Can Parietin Transfer Energy Radiatively to Photosynthetic Pigments?
Abstract
:1. Introduction
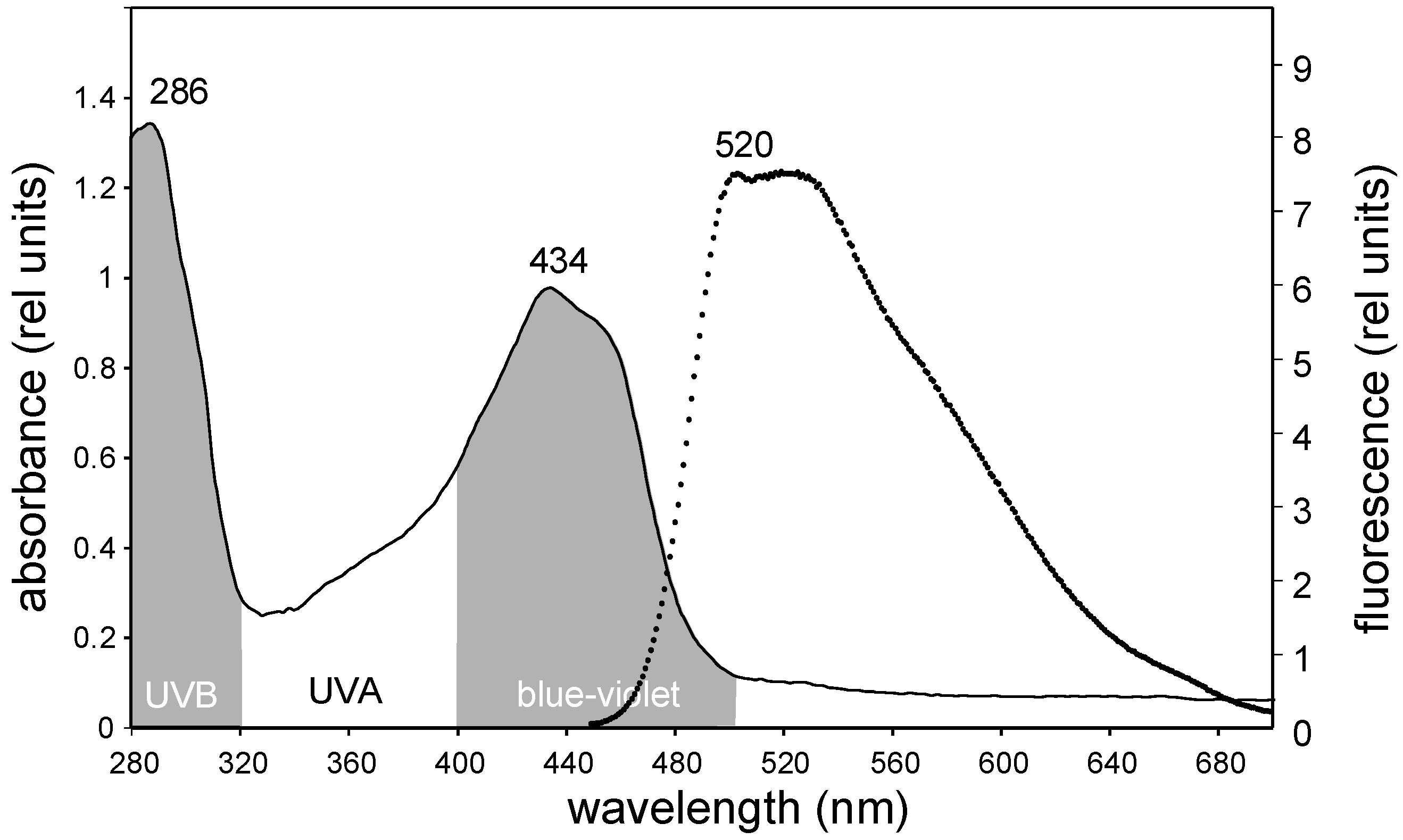
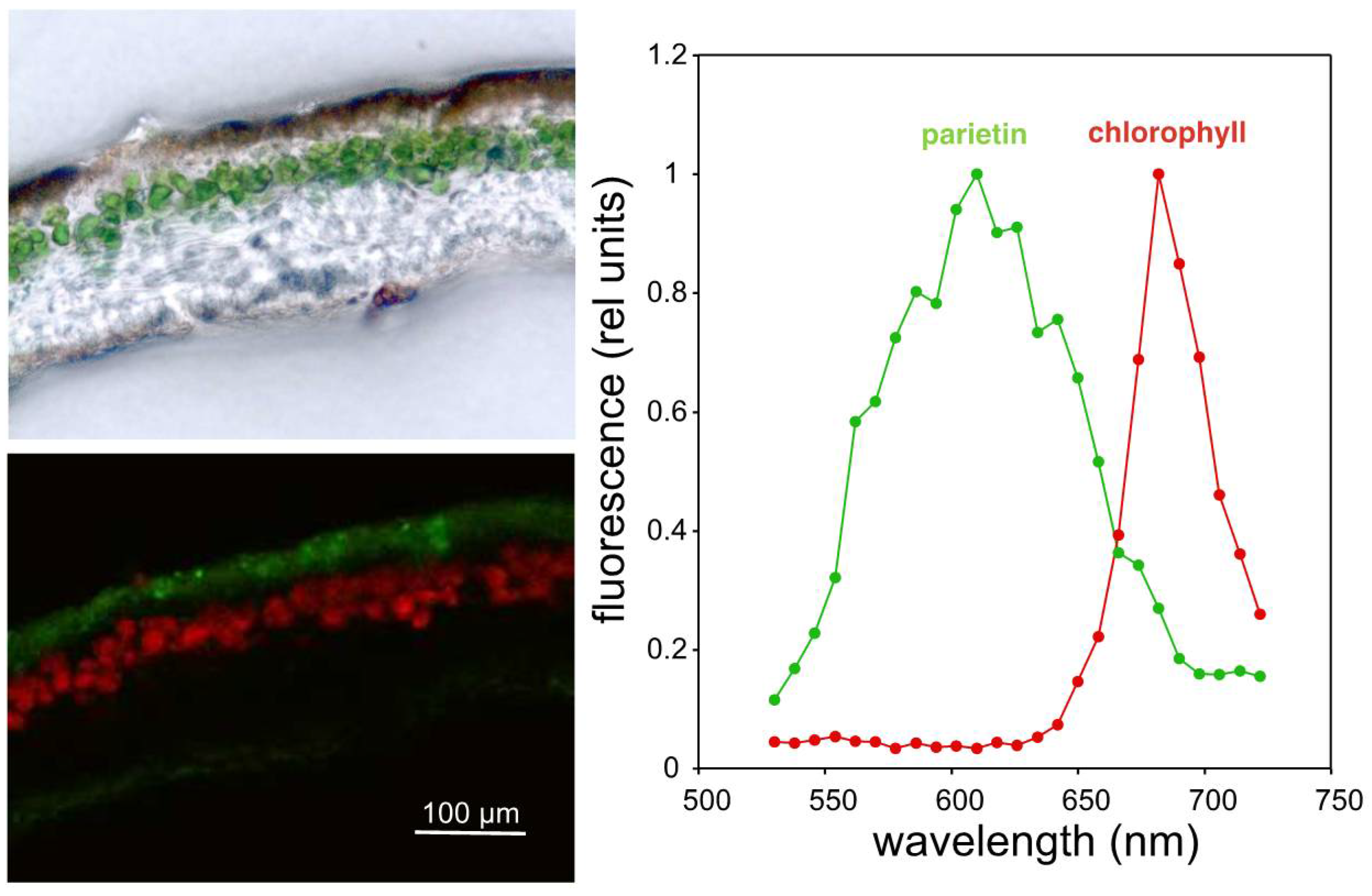
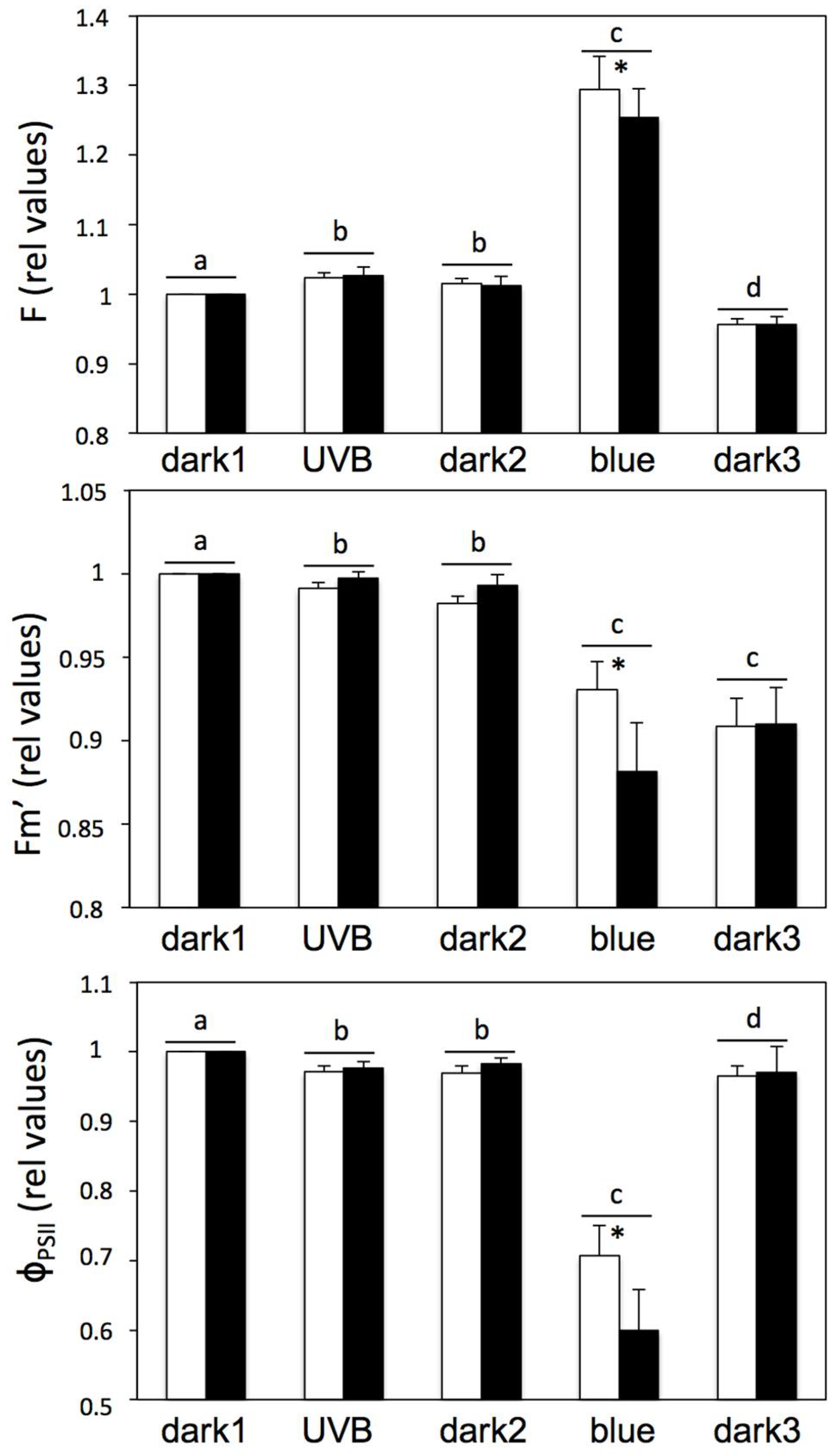
2. Results and Discussion
3. Materials and Methods
3.1. Lichen Collection
3.2. Chlorophyll Fluorescence
3.3. Confocal Microscopy
3.4. Fluorescence and Absorbance Spectra
3.5. UV-B and Blue Light Experiments
3.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lawrey, J.D. Biological role of lichen substances. Bryologist 1986, 89, 111–122. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Gauslaa, Y. Parietin, a photoprotective secondary product of the lichen Xanthoria parietina. Oecologia 1996, 108, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Gauslaa, Y. Lichen palatability depends on investments in herbivore defence. Oecologia 2005, 143, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Rigano, D.; Loppi, S.; Di Santi, A.; Nebbioso, A.; Sorbo, S.; Conte, B.; Paoli, L.; De Ruberto, F.; Molinari, A.M.; et al. Antiproliferative, antibacterial and antifungal activity of the lichen Xanthoria parietina and its secondary metabolite parietin. Int. J. Mol. Sci. 2015, 16, 7861–7875. [Google Scholar] [CrossRef] [PubMed]
- Gauslaa, Y.; McEvoy, M. Seasonal changes in solar radiation drive acclimation to the sun-screening compound parietin in the lichen Xanthoria parietina. Basic Appl. Ecol. 2005, 6, 75–82. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Ustvedt, E.M. Is parietin a UV-B or a blue-light screening pigment in the lichen Xanthoria parietina? Photochem. Photobiol. 2003, 2, 424–432. [Google Scholar] [CrossRef]
- Larsson, P.; Vecerová, K.; Cempírkova, H.; Solhaug, K.A.; Gauslaa, Y. Does UV-B influence biomass growth in lichens deficient in sun-screening pigments? Environ. Exp. Bot. 2009, 67, 215–221. [Google Scholar] [CrossRef]
- Hakala-Yatkin, M.; Mäntysaari, M.; Mattila, H.; Tyystjärvi, E. Contributions of visible and ultraviolet parts of sunlight to photoinhibition. Plant Cell Physiol. 2010, 51, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, K.A.; Gauslaa, Y.; Nybakker, L.; Bilger, W. UV-induction of sun-screening pigments in lichens. New Phytol. 2003, 158, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Vráblíková, H.; McEvoy, M.; Solhaug, K.A.; Barták, M.; Gauslaa, Y. Annual variation in photoacclimation and photoprotection of the photobiont in the foliose lichen Xanthoria parietina. J. Photochem. Photobiol. 2006, 83, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, K.A.; Gauslaa, Y. Photosynthates stimulate the UV-B induced fungal anthraquinone synthesis in the foliose lichen Xanthoria parietina. Plant Cell Environ. 2004, 27, 157–176. [Google Scholar] [CrossRef]
- Sancho, L.G.; de la Torre, R.; Horneck, G.; Acaso, C.; de los Ríos, A.; Pintado, A.; Wierzschos, J.; Schuster, M. Lichen survive in space: Results from the 2005 lichens experiment. Astrobiology 2007, 7, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Gaya, E.; Fernández-Brime, S.; Vargas, R.; Lachlan, R.F.; Gueidan, C.; Ramírez-Mejía, M.; Lutzoni, F. The adaptive radiation of lichen-forming Teloschistaceae is associated with sunscreening pigments and a bark-to-rock substrate shift. Proc. Natl. Acad. Sci. USA 2018, 112, 11600–11605. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.E., Jr. Fluorescence of lichen depsides and depsidones as a taxonomic criterion. Castanea 1956, 21, 30–32. [Google Scholar]
- Rao, D.N.; LeBlanc, B.F. A possible role of atranorin in the lichen thallus. Bryologist 1965, 68, 284–289. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Fernández-Marín, B.; Duke, S.O.; Hernández, A.; López-Arbeloa, F.; Becerril, J.M. Autofluorescence: Biological functions and technical applications. Plant Sci. 2015, 236, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Hoque, E.; Remus, G. Natural UV-screening mechanisms of Norway spruce (Picea abies (L.) Karst.) needles. Photochem. Photobiol. 1999, 69, 177–192. [Google Scholar] [PubMed]
- Hura, T.; Grzesiak, S.; Hura, K.; Thiemt, E.; Tokarz, K.; Wedzony, M. Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: Accumulation of ferulic acid correlates with drought tolerance. Ann. Bot. 2007, 100, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Schweiger, J. Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. J. Plant Physiol. 1998, 152, 272–282. [Google Scholar] [CrossRef]
- Morales, F.; Cerovic, Z.G.; Moya, I. Time-resolved blue-green fluorescence of sugar beet (Beta vulgaris L.) leaves. Spectroscopic evidence for the presence of ferulic acid as the main fluorophore of the epidermis. Biochim. Biophys. Acta 1996, 1273, 251–262. [Google Scholar] [CrossRef]
- Piattelli, M.; Giudici de Nicola, M. Anthraquinone pigments from Xanthoria parietina. Phytochemistry 1968, 7, 1183–1187. [Google Scholar] [CrossRef]
- Alboresi, A.; Gerotto, C.; Cazzaniga, S.; Bassi, R.; Morosinotto, T. A Red-shifted antenna protein associated with photosystem II in Physcomitrella patens. J. Biol. Chem. 2011, 289, 28978–28987. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meterol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Hidalgo, M.E.; Fernández, E.; Ponce, M.; Rubio, C.; Quilhot, W. Photophysical, photochemical, and thermodynamic properties of shikimic acid derivatives: Calycin and rhizocarpic acid (lichens). J. Photochem. Photobiol. 2002, 66, 213–217. [Google Scholar] [CrossRef]
- Kauppi, K.; Verseghy-Patay, K. Determination of the distribution of lichen substances in the thallus by fluorescence microscopy. Ann. Bot. Fenn. 1990, 27, 189–202. [Google Scholar]
- Solhaug, K.A.; Larsson, P. Light screening in lichen cortices can be quantified by chlorophyll fluorescence techniques for both reflecting and absorbing pigments. Planta 2010, 231, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Candotto Carniel, F.; Pellegrini, E.; Bove, F.; Grosera, M.; Adami, G.; Nali, C.; Lorenzini, G.; Tretiach, M. Acetone washing for the removal of lichen substances affects membrane permeability. Lichenologist 2017, 49, 387–395. [Google Scholar] [CrossRef]
- Mathey, A.; Lukins, P.B. Spatial distribution of perylenquinones in lichens and extended quinines in quincyte using confocal fluorescence microscopy. Micron 2001, 32, 107–113. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lozano, J.A.; Utrillas, M.P.; Núnez, J.A.; Esteve, A.R.; Gómez-Amo, J.L.; Estellés, V.; Pedrós, R. Measurement and Analysis of Broadband UVB Solar Radiation in Spain. Photochem. Photobiol. 2012, 88, 1489–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Marín, B.; Artetxe, U.; Becerril, J.M.; Martínez-Abaigar, J.; Núñez-Olivera, E.; García-Plazaola, J.I. Can Parietin Transfer Energy Radiatively to Photosynthetic Pigments? Molecules 2018, 23, 1741. https://doi.org/10.3390/molecules23071741
Fernández-Marín B, Artetxe U, Becerril JM, Martínez-Abaigar J, Núñez-Olivera E, García-Plazaola JI. Can Parietin Transfer Energy Radiatively to Photosynthetic Pigments? Molecules. 2018; 23(7):1741. https://doi.org/10.3390/molecules23071741
Chicago/Turabian StyleFernández-Marín, Beatriz, Unai Artetxe, José María Becerril, Javier Martínez-Abaigar, Encarnación Núñez-Olivera, and José Ignacio García-Plazaola. 2018. "Can Parietin Transfer Energy Radiatively to Photosynthetic Pigments?" Molecules 23, no. 7: 1741. https://doi.org/10.3390/molecules23071741





