Application of Spray Drying Particle Engineering to a High-Functionality/Low-Solubility Milk Thistle Extract: Powders Production and Characterization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Silymarin Content
2.2. Microencapsulation Process
2.3. Powder Characterization
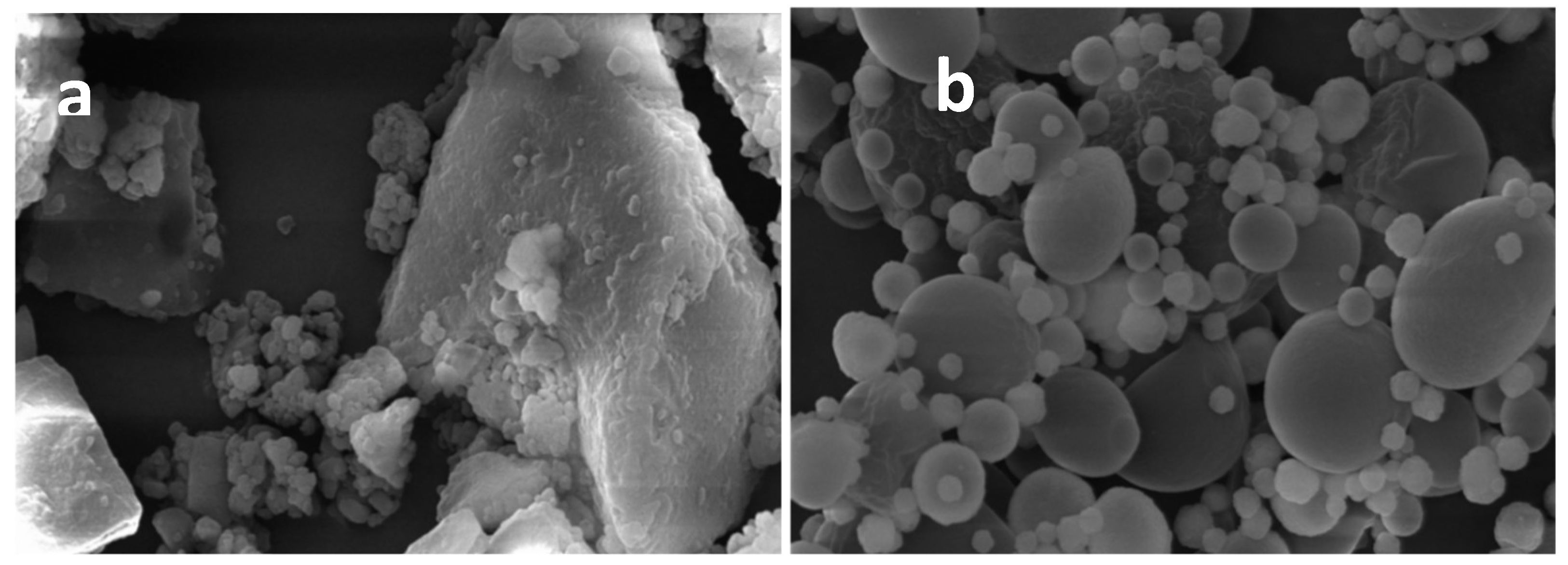
2.3.1. Dimensions and Morphology
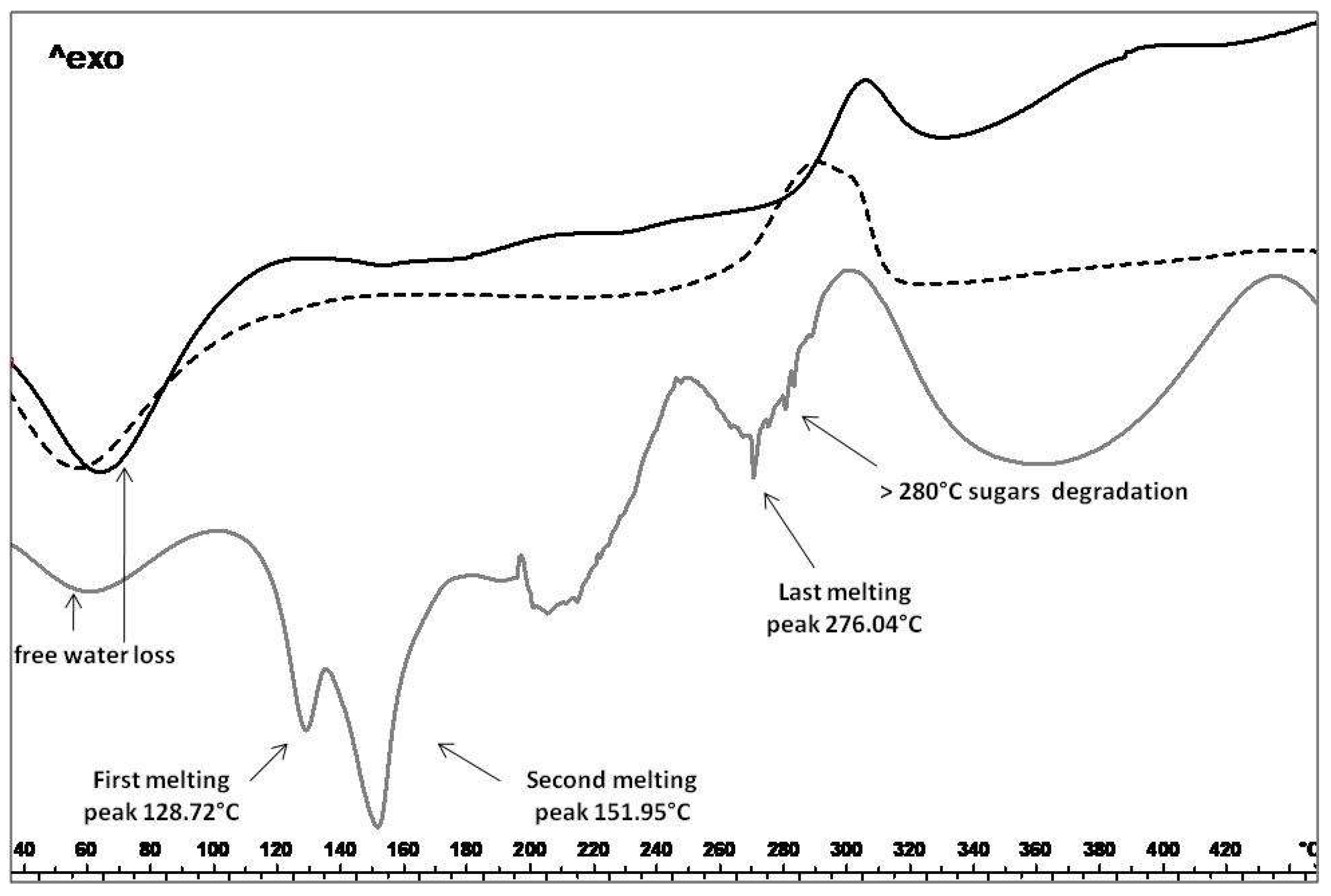
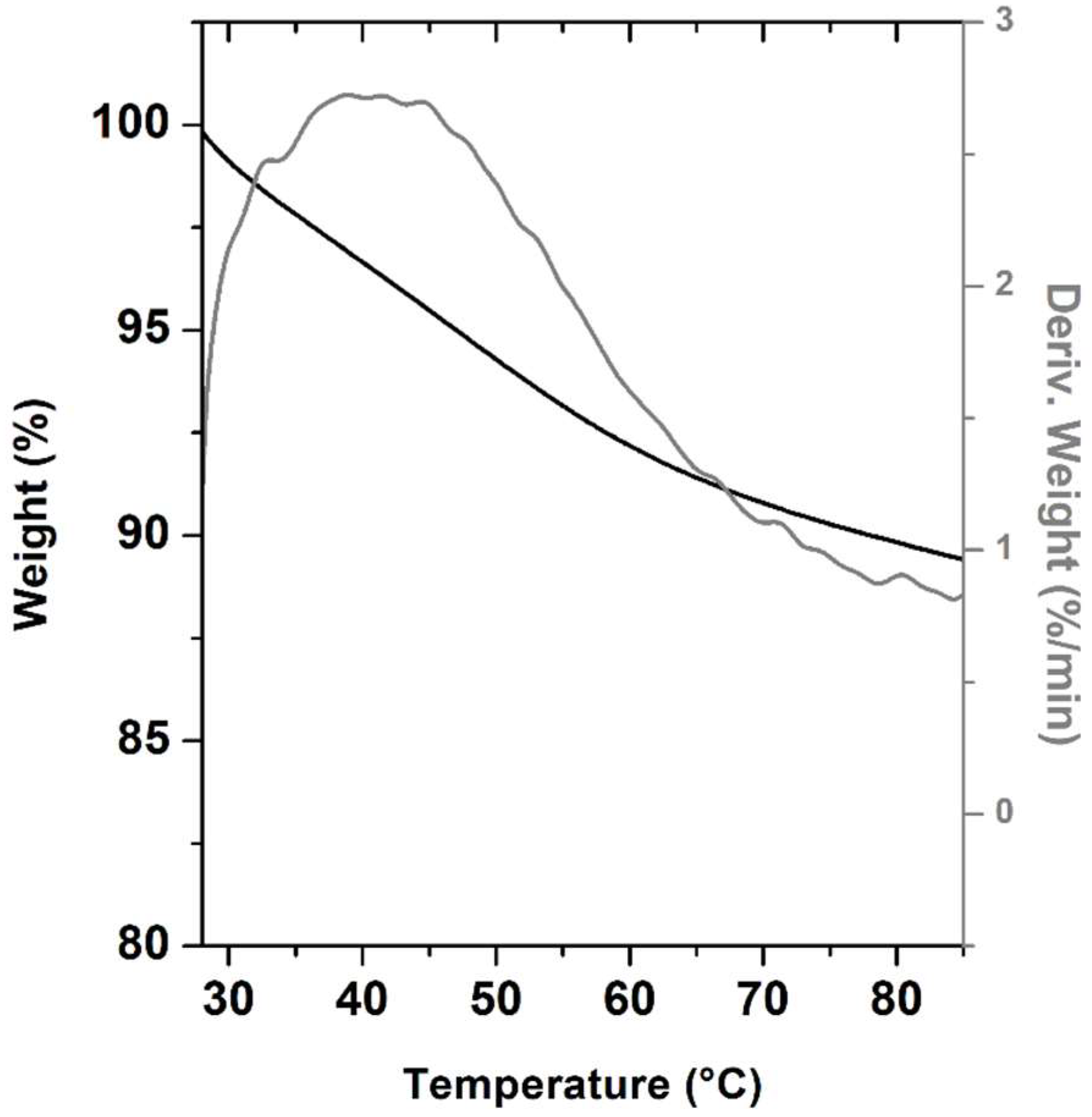
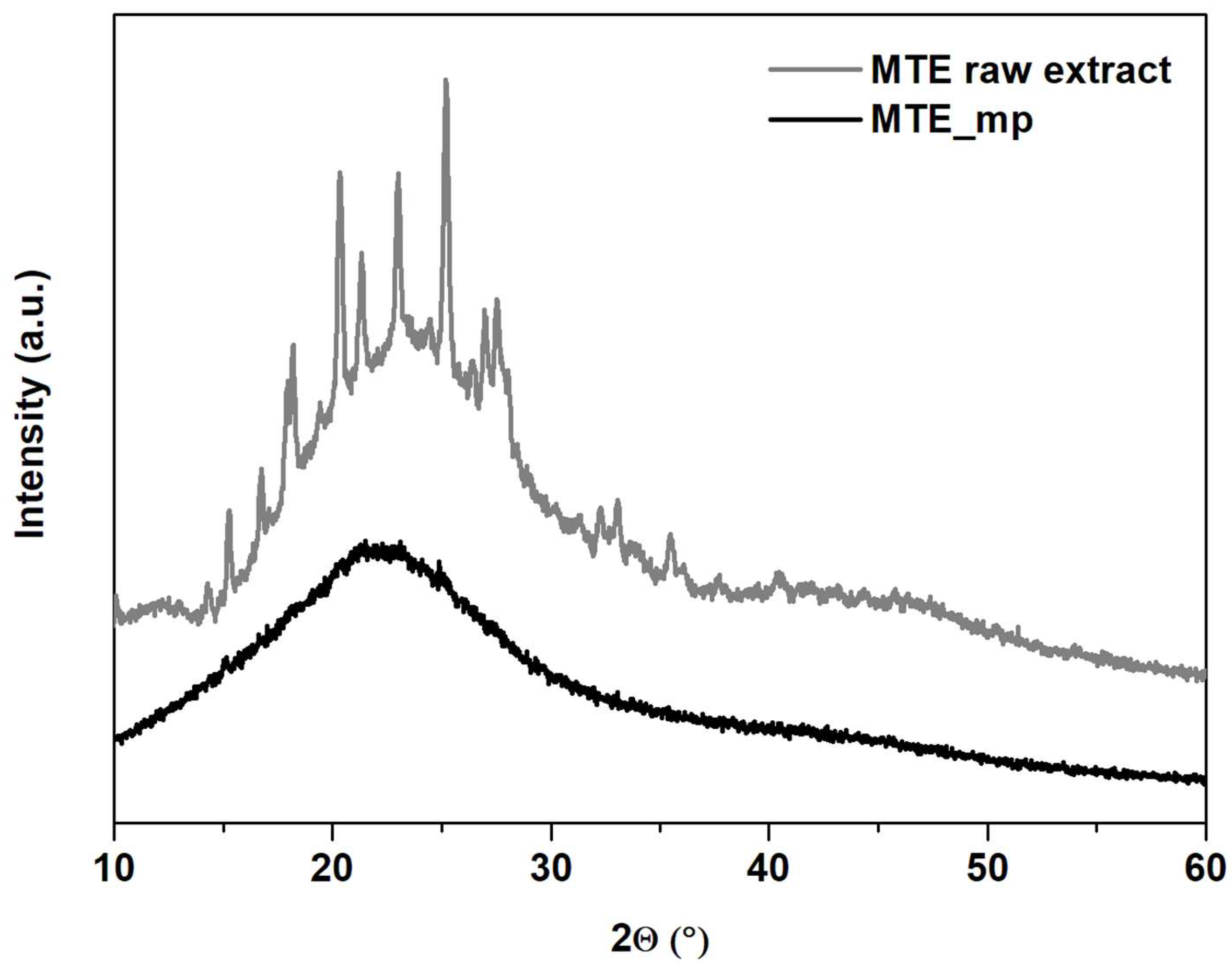
2.3.2. Thermal Analysis, TGA and PXRD
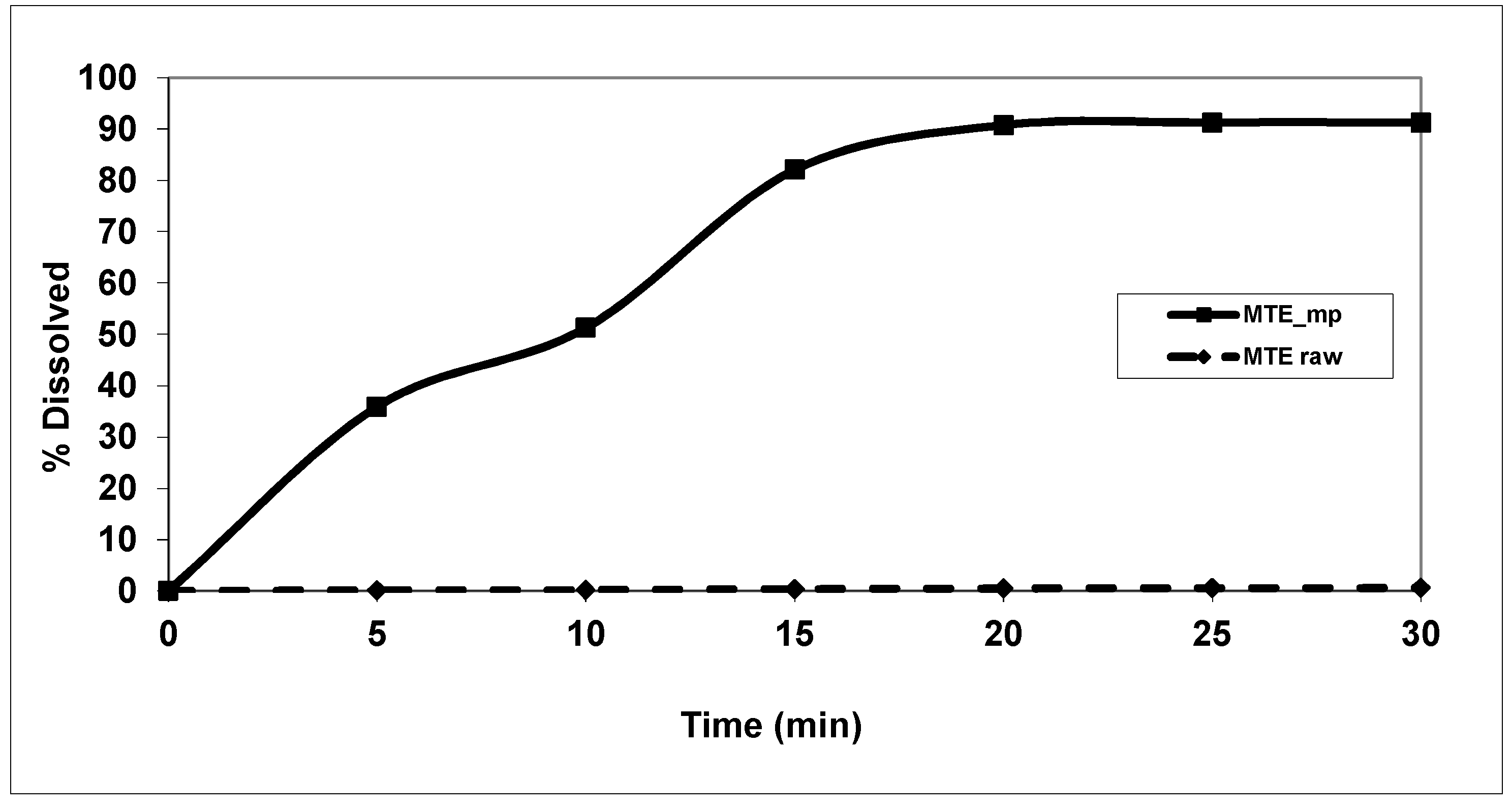
2.4. Solubility and Dissolution Profiles
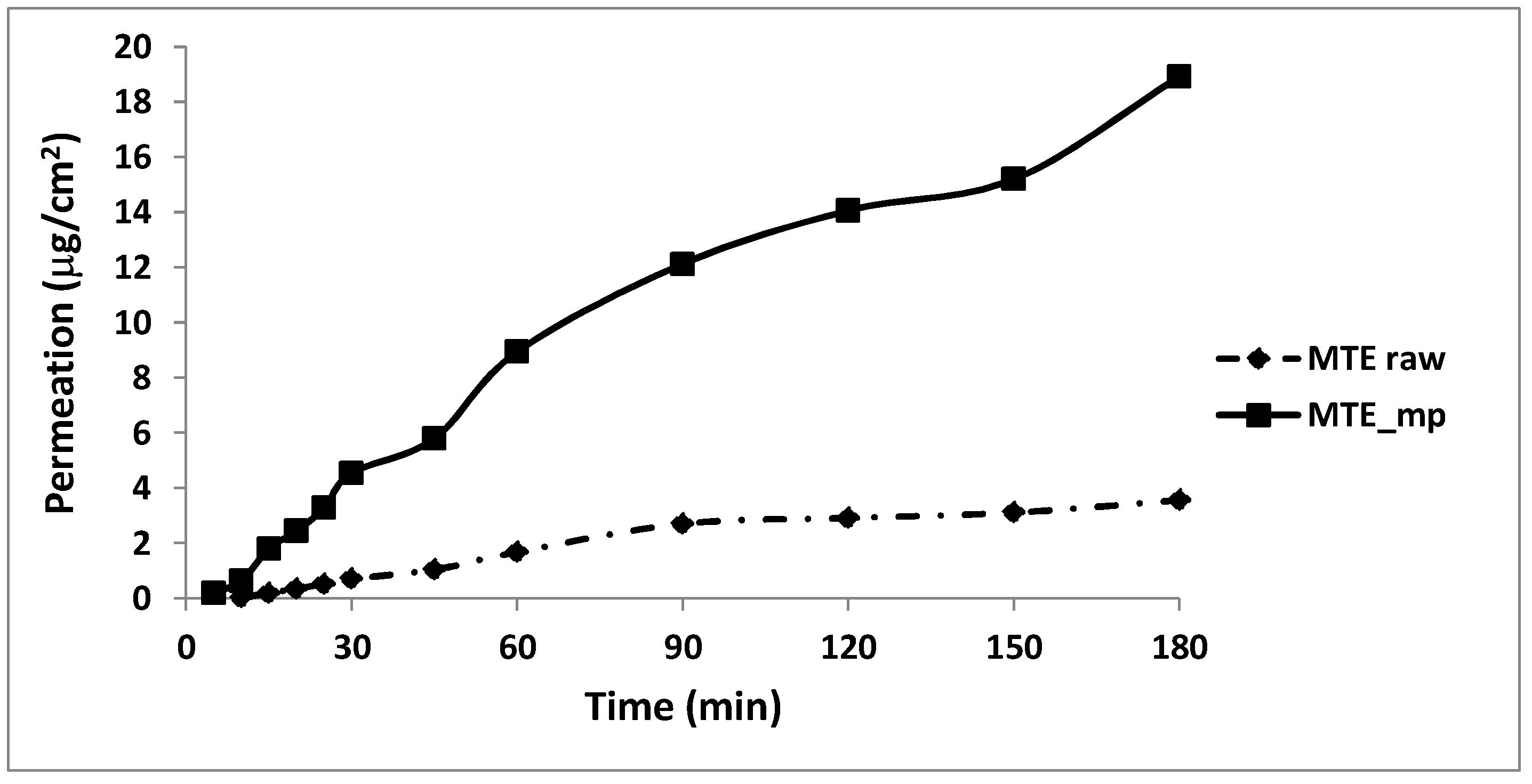
2.5. Permeation Studies
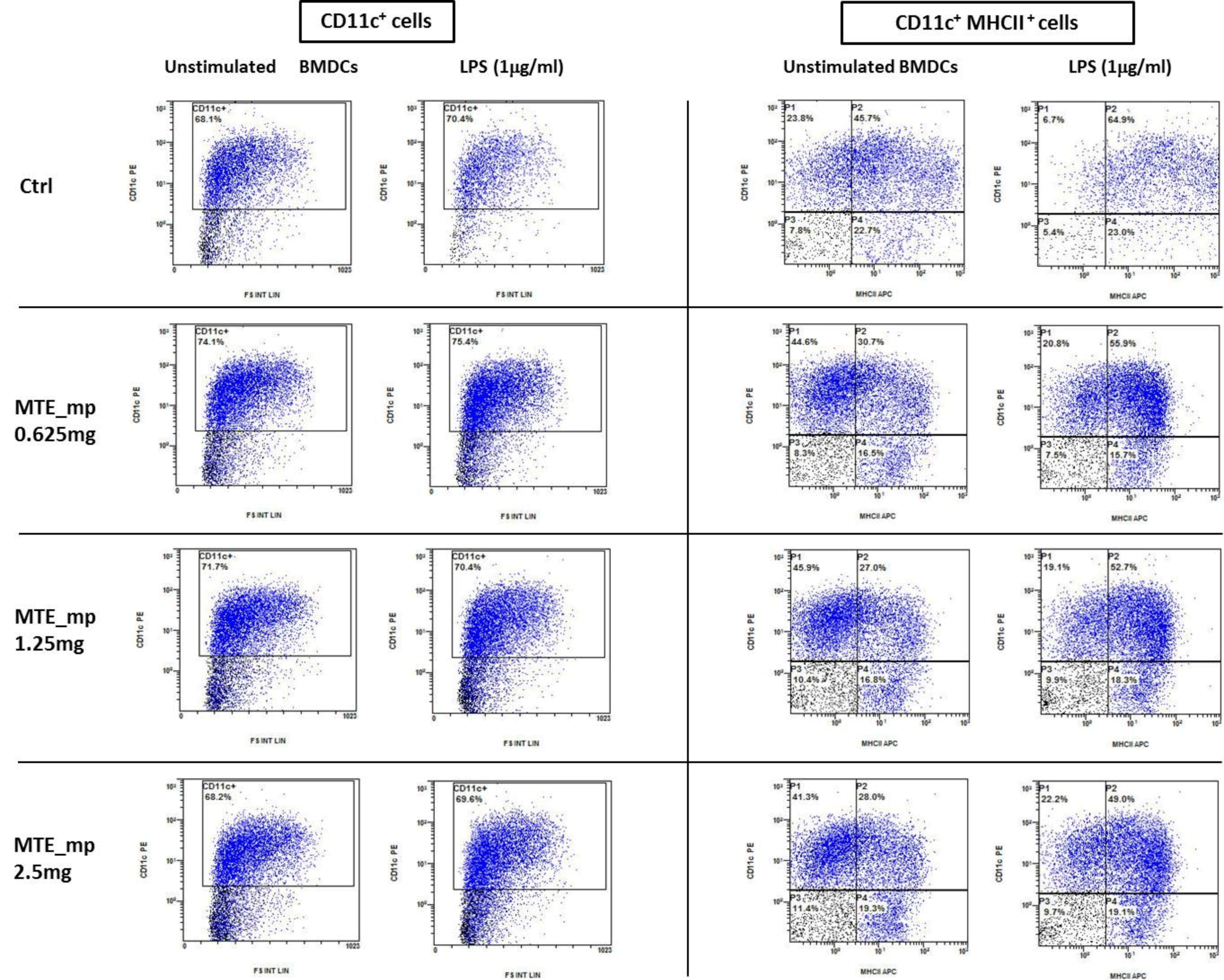
2.6. Biological Assays
2.7. Stability Studies and Antioxidant Activity
3. Materials and Methods
3.1. Chemicals
3.2. Liquid Feed Preparation and Spray Drying Conditions
3.3. Powders Characterization
3.3.1. Quantitative Analysis
3.3.2. Yield and Loading Efficiency
3.3.3. Particle Size Analyses
3.3.4. Morphology
3.3.5. Differential Scanning Calorimetry (DSC)
3.3.6. X-ray Analysis
3.3.7. Thermogravimetric Analysis
3.4. Solubility, Dissolution Tests, and Permeation Profile
- VR is the receiver volume;
- Cn is silybin concentration in the receiver at the time n;
- VP is the volume of the removed sample;
- Ci is silybin concentration in the receiver at the time n-1;
3.5. Generation and Culture of DC
3.6. Enzyme-Linked Immunosorbent Assay (ELISA)
3.7. Antioxidant Activity Preservation—Stability Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Z.P.; Sun, J.; Chen, H.X.; Xiao, Y.Y.; Liu, D.; Chen, J.; Cai, H.; Cai, B.C. Comparative pharmacokinetics and bioavailability studies of quercetin, kaempferol and isorhamnetin after oral administration of Ginkgo biloba extracts, Ginkgo biloba extract phospholipid complexes and Ginkgo biloba extract solid dispersions in rats. Fitoterapia 2010, 81, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Lauro, M.R.; Tirendi, G.G.; Fassari, G.E.; Carbone, C.; Bonina, F.; Puglisi, G. Modern drug delivery strategies applied to natural active compounds. Expert Opin. Drug Deliv. 2017, 14, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Picerno, P.; Mencherini, T.; Villecco, F.; D’Ursi, A.M.; Aquino, R.P.; Lauro, M.R. Flavonoid microparticles by spray-drying: Influence of enhancers of the dissolution rate on properties and stability. J. Food Eng. 2011, 103, 188–196. [Google Scholar] [CrossRef]
- Cho, H.J.; Jee, J.P.; Kang, J.Y.; Shin, D.Y.; Choi, H.G.; Maeng, H.J.; Cho, K.H. Cefdinir solid dispersion composed of hydrophilic polymers with enhanced solubility, dissolution, and bioavailability in rats. Molecules 2017, 22, 280. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, P.; De Cicco, F.; Sansone, F.; Aquino, R.P.; Adami, R.; Ricci, M.; Giovagnoli, S. Alginate beads as a carrier for omeprazole/SBA-15 inclusion compound: A step towards the development of personalized paediatric dosage forms. Carbohydr. Polym. 2015, 133, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Cerciello, A.; Auriemma, G.; Del Gaudio, P.; Sansone, F.; Aquino, R.P.; Russo, P. A novel core-shell chronotherapeutic system for the oral administration of ketoprofen. J. Drug Deliv. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Robert, P.; Fredes, C. The encapsulation of anthocyanins from berry-type fruits. Trends in foods. Molecules 2015, 20, 5875–5888. [Google Scholar] [CrossRef] [PubMed]
- Zokti, J.; Sham Baharin, B.; Mohammed, A.; Abas, F. Green Tea Leaves Extract: Microencapsulation, Physicochemical and Storage Stability Study. Molecules 2016, 21, 940. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Becerra, C.; Parada, J.; Robert, P. The microencapsulation of maqui (Aristotelia chilensis (Mol.) Stuntz) juice by spray-drying and freeze-drying produces powders with similar anthocyanin stability and bioaccessibility. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Endo, E.H.; Ueda-Nakamura, T.; Nakamura, C.V.; Filho, B.P.D. Activity of spray-dried microparticles containing pomegranate peel extract against Candida albicans. Molecules 2012, 17, 10094–10107. [Google Scholar] [CrossRef] [PubMed]
- Lauro, M.R.; Crascì, L.; Giannone, V.; Ballistreri, G.; Fabroni, S.; Sansone, F.; Rapisarda, P.; Panico, A.M.; Puglisi, G. An alginate/cyclodextrin spray drying matrix to improve shelf life and antioxidant efficiency of a blood orange by-product extract rich in polyphenols: MMPs inhibition and antiglycation activity in dysmetabolic diseases. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Lauro, M.R.; Crascí, L.; Sansone, F.; Cardile, V.; Panico, A.M.; Puglisi, G. Development and in Vitro Evaluation of an Innovative “dietary Flavonoid Supplement” on Osteoarthritis Process. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Qavami, N.; Naghdi Badi, H.; Labbafi, M.R.; Mehrafarin, A. A Review on Pharmacological, Cultivation and Biotechnology Aspects of Milk Thistle (Silybum marianum (L.) Gaertn.). J. Med. Plants 2013, 3, 19–37. [Google Scholar]
- Sabiu, S.; Sunmonu, T.O.; Ajani, E.O.; Ajiboye, T.O. Combined administration of silymarin and vitamin C stalls acetaminophen-mediated hepatic oxidative insults in Wistar rats. Braz. J. Pharmacogn. 2015, 25, 29–34. [Google Scholar] [CrossRef]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. 2011, 16, 239–249. [Google Scholar] [PubMed]
- Snima, K.S.; Arunkumar, P.; Jayakumar, R.; Lakshmanan, V.K. Silymarin encapsulated Poly(d,l-lactic-co-glycolic acid) nanoparticles: A prospective candidate for prostate cancer therapy. J. Biomed. Nanotechnol. 2014, 10, 559–570. [Google Scholar] [CrossRef] [PubMed]
- KÖksal, E.; GÜLÇİN, İ.; Beyza, S.; Sarikaya, Ö.; Bursal, E. In vitro antioxidant activity of silymarin. J. Enzyme Inhib. Med. Chem. 2009, 24, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.M. Bioavailability and activity of phytosome complexes from botanical polyphenols: The silymarin, curcumin, green tea, and grape seed extracts. Altern. Med. Rev. 2009, 14, 226–246. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.M. A Novel Pharmaceutical Formulation (Niosomes) of the Hepatoprotective Drug Silymarin. Ph.D. Thesis, Cairo University, Giza, Egypt, 2008. [Google Scholar]
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Increasing bioavailability of silymarin using a buccal liposomal delivery system: Preparation and experimental design investigation. Int. J. Pharm. 2006, 308, 140–148. [Google Scholar] [CrossRef] [PubMed]
- El-Ridy, M.S.; Badawi, A.A.; Safar, M.M.; Mohsen, A.M. Niosomes as a novel pharmaceutical formulation encapsulating the hepatoprotective drug silymarin. Int. J. Pharm. Pharm. Sci. 2012, 4, 549–559. [Google Scholar]
- Elmowafy, M.; Viitala, T.; Ibrahim, H.M.; Abu-Elyazid, S.K.; Samy, A.; Kassem, A.; Yliperttula, M. Silymarin loaded liposomes for hepatic targeting: In vitro evaluation and HepG2 drug uptake. Eur. J. Pharm. Sci. 2013, 50, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Voinovich, D.; Perissutti, B.; Grassi, M.; Passerini, N.; Bigotto, A. Solid State Mechanochemical Activation of Silybum marianum Dry Extract With Betacyclodextrins: Characterization and Bioavailability of the Coground Systems. J. Pharm. Sci. 2009, 98, 4119–4129. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Jia, W.; Li, S.; Xu, Z.; Sun, X.; Wang, X.; Zhang, Y.; Xie, G. A new silymarin preparation based on solid dispersion technique. Adv. Ther. 2005, 22, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Shen, Z.G.; Wang, J.X.; Zhang, H.X.; Zhao, H.; Chen, J.F.; Yun, J. Micronization of silybin by the emulsion solvent diffusion method. Int. J. Pharm. 2009, 376, 116–122. [Google Scholar] [CrossRef] [PubMed]
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance. Int. J. Pharm. 2006, 319, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.M.; Asfour, M.H.; Salama, A.A.A. Improved hepatoprotective activity of silymarin via encapsulation in the novel vesicular nanosystem bilosomes. Drug Dev. Ind. Pharm. 2017, 43, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Esposito, T.; Mencherini, T.; Lauro, M.R.; Del Gaudio, P.; Picerno, P.; Pepe, G.; Aquino, R.P. Particle technology applied to a lactose/NaCMC blend: Production and characterization of a novel and stable spray-dried ingredient. Powder Technol. 2018, 329. [Google Scholar] [CrossRef]
- Sansone, F.; Picerno, P.; Mencherini, T.; Russo, P.; Gasparri, F.; Giannini, V.; Lauro, M.R.; Puglisi, G.; Aquino, R.P. Enhanced technological and permeation properties of a microencapsulated soy isoflavones extract. J. Food Eng. 2013, 115, 298–305. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray Drying Technique. I: Hardware and Process Parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, R.; Orsat, V. Spray Drying for the Production of Nutraceutical Ingredients—A Review. Food Bioprocess Technol. 2012, 5, 3–14. [Google Scholar] [CrossRef]
- Eri, R.; Chieppa, M. Messages from the inside. The dynamic environment that favors intestinal homeostasis. Front. Immunol. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.P.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Niess, J.H. CX3CR1-Mediated Dendritic Cell Access to the Intestinal Lumen and Bacterial Clearance. Science 2005, 307, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Chieppa, M.; Rescigno, M.; Huang, A.Y.C.; Germain, R.N. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J. Exp. Med. 2006, 203, 2841–2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delvecchio, F.R.; Vadrucci, E.; Cavalcanti, E.; De Santis, S.; Kunde, D.; Vacca, M.; Myers, J.; Allen, F.; Bianco, G.; Huang, A.Y.; et al. Polyphenol administration impairs T-cell proliferation by imprinting a distinct dendritic cell maturational profile. Eur. J. Immunol. 2015, 45, 2638–2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Santis, S.; Kunde, D.; Serino, G.; Galleggiante, V.; Caruso, M.L.; Mastronardi, M.; Cavalcanti, E.; Ranson, N.; Pinto, A.; Campiglia, P.; et al. Secretory leukoprotease inhibitor is required for efficient quercetin-mediated suppression of TNF beta secretion. Oncotarget 2016, 7, 75800–75809. [Google Scholar] [CrossRef] [PubMed]
- Gleason, M.N.; Gosselin, R.E.; Hodge, I.C. Clinical Toxicology of Commercial Products, 5th ed.; Williams and Wilkins: Baltimore, MD, USA, 1984; p. II-274. [Google Scholar]
- Jain, A.; Ran, Y.; Yalkowsky, S.H. Effect of pH-sodium lauryl sulfate combination on solubilization of PG-300995 (an anti-HIV agent): A technical note. AAPS PharmSciTech 2004, 5, e45. [Google Scholar] [CrossRef] [PubMed]
- Rege, B.D.; Yu Lawrence, X.; Hussain, A.S.; Polli, J.E. Effect of common excipients on Caco-2 transport of low-permeability drugs. J. Pharm. Sci. 2001, 90, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Mencherini, T.; Picerno, P.; Esposito, T.; Del Gaudio, P.; Russo, P.; Pepe, G.; Lauro, M.R.; Aquino, R.P. Microencapsulation by spray drying of Lannea microcarpa extract: Technological characteristics and antioxidant activity. J. Pharm. Pharmacogn. Res. 2014, 2, 100–109. [Google Scholar]
- Ma, Y.; He, S.; Ma, X.; Hong, T.; Li, Z.; Park, K.; Wang, W. Silymarin-loaded nanoparticles based on stearic acid-modified Bletilla striata polysaccharide for hepatic targeting. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Farmacopea Ufficiale Italiana, 12th ed.; Istituto poligrafico dello stato: Roma, Italy, 2008.
- Sansone, F.; Esposito, T.; Mencherini, T.; Piccinelli, A.L.; Gazzerro, P.; Picerno, P.; Russo, P.; Del Gaudio, P.; Essolito, M.; Campiglia, P.; et al. Annurca peel extract: From the chemical composition, through the functional activity, to the formulation and characterisation of a topical oil-in-water emulsion. Nat. Prod. Res. 2016, 30. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Chieppa, M. Gut-level decisions in peace and war. Nat. Med. 2005, 11, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Fordham, J.B.; Raza Naqvi, A.; Nares, S. Leukocyte production of inflammatory mediators is inhibited by the antioxidants phloretin, silymarin, hesperetin, and resveratrol. Mediators Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Pagliarulo, C.; Sansone, F.; Moccia, S.; Russo, G.L.; Aquino, R.P.; Salvatore, P.; Di Stasio, M.; Volpe, M.G. Preservation of Strawberries with an Antifungal Edible Coating Using Peony Extracts in Chitosan. Food Bioprocess Technol. 2016. [Google Scholar] [CrossRef]
- Lovelace, E.S.; Maurice, N.J.; Miller, H.W.; Slichter, C.K.; Harrington, R.; Magaret, A.; Prlic, M.; De Rosa, S.; Polyak, S.J. Silymarin suppresses basal and stimulusinduced activation, exhaustion, differentiation, and inflammatory markers in primary human immune cells. PLoS ONE 2017, 12, e0171139. [Google Scholar] [CrossRef] [PubMed]
- Sansone, F.; Picerno, P.; Mencherini, T.; Porta, A.; Lauro, M.R.; Russo, P.; Aquino, R.P. Technological properties and enhancement of antifungal activity of a Paeonia rockii extract encapsulated in a chitosan-based matrix. J. Food Eng. 2014, 120, 260–267. [Google Scholar] [CrossRef]
- Abraham, J. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. In Handbook of Transnational Economic Governance Regimes; Brill: Leiden, The Netherlands, 2009; pp. 1041–1054. [Google Scholar]
- Esposito, T.; Sansone, F.; Franceschelli, S.; Del Gaudio, P.; Picerno, P.; Aquino, R.; Mencherini, T. Hazelnut (Corylus avellana L.) Shells Extract: Phenolic Composition, Antioxidant Effect and Cytotoxic Activity on Human Cancer Cell Lines. Int. J. Mol. Sci. 2017, 18, 392. [Google Scholar] [CrossRef] [PubMed]
- Piccinelli, A.L.; Pagano, I.; Esposito, T.; Mencherini, T.; Porta, A.; Petrone, A.M.; Gazzerro, P.; Picerno, P.; Sansone, F.; Rastrelli, L.; et al. HRMS Profile of a Hazelnut Skin Proanthocyanidin-rich Fraction with Antioxidant and Anti-Candida albicans Activities. J. Agric. Food Chem. 2016, 64, 585–595. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds MTE_mp powders are available from the authors. |


| Samples | Yield % | a TEC % | b TSC % | c ASC % | d AEC % | e EE % | d50 μm (span) |
|---|---|---|---|---|---|---|---|
| NaCMC | - | - | - | - | - | - | 21.1 (1.1 ± 0.2) |
| MTE | - | - | - | 91.1 ± 2.3 * | - | - | 19.1 (2.5 ± 0.9) |
| Blank_mp | 74.9 ± 3.7 * | - | - | - | - | - | 3.9 (1.9 ± 0.4) |
| MTE_mp | 69.7 ± 4.1 * | 50 | 45.5 | 38.6 ± 0.6 * | 42.4 ± 0.6 * | 84.9 ± 0.6 * | 4.4 (2.7 ± 0.8) |
| Materials | Months | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 6 | |
| DPPH test EC50 µg/mL | ||||
| MTE raw extract | 25.2 ± 1.2 | 51.1 ± 1.3 | 70.2 ± 1.9 | 71.2 ± 2.9 |
| MTE_mp | 26.3± 0.9 | 25.1 ± 1.4 | 26.8 ± 1.0 | 27.2 ± 1.5 |
| α-tocopherol | 10.1 ± 1.3 | 10.2 ± 1.1 | 10.1 ± 1.1 | 10.3 ± 1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansone, F.; Esposito, T.; Lauro, M.R.; Picerno, P.; Mencherini, T.; Gasparri, F.; De Santis, S.; Chieppa, M.; Cirillo, C.; Aquino, R.P. Application of Spray Drying Particle Engineering to a High-Functionality/Low-Solubility Milk Thistle Extract: Powders Production and Characterization. Molecules 2018, 23, 1716. https://doi.org/10.3390/molecules23071716
Sansone F, Esposito T, Lauro MR, Picerno P, Mencherini T, Gasparri F, De Santis S, Chieppa M, Cirillo C, Aquino RP. Application of Spray Drying Particle Engineering to a High-Functionality/Low-Solubility Milk Thistle Extract: Powders Production and Characterization. Molecules. 2018; 23(7):1716. https://doi.org/10.3390/molecules23071716
Chicago/Turabian StyleSansone, Francesca, Tiziana Esposito, Maria Rosaria Lauro, Patrizia Picerno, Teresa Mencherini, Franco Gasparri, Stefania De Santis, Marcello Chieppa, Claudia Cirillo, and Rita Patrizia Aquino. 2018. "Application of Spray Drying Particle Engineering to a High-Functionality/Low-Solubility Milk Thistle Extract: Powders Production and Characterization" Molecules 23, no. 7: 1716. https://doi.org/10.3390/molecules23071716





