Design, Synthesis and Characterization of Novel Co-Polymers Decorated with Peptides for the Selective Nanoparticle Transport across the Cerebral Endothelium
Abstract
:1. Introduction
2. Results
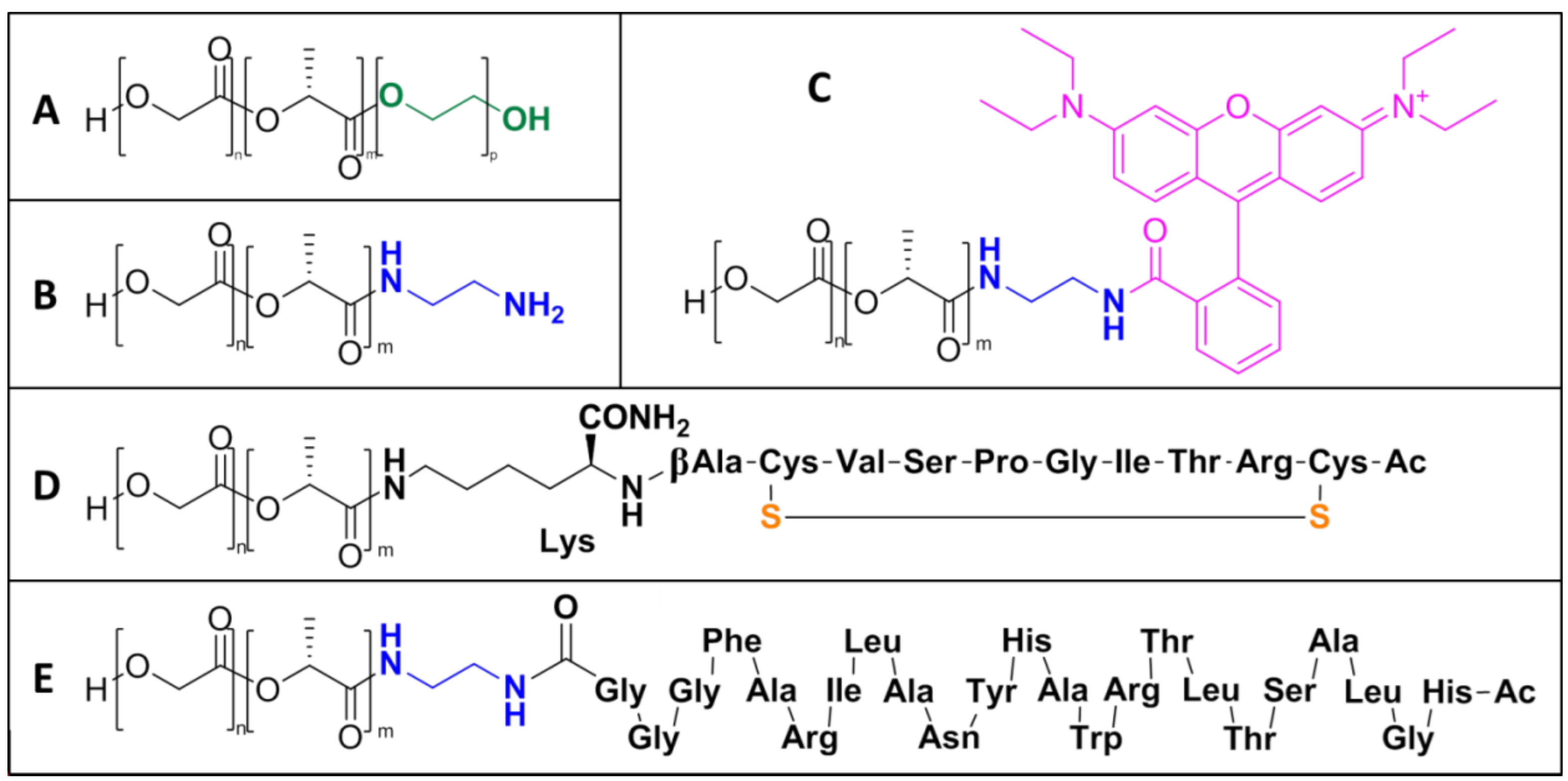
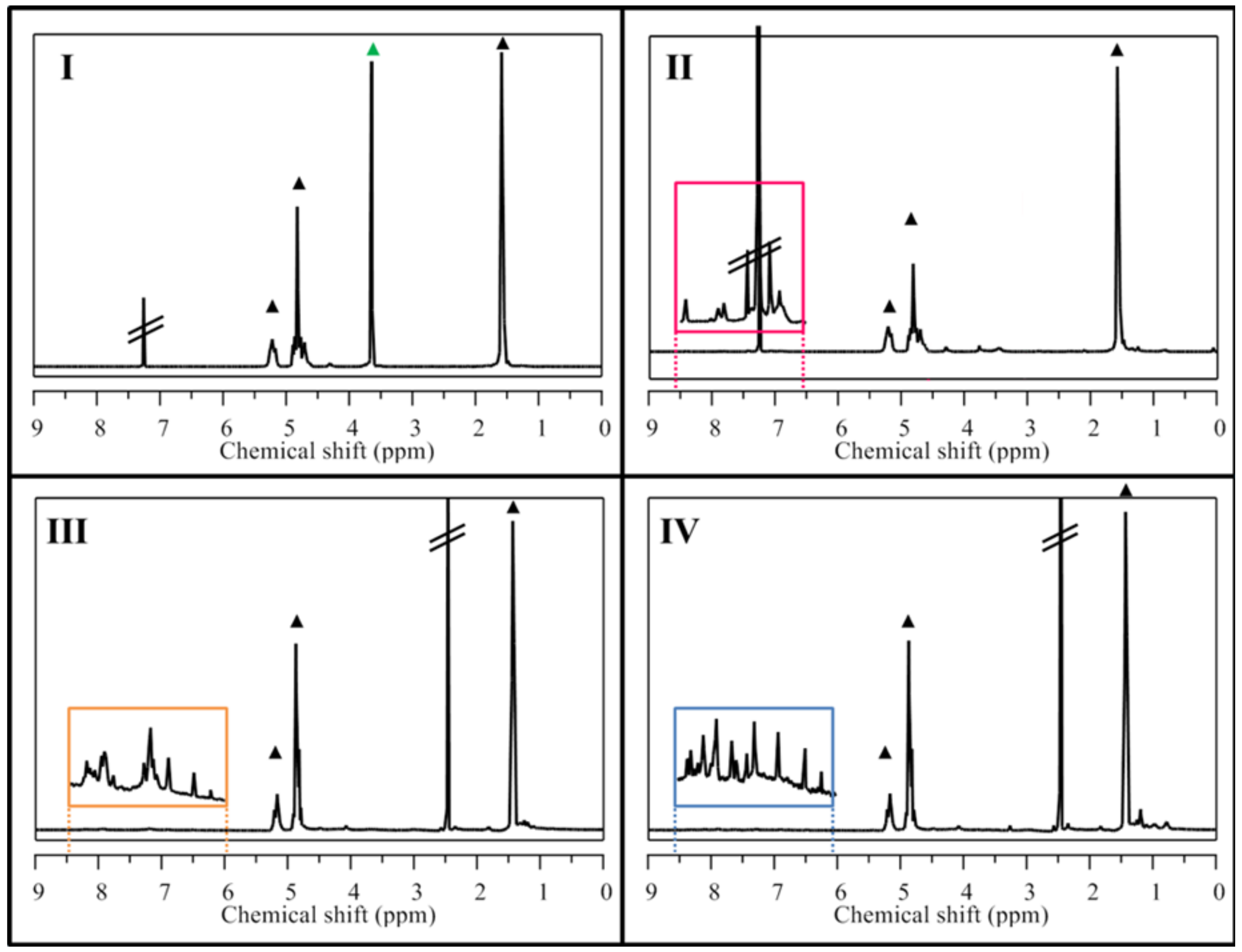
2.1. NP Building Blocks and NMR Analysis
2.2. NP Characterization
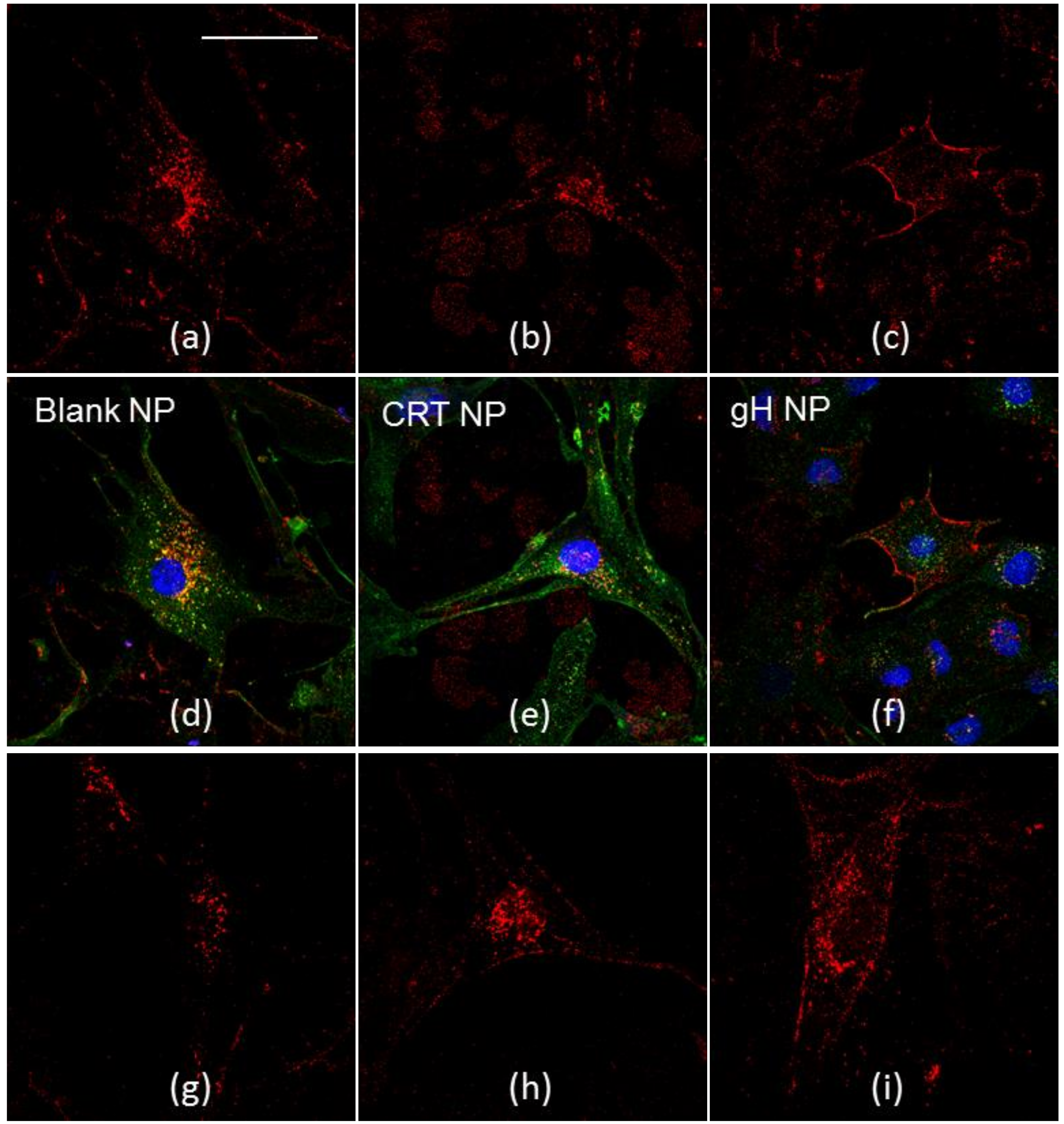
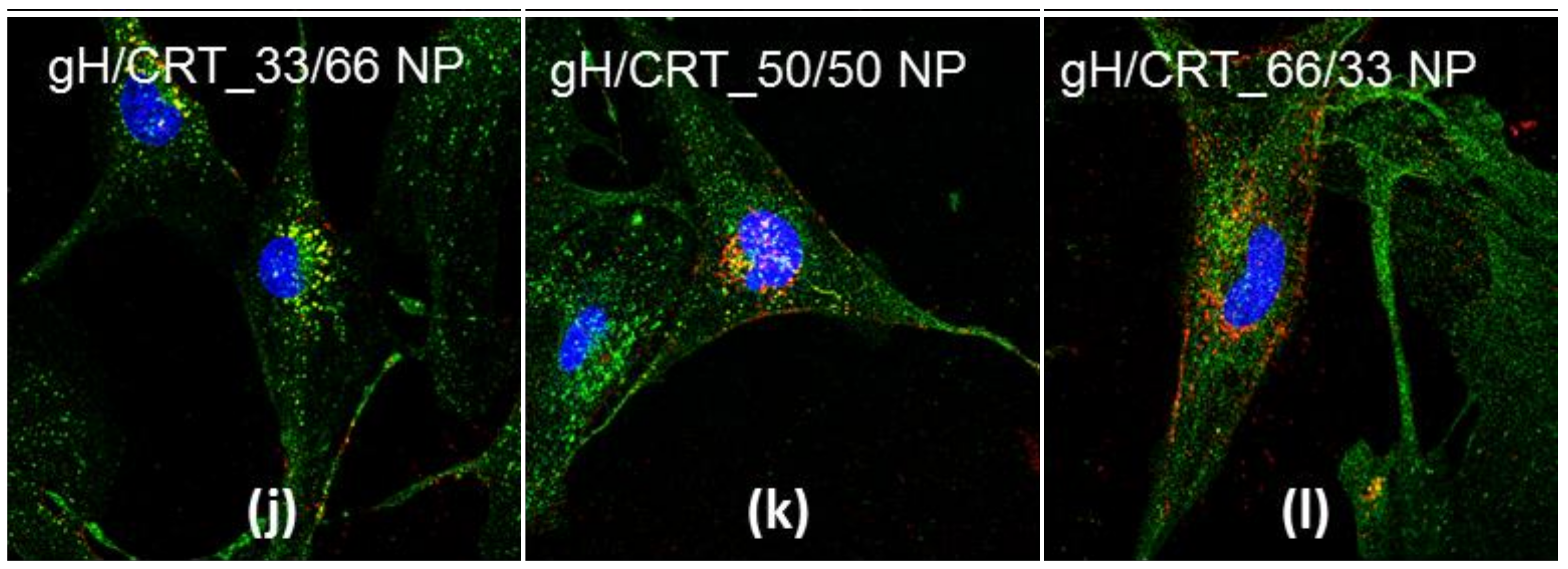
2.3. NP Intracellular Distribution
2.4. NP Transport across the BBB Endothelium
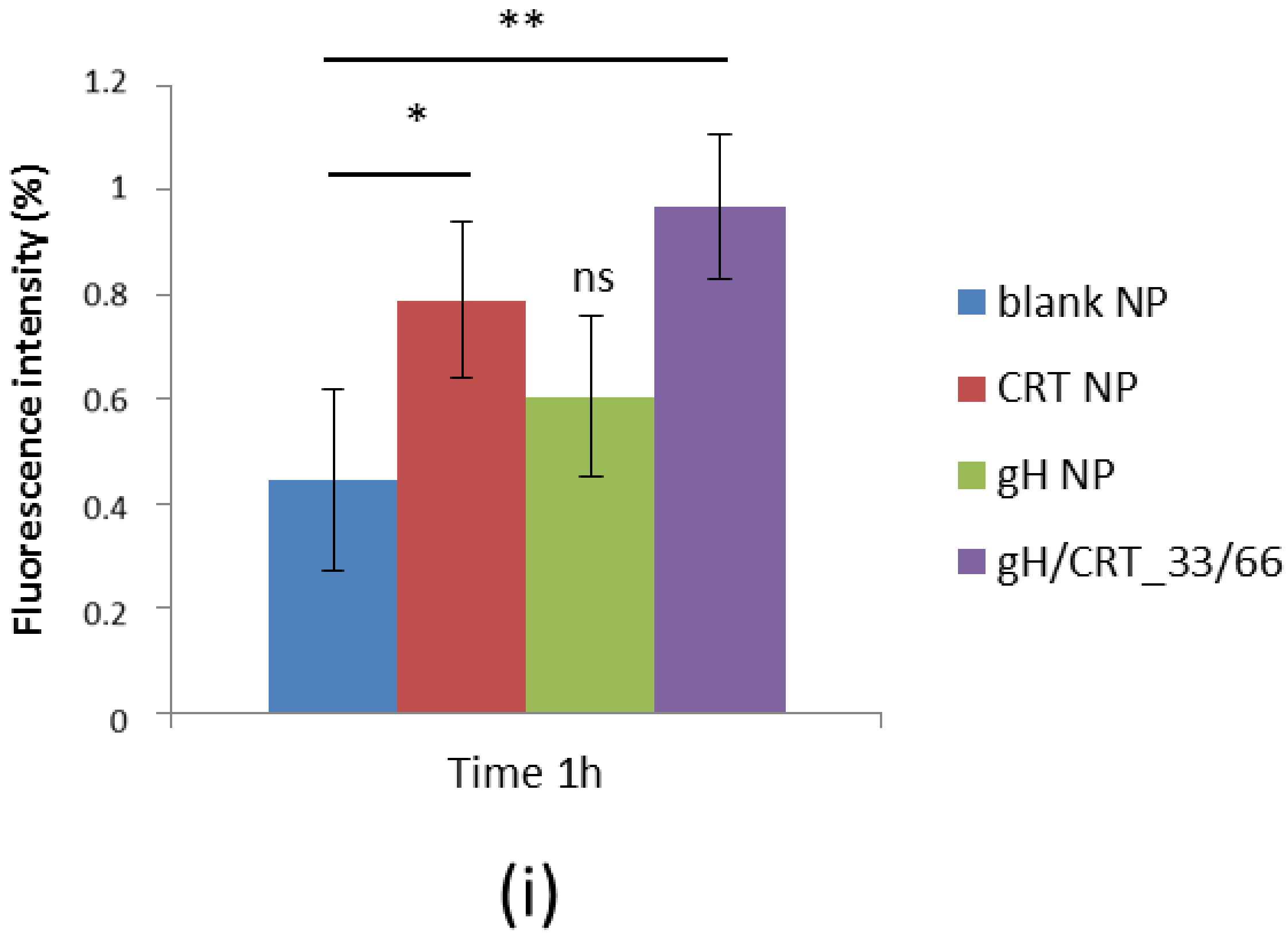
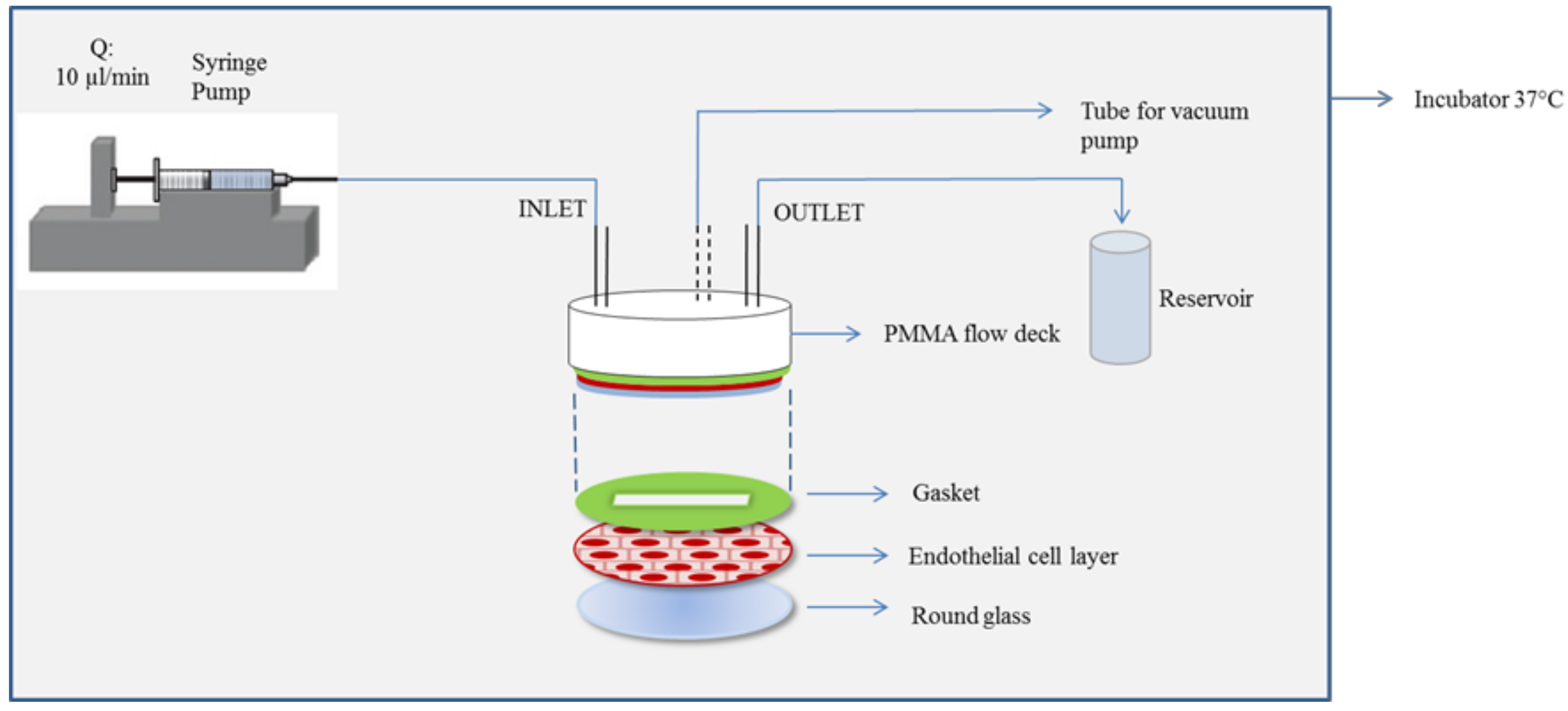
2.5. NP Adhesion to the BBB Endothelium in Flow Conditions
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Peptide Synthesis and Characterization
4.3. Peptide Cyclization
4.4. Synthesis of Co-Polymers and Conjugates
4.4.1. PELGA
4.4.2. PLGA-Amine
4.4.3. PLGA-Rhodamine
4.4.4. PLGA-Peptide Conjugates
4.5. Nuclear Magnetic Resonance (NMR) Analysis
4.6. NP Preparation
4.7. Cell Culture
4.8. Cellular Uptake and Co-Localizations
4.9. Permeability
4.10. Glycotech
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Poly(lactic-co-glycolic acid) | PLGA |
| Polyethylene glycol | PEG |
| Blood-brain barrier | BBB |
| Central nervous system | CNS |
| Receptor-mediated transcytosis | RMT |
| Transferrin | Tf |
| Transferrin receptor | TfR |
| Nanoparticle | NP |
| Trans Endothelial Electrical resistance | TEER |
| Ac-CRTIGPSVC-βAK-CONH2 | CRT |
| Ac-HGLASTLTRWAHYNALIRAFGGG-COOH | gH |
References
- Farokhzad, O.; Langer, R. Nanomedicine: Developing smarter therapeutic and diagnostic modalities. Adv. Drug Deliv. Rev. 2006, 58, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Veszelka, S.; Bocsik, A.; Walter, F.R.; Hantosi, D.; Deli, M.A. Blood-brain- barrier co-culture models to study nanoparticle penetration: Focus on co-culture systems. Acta Biol. Szeged. Rev. 2015, 59, 1–12. [Google Scholar]
- Mc Carthy, D.J.; Malhotra, M.; O’Mahony, A.M.; Cryan, J.F.; O’Driscoll, C.M. Nanoparticles and the blood-brain barrier: Advancing from in-vitro models towards therapeutic significance. Pharm. Res. 2015, 32, 1161–1185. [Google Scholar] [CrossRef] [PubMed]
- Iversen, T.G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Vitiello, M.; Falanga, A.; Cantisani, M.; Incoronato, N.; Galdiero, M. Intracellular delivery: Exploiting viral membranotropic peptides. Curr. Drug Metab. 2012, 13, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Falanga, A.; Vitiello, M.; D’Isanto, M.; Collins, C.; Orrei, V.; Browne, H.; Pedone, C.; Galdiero, M. Evidence for a role of the membrane-proximal region of herpes simplex virus type 1 glycoprotein H in membrane fusion and virus inhibition. ChemBioChem 2007, 8, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, S.; Vitiello, M.; D’Isanto, M.; Falanga, A.; Cantisani, M.; Browne, H.; Pedone, C.; Galdiero, M. The identification and characterization of fusogenic domains in herpes virus glycoprotein B molecules. ChemBioChem 2008, 9, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Galdiero, M.; Galdiero, S. Membranotropic Cell Penetrating Peptides: The Outstanding Journey. Int. J. Mol. Sci. 2015, 16, 25323–25337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galdiero, S.; Falanga, A.; Vitiello, M.; D’Isanto, M.; Cantisani, M.; Kampanaraki, A.; Benedetti, E.; Browne, H.; Galdiero, M. Peptides containing membrane-interacting motifs inhibit herpes simplex virus type 1 infectivity. Peptides 2008, 29, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, D.; Muscetti, O.; Falanga, A.; Fusco, S.; Belli, V.; Perillo, E.; Battista, E.; Panzetta, V.; Galdiero, S.; Netti, P.A. Surface decoration with gH625-membranotropic peptides as a method to escape the endo-lysosomal compartment and reduce nanoparticle toxicity. Nanotechnology 2015, 26, 415101. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, D.; Falanga, A.; Muscetti, O.; Tarallo, R.; Fusco, S.; Galdiero, M.; Galdiero, S.; Netti, P.A. Shuttle-Mediated Nanoparticle Delivery to the Blood-Brain Barrier. Small 2013, 9, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.P.; Pitingolo, G.; Celentano, M.; Cosentino, A.; Melone, P.; Vecchione, R.; Guarnieri, D.; Netti, P.A.; Federico, N. Shuttle-Mediated Nanoparticle Transport Across an In Vitro Brain Endothelium Under Flow Conditions. Biotechnol. Bioeng. 2016, 9999, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, D.; Melone, P.; Moglianetti, M.; Marotta, R.; Netti, P.A.; Pompa, P.P. Particle size affects cytosolic delivery of membranotropic peptide-functionalized platinum nanozymes. Nanoscale 2017, 11288–11296. [Google Scholar] [CrossRef] [PubMed]
- Fotticchia, T.; Vecchione, R.; Scognamiglio, P.L.; Guarnieri, D.; Calcagno, V.; Di Natale, C.; Attanasio, C.; De Gregorio, M.; Di Cicco, C.; Quagliariello, V.; et al. Enhanced Drug Delivery into Cell Cytosol via Glycoprotein H-Derived Peptide Conjugated Nanoemulsions. ACS Nano 2017, 11, 9802–9813. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R.; Shusta, E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007, 24, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Van Rooy, I.; Mastrobattista, E.; Storm, G.; Hennink, W.E.; Schiffelers, R.M. Comparison of five different targeting ligands to enhance accumulation of liposomes into the brain. J. Control. Release 2011, 150, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.L.; Wu, X.Y.; Bendayan, R. Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Grossniklaus, H.E.; Kang, S.J.; Edelhauser, H.F.; Ambati, B.K.; Kompella, U.B. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009, 16, 645–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, Y.; Chen, N.; Yu, H.; Mu, H.; He, B.; Hua, H.; Wang, A.; Sun, K. Topical ocular delivery to laser-induced choroidal neovascularization by dual internalizing RGD and TAT peptide-modified nanoparticles. Int. J. Nanomed. 2017, 12, 1353–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Jothar, L.; Doulkeridou, S.; Schiffelers, R.M.; Sastre Torano, J.; Oliveira, S.; van Nostrum, C.F.; Hennink, W.E. Insights into maleimide-thiol conjugation chemistry: Conditions for efficient surface functionalization of nanoparticles for receptor targeting. J. Control. Release 2018. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.M.; Shusta, E.V. Targeting Receptor-Mediated Transport for Delivery of Biologics Across the Blood-Brain Barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Béduneau, A.; Saulnier, P.; Benoit, J.P. Active targeting of brain tumors using nanocarriers. Biomaterials 2007, 28, 4947–4967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huile, G.; Shuaiqi, P.; Zhi, Y.; Shijie, C.; Chen, C.; Xinguo, J.; Shun, S.; Zhiqing, P.; Yu, H. A cascade targeting strategy for brain neuroglial cells employing nanoparticles modified with angiopep-2 peptide and EGFP-EGF1 protein. Biomaterials 2011, 32, 8669–8675. [Google Scholar] [CrossRef] [PubMed]
- Staquicini, F.I.; Ozawa, M.G.; Moya, C.A.; Driessen, W.H.P.; Barbu, E.M.; Nishimori, H.; Soghomonyan, S.; Flores, L.G.; Liang, X.; Paolillo, V.; et al. Systemic combinatorial peptide selection yields a non-canonical iron-mimicry mechanism for targeting tumors in a mouse model of human glioblastoma. J. Clin. Invest. 2011, 121, 161–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fotticchia, I.; Guarnieri, D.; Fotticchia, T.; Falanga, A.P.; Vecchione, R.; Giancola, C.; Netti, P.A. Energetics of ligand-receptor binding affinity on endothelial cells: An in vitro model. Colloids Surfaces B Biointerfaces 2016, 144, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ponka, P.; Lok, C.N. The transferrin receptor: Role in health and disease. Int. J. Biochem. Cell Biol. 1999, 31, 1111–1137. [Google Scholar] [CrossRef]
- Kang, T.; Jiang, M.; Jiang, D.; Feng, X.; Yao, J.; Song, Q.; Chen, H.; Gao, X.; Chen, J. Enhancing Glioblastoma-Specific Penetration by Functionalization of Nanoparticles with an Iron-Mimic Peptide Targeting Transferrin/Transferrin Receptor Complex. Mol. Pharm. 2015, 12, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Q.; Shao, X.; Qian, Y.; Zhang, Q. Phage-displayed peptide-conjugated biodegradable nanoparticles enhanced brain drug delivery. Mater. Lett. 2016, 167, 213–217. [Google Scholar] [CrossRef]
- Lee, S.U.J.; Han, B.O.R.; Park, S.Y.; Han, D.K.; Kim, S.C. Sol-gel transition behavior of biodegradable three-arm and four-arm star-shaped PLGA-PEG block copolymer aqueous solution. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 888–899. [Google Scholar] [CrossRef]
- Cantisani, M.; Guarnieri, D.; Biondi, M.; Belli, V.; Profeta, M.; Raiola, L.; Netti, P.A. Biocompatible nanoparticles sensing the matrix metallo-proteinase 2 for the on-demand release of anticancer drugs in 3D tumor spheroids. Colloids Surfaces B Biointerfaces 2015, 135, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Wehrstedt, K.D.; Wandrey, P.A.; Heitkamp, D. Explosive properties of 1-hydroxybenzotriazoles. J. Hazard. Mater. 2005, 126, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Subirós-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion. Chem. A Eur. J. 2009, 15, 9394–9403. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Morris, A.P.; O’Neil, R.G. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007, 1130, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omidi, Y.; Campbell, L.; Barar, J.; Connell, D.; Akhtar, S.; Gumbleton, M. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake and transport studies. Brain Res. 2003, 990, 95–112. [Google Scholar] [CrossRef]
- Booth, R.; Kim, H. Characterization of a microfluidic in vitro model of the blood-brain barrier (μBBB). Lab Chip 2012, 12, 1784. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Ferrari, M. The role of specific and non-specific interactions in receptor-mediated endocytosis of nanoparticles. Biomaterials 2007, 28, 2915–2922. [Google Scholar] [CrossRef] [PubMed]
- van Rooy, I.; Cakir-Tascioglu, S.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Storm, G.; Hennink, W.E.; Schiffelers, R.M.; Mastrobattista, E. Identification of Peptide Ligands for Targeting to the Blood-Brain Barrier. Pharm. Res. 2010, 27, 673–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biondi, M.; Guarnieri, D.; Yu, H.; Belli, V.; Netti, P.A. Sub-100 nm biodegradable nanoparticles: In vitro release features and toxicity testing in 2D and 3D cell cultures. Nanotechnology 2013, 24, 045101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ma, C.; Bai, E.; Yang, K.; Xu, R. Liposome for Targeted Drug Delivery To Glioma. Int. J. Clin. Exp. Med. 2015, 8, 1658–1668. [Google Scholar] [PubMed]
- Salvati, E.; Re, F.; Sesana, S.; Cambianica, I.; Sancini, G.; Masserini, M.; Gregori, M. Liposomes functionalized to overcome the blood-brain barrier and to target amyloid-β peptide: The chemical design affects the permeability across an in vitro model. Int. J. Nanomed. 2013, 8, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Venkatraman, S.S.; Yang, Y.-Y.; Guo, K.; Lu, J.; He, B.; Moochhala, S.; Kan, L. Polymeric micelles anchored with TAT for delivery of antibiotics across the blood-brain barrier. Biopolymers 2008, 90, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Vivès, E.; Richard, J.-P.; Rispal, C.; Lebleu, B. TAT peptide internalization: seeking the mechanism of entry. Curr. Protein Pept. Sci. 2003, 4, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.P.; Melikov, K.; Brooks, H.; Prevot, P.; Lebleu, B.; Chernomordik, L.V. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J. Biol. Chem. 2005, 280, 15300–15306. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv. Drug Deliv. Rev. 2008, 60, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xiong, C.; Zhang, R.; Shi, L.; Huang, M.; Zhang, G.; Song, S.; Huang, Q.; Liu, G.Y.; Li, C. Receptor-mediated transcytosis: A mechanism for active extravascular transport of nanoparticles in solid tumors. J. Control. Release 2012, 161, 959–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Yang, Z.; Zhang, S.; Cao, S.; Pang, Z.; Yang, X.; Jiang, X. Glioma-homing peptide with a cell-penetrating effect for targeting delivery with enhanced glioma localization, penetration and suppression of glioma growth. J. Control. Release 2013, 172, 921–928. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: All NP preparations are available from the authors. |


| NP | HD (nm) ± SD | PDI | Z-Potential (mV) ± SD |
|---|---|---|---|
| Blank | 88.24 ± 1.62 | 0.15 | −27.40 ± 0.66 |
| CRT | 92.06 ± 1.53 | 0.15 | −26.10 ± 1.17 |
| gH | 84.53 ± 0.60 | 0.14 | −13.83 ± 0.11 |
| gH/CRT_66/33 | 80 ± 1 | 0.16 | −22.4 ± 0.5 |
| gH/CRT_50/50 | 78 ± 2 | 0.14 | −23.8 ± 0.9 |
| gH/CRT_33/66 | 76 ± 1 | 0.13 | −20.0 ± 0.5 |
| NP | PELGA (mg) | PLGA-Rho (mg) | PLGA-gH (mg) | PLGA-CRT (mg) | PLGA (mg) | mg tot |
|---|---|---|---|---|---|---|
| Blank | 1 | 0.4 | - | - | 0.8 | 2.2 |
| gH | 1 | 0.4 | 0.4 | - | 0.4 | 2.2 |
| CRT | 1 | 0.4 | - | 0.4 | 0.4 | 2.2 |
| gH/CRT_66/33 | 1 | 0.4 | 0.4 | 0.2 | 0.2 | 2.2 |
| gH/CRT_50/50 | 1 | 0.4 | 0.4 | 0.4 | - | 2.2 |
| gH/CRT_33/66 | 1 | 0.4 | 0.2 | 0.4 | 0.2 | 2.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falanga, A.P.; Melone, P.; Cagliani, R.; Borbone, N.; D’Errico, S.; Piccialli, G.; Netti, P.A.; Guarnieri, D. Design, Synthesis and Characterization of Novel Co-Polymers Decorated with Peptides for the Selective Nanoparticle Transport across the Cerebral Endothelium. Molecules 2018, 23, 1655. https://doi.org/10.3390/molecules23071655
Falanga AP, Melone P, Cagliani R, Borbone N, D’Errico S, Piccialli G, Netti PA, Guarnieri D. Design, Synthesis and Characterization of Novel Co-Polymers Decorated with Peptides for the Selective Nanoparticle Transport across the Cerebral Endothelium. Molecules. 2018; 23(7):1655. https://doi.org/10.3390/molecules23071655
Chicago/Turabian StyleFalanga, Andrea P., Pietro Melone, Roberta Cagliani, Nicola Borbone, Stefano D’Errico, Gennaro Piccialli, Paolo A. Netti, and Daniela Guarnieri. 2018. "Design, Synthesis and Characterization of Novel Co-Polymers Decorated with Peptides for the Selective Nanoparticle Transport across the Cerebral Endothelium" Molecules 23, no. 7: 1655. https://doi.org/10.3390/molecules23071655





