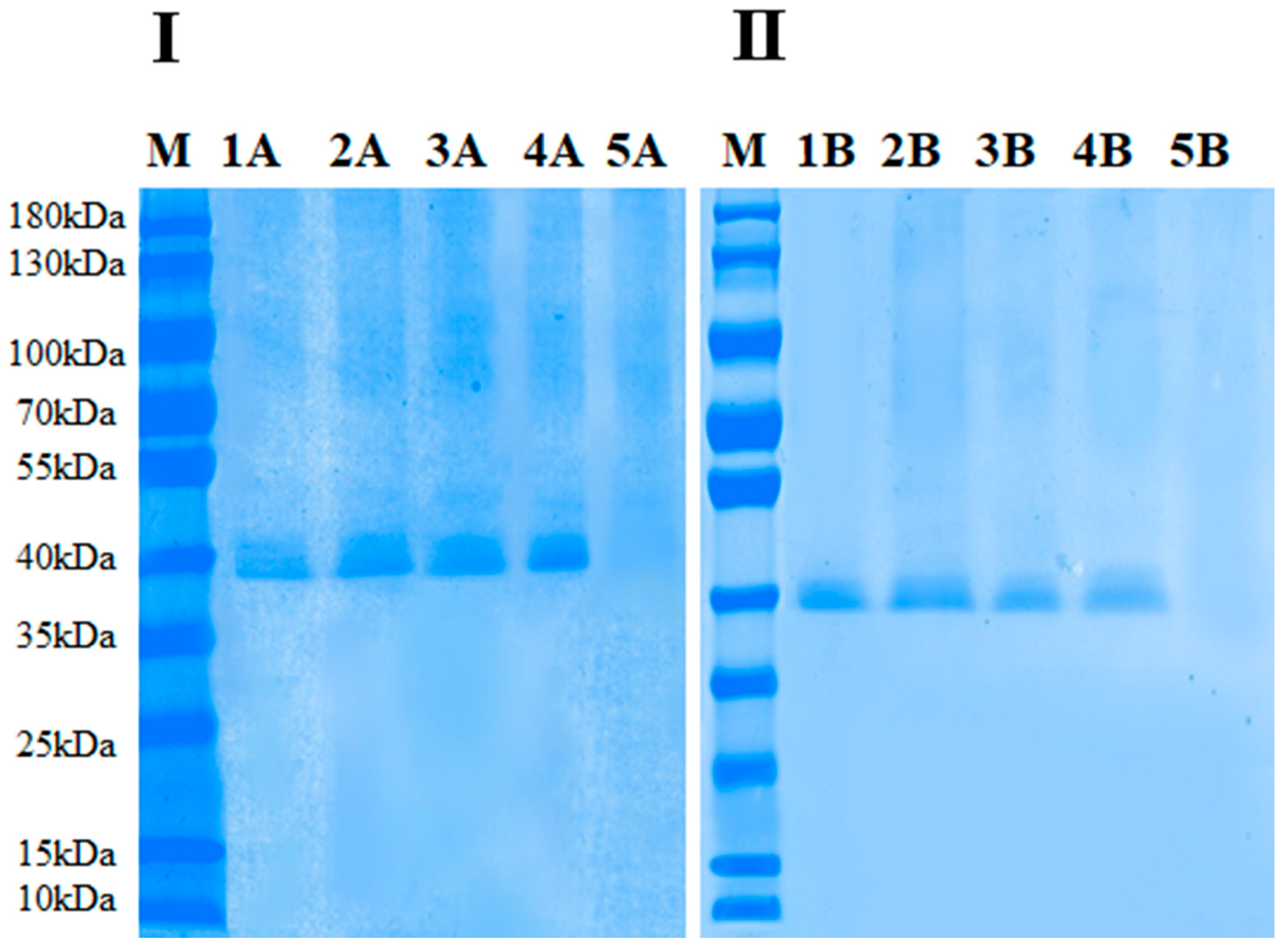
2.4. PPO Extraction and Purification
2.5. PPO Activity
2.6. Protein Determination
2.7. Electrophoretic Analysis of Thermal and HPCD-Treated PPO Enzymes
2.8. Structural Analysis of Untreated and Treated (Thermal and HPCD) PPO
2.8.1. Circular Dichroism Spectral Measurement
2.8.2. Fluorescence Spectral Measurement
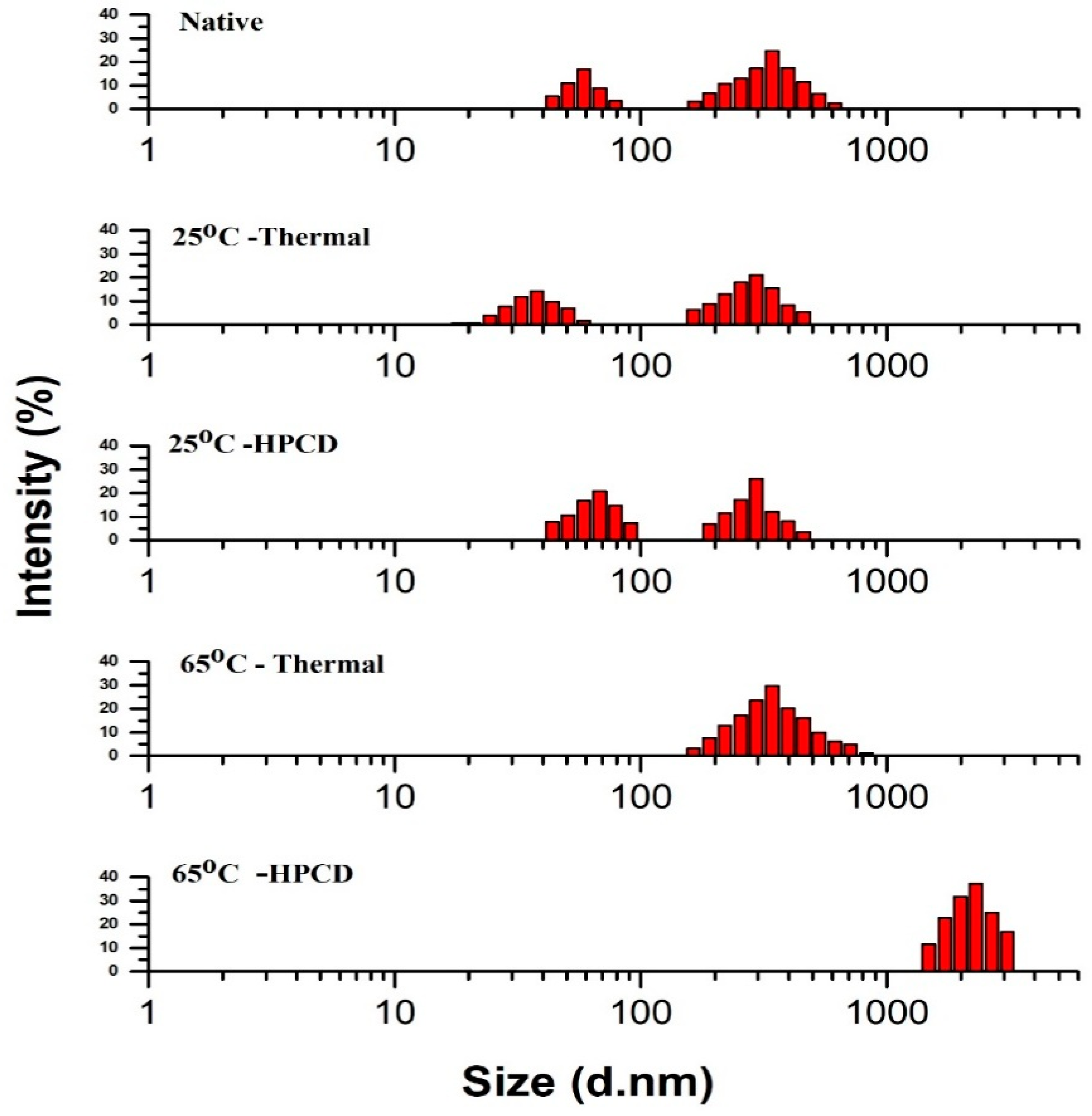
2.8.3. PSD Measurement
2.9. Statistical Analysis
3. Results and Discussion
3.1. Changes in Color, pH and TSS of Quince Juice
3.2. Activation & Inactivation of Crude PPO Enzyme by Thermal and HPCD Treatments
3.3. Effect of Thermal and HPCD Processing on Browning Degree of Quince Juice
3.4. Particle Size Distribution of Thermal- and HPCD-Treated PPO
3.5. Effect of Thermal and HPCD Treatments on the Secondary Structure of PPO
3.6. Fluorescence Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elmizadeh, A.; Shahedi, M.; Hamdami, N. Comparison of electrohydrodynamic and hot-air drying of the quince slices. Innov. Food Sci. Emerg. Technol. 2017, 43, 130–135. [Google Scholar] [CrossRef]
- Carbonell-Barrachina, Á.A.; Szychowski, P.J.; Vásquez, M.V.; Hernández, F.; Wojdyło, A. Technological aspects as the main impact on quality of quince liquors. Food Chem. 2015, 167, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Teleszko, M.; Oszmiański, J. Antioxidant property and storage stability of quince juice phenolic compounds. Food Chem. 2014, 152, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Plazzotta, S.; Spilimbergo, S.; Nicoli, M.C. Impact of high-pressure carbon dioxide on polyphenoloxidase activity and stability of fresh apple juice. LWT Food Sci. Technol. 2017, 85, 363–371. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Barba, F.J.; Skąpska, S.; Lorenzo, J.M.; Zambon, A.; Spilimbergo, S. Enzymatic, physicochemical, nutritional and phytochemical profile changes of apple (Golden Delicious L.) juice under supercritical carbon dioxide and long-term cold storage. Food Chem. 2018, 268, 279–286. [Google Scholar] [CrossRef]
- Bibhuti Bhusan Mishra, B. Polyphonel oxidases: Biochemical and molecular characterization, distribution, role and its control. Enzym. Eng. 2016, 5, 141–149. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Kruszewski, B.; Skapska, S. The effect of high pressure techniques on the stability of anthocyanins in fruit and vegetables. Int. J. Mol. Sci. 2017, 18, 277. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Pälijärvi, M.; Karonen, M.; Salminen, J.P. Oxidatively active plant phenolics detected by UHPLC-DAD-MS after enzymatic and alkaline oxidation. J. Chem. Ecol. 2018, 44, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Huang, X.; Liu, S.; Tang, M.; Hu, W.; Pan, S. Characterization of germin-like protein with polyphenol oxidase activity from Satsuma mandarine. Biochem. Biophys. Res. Commun. 2014, 449, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, K.; Kruszewski, B.; Woźniak, Ł.; Skąpska, S. The application of supercritical carbon dioxide for the stabilization of native and commercial polyphenol oxidases and peroxidases in cloudy apple juice (cv. Golden Delicious). Innov. Food Sci. Emerg. Technol. 2017, 39, 42–48. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Huang, X.; Yang, W.; Hu, W.; Pan, S. Effect of ultrasonic processing on the changes in activity, aggregation and the secondary and tertiary structure of polyphenol oxidase in oriental sweet melon (Cucumis melo var. makuwa Makino). J. Sci. Food Agric. 2017, 97, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, K.; Krzyżanowska, J.; Woźniak, Ł.; Skąpska, S. Kinetic modelling of polyphenol oxidase, peroxidase, pectin esterase, polygalacturonase, degradation of the main pigments and polyphenols in beetroot juice during high pressure carbon dioxide treatment. LWT Food Sci. Technol. 2017, 85, 412–417. [Google Scholar] [CrossRef]
- Marszałek, K.; Skąpska, S.; Woźniak, Ł.; Sokołowska, B. Application of supercritical carbon dioxide for the preservation of strawberry juice: Microbial and physicochemical quality, enzymatic activity and the degradation kinetics of anthocyanins during storage. Innov. Food Sci. Emerg. Technol. 2015, 32, 101–109. [Google Scholar] [CrossRef]
- Ferrentino, G.; Spilimbergo, S. High pressure carbon dioxide pasteurization of solid foods: Current knowledge and future outlooks. Trends Food Sci. Technol. 2011, 22, 427–441. [Google Scholar] [CrossRef]
- Murtaza, A.; Muhammad, Z.; Iqbal, A.; Ramzan, R.; Liu, Y.; Hu, W.; Pan, S. Aggregation and conformational changes in native and thermally treated polyphenol oxidase from apple juice (Malus domestica). Front. Chem. 2018, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, K.; Krzyżanowska, J.; Woźniak, Ł.; Skąpska, S. Kinetic modelling of tissue enzymes inactivation and degradation of pigments and polyphenols in cloudy carrot and celery juices under supercritical carbon dioxide. J. Supercrit. Fluids 2016, 117, 26–32. [Google Scholar] [CrossRef]
- Liao, H.; Hu, X.; Liao, X.; Chen, F.; Wu, J. Inactivation of Escherichia coli inoculated into cloudy apple juice exposed to dense phase carbon dioxide. Int. J. Food Microbiol. 2007, 118, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Huang, N.; Cheng, X.; Hu, W.; Pan, S. Inactivation, aggregation, secondary and tertiary structural changes of germin-like protein in Satsuma mandarine with high polyphenol oxidase activity induced by ultrasonic processing. Biophys. Chem. 2015, 197, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.; Hu, W.; Liao, X. Changes in the activity, dissociation, aggregation, and the secondary and tertiary structures of a thaumatin-like protein with a high polyphenol oxidase activity induced by high pressure CO2. Innov. Food Sci. Emerg. Technol. 2014, 23, 68–78. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Wang, Y.; Zhou, L.; Leng, X.; Liao, X.; Hu, X. Aggregation and homogenization, surface charge and structural change, and inactivation of mushroom tyrosinase in an aqueous system by subcritical/supercritical carbon dioxide. Langmuir 2011, 27, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.L.; Zeng, W.C.; Lu, X.L. Influence of ultrasound to the activity of tyrosinase. Ultrason. Sonochem. 2013, 20, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhao, J.; Wen, X.; Ni, Y. Purification and structural analysis of membrane-bound polyphenol oxidase from Fuji apple. Food Chem. 2015, 183, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhou, L.; Xu, Z.; Zhang, Y.; Liao, X. Enzyme inactivation in food processing using high pressure carbon dioxide technology. Crit. Rev. Food Sci. Nutr. 2013, 53, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Cundari, T.R.; Wilson, A.K.; Drummond, M.L.; Gonzalez, H.E.; Jorgensen, K.R.; Payne, S.; Braunfeld, J.; De Jesus, M.; Johnson, V.M. CO2-formatics: How do proteins bind carbon dioxide? J. Chem. Inf. Model. 2009, 49, 2111–2115. [Google Scholar] [CrossRef] [PubMed]
- Derardja, A.E.; Pretzler, M.; Kampatsikas, I.; Barkat, M.; Rompel, A. Purification and characterization of latent polyphenol oxidase from apricot (Prunus armeniaca L.). J. Agric. Food Chem. 2017, 65, 8203–8212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, X.; Zhao, X.; Song, H. Combined effect of high pressure carbon dioxide and mild heat treatment on overall quality parameters of watermelon juice. Innov. Food Sci. Emerg. Technol. 2012, 13, 112–119. [Google Scholar] [CrossRef]
- Waliszewski, K.N.; Márquez, O.; Pardio, V.T. Quantification and characterisation of polyphenol oxidase from vanilla bean. Food Chem. 2009, 117, 196–203. [Google Scholar] [CrossRef]
- Keenan, D.F.; Rößle, C.; Gormley, R.; Butler, F.; Brunton, N.P. Effect of high hydrostatic pressure and thermal processing on the nutritional quality and enzyme activity of fruit smoothies. LWT Food Sci. Technol. 2012, 45, 50–57. [Google Scholar] [CrossRef]
- Niu, S.; Xu, Z.; Fang, Y.; Zhang, L.; Yang, Y.; Liao, X.; Hu, X. Comparative study on cloudy apple juice qualities from apple slices treated by high pressure carbon dioxide and mild heat. Innov. Food Sci. Emerg. Technol. 2010, 11, 91–97. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Hu, X.; Liao, X.; He, J. Comparison of the inactivation kinetics of pectin methylesterases from carrot and peach by high-pressure carbon dioxide. Food Chem. 2009, 115, 449–455. [Google Scholar] [CrossRef]
- Leeb, E.; Götz, A.; Letzel, T.; Cheison, S.C.; Kulozik, U. Influence of denaturation and aggregation of β-lactoglobulin on its tryptic hydrolysis and the release of functional peptides. Food Chem. 2015, 187, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Zisu, B.; Palmer, M.; Kentish, S.; Ashokkumar, M. Ultrasonics sonochemistry effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason. Sonochem. 2011, 18, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, W.; Zou, L.; Xiong, Z.; Hu, X.; Chen, J. Aggregation and conformational change of mushroom (Agaricus bisporus) polyphenoloxidase subjected to thermal treatment. Food Chem. 2017, 214, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, W.; Xiong, Z.; Zou, L.; Liu, J.; Zhong, J.; Chen, J. Effect of ultrasound combined with malic acid on the activity and conformation of mushroom (Agaricus bisporus) polyphenoloxidase. Enzym. Microb. Technol. 2016, 90, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ioniţǎ, E.; Aprodu, I.; Stǎnciuc, N.; Râpeanu, G.; Bahrim, G. Advances in structure-function relationships of tyrosinase from Agaricus bisporus–Investigation on heat-induced conformational changes. Food Chem. 2014, 156, 129–136. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| Treatment | Temp | pH | °Brix |
|---|---|---|---|
| Control (Untreated) | 4.74 ± 0.23a | 11.55 ± 0.22a | |
| Thermal-treated (20 min) | 25 | 4.75 ± 0.18a | 11.54 ± 0.18a |
| 35 | 4.73 ± 0.24a | 11.56 ± 0.2a | |
| 45 | 4.73 ± 0.13a | 11.54 ± 0.2a | |
| 55 | 4.70 ± 0.22a | 11.51 ± 0.19a | |
| 65 | 4.69 ± 0.17a | 11.49 ± 0.19a | |
| HPCD-treated (20 MPa, 20 min) | 25 | 4.23 ± 0.21ab | 11.25 ± 0.37a |
| 35 | 4.23 ± 0.23ab | 11.19 ± 0.16a | |
| 45 | 4.20 ± 0.19ab | 11.10 ± 0.15a | |
| 55 | 4.18 ± 0.14ab | 10.98 ± 0.17a | |
| 65 | 4.11 ± 0.11b | 10.92 ± 0.21a | |
| Treatments | Secondary Structure Contents | |||||
|---|---|---|---|---|---|---|
| α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) | PPO Activity (%) | Concentration (%) | |
| Native | 39.34 ± 1.42a | 17.9 ± 1.13d | 26.41 ± 0.59a | 16.35 ± 0.75d | 24.23 ± 0.76d | 8.57 ± 0.67b |
| 25 °C thermal | 38.96 ± 0.96b | 18.4 ± 0.78cd | 24.48 ± 0.82ab | 18.16 ± 1.12cd | 42.58 ± 1.02a | 12.78 ± 0.93a |
| 25 °C HPCD | 35.26 ± 0.78b | 20.5 ± 0.53b | 23.85 ± 0.77b | 20.39 ± 0.82c | 36.88 ± 0.64b | 4.04 ± 0.15c |
| 65 °C thermal | 30.81 ± 1.77c | 23.4 ± 0.35b | 21.21 ± 1.11c | 24.58 ± 0.66b | 29.53 ± 1.2c | 3.91 ± 0.09c |
| 65 °C HPCD | 24.11 ± 0.97d | 27.7 ± 0.77a | 18.4 ± 1.15d | 29.79 ± 0.92a | 07.40 ± 1.33e | 0.27 ± 0.015d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, A.; Murtaza, A.; Muhammad, Z.; Elkhedir, A.E.; Tao, M.; Xu, X. Inactivation, Aggregation and Conformational Changes of Polyphenol Oxidase from Quince (Cydonia oblonga Miller) Juice Subjected to Thermal and High-Pressure Carbon Dioxide Treatment. Molecules 2018, 23, 1743. https://doi.org/10.3390/molecules23071743
Iqbal A, Murtaza A, Muhammad Z, Elkhedir AE, Tao M, Xu X. Inactivation, Aggregation and Conformational Changes of Polyphenol Oxidase from Quince (Cydonia oblonga Miller) Juice Subjected to Thermal and High-Pressure Carbon Dioxide Treatment. Molecules. 2018; 23(7):1743. https://doi.org/10.3390/molecules23071743
Chicago/Turabian StyleIqbal, Aamir, Ayesha Murtaza, Zafarullah Muhammad, Abdeen E. Elkhedir, Mingfang Tao, and Xiaoyun Xu. 2018. "Inactivation, Aggregation and Conformational Changes of Polyphenol Oxidase from Quince (Cydonia oblonga Miller) Juice Subjected to Thermal and High-Pressure Carbon Dioxide Treatment" Molecules 23, no. 7: 1743. https://doi.org/10.3390/molecules23071743