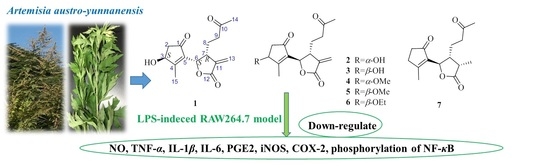
1,10-Secoguaianolides from Artemisia austro-yunnanensis and Their Anti-Inflammatory Effects
Abstract
:1. Introduction
2. Results and Discussion
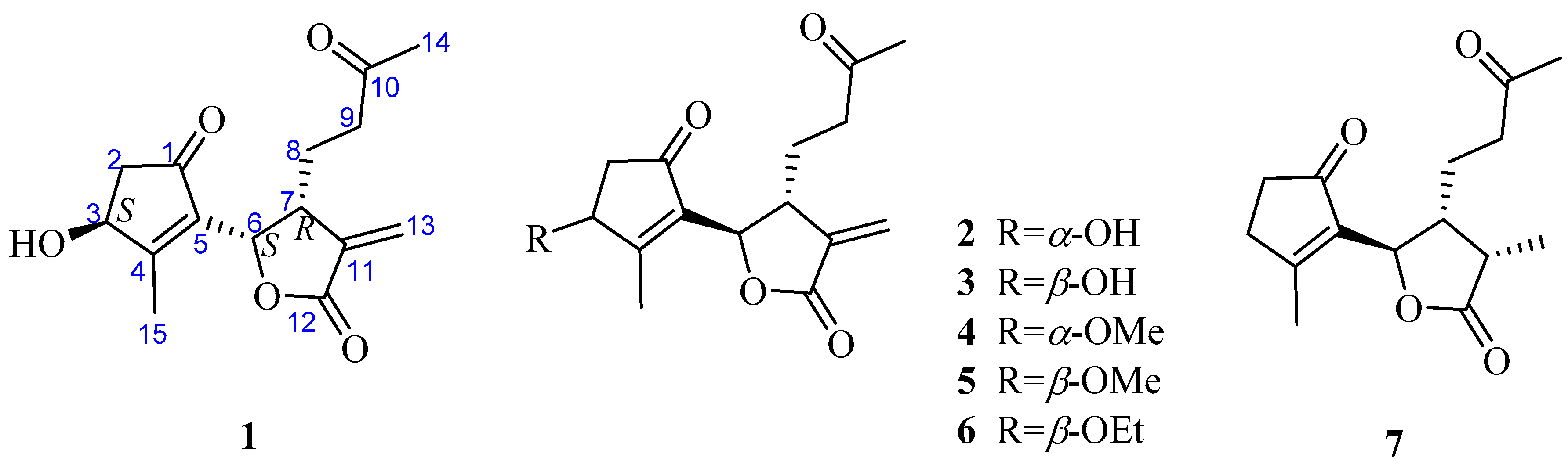
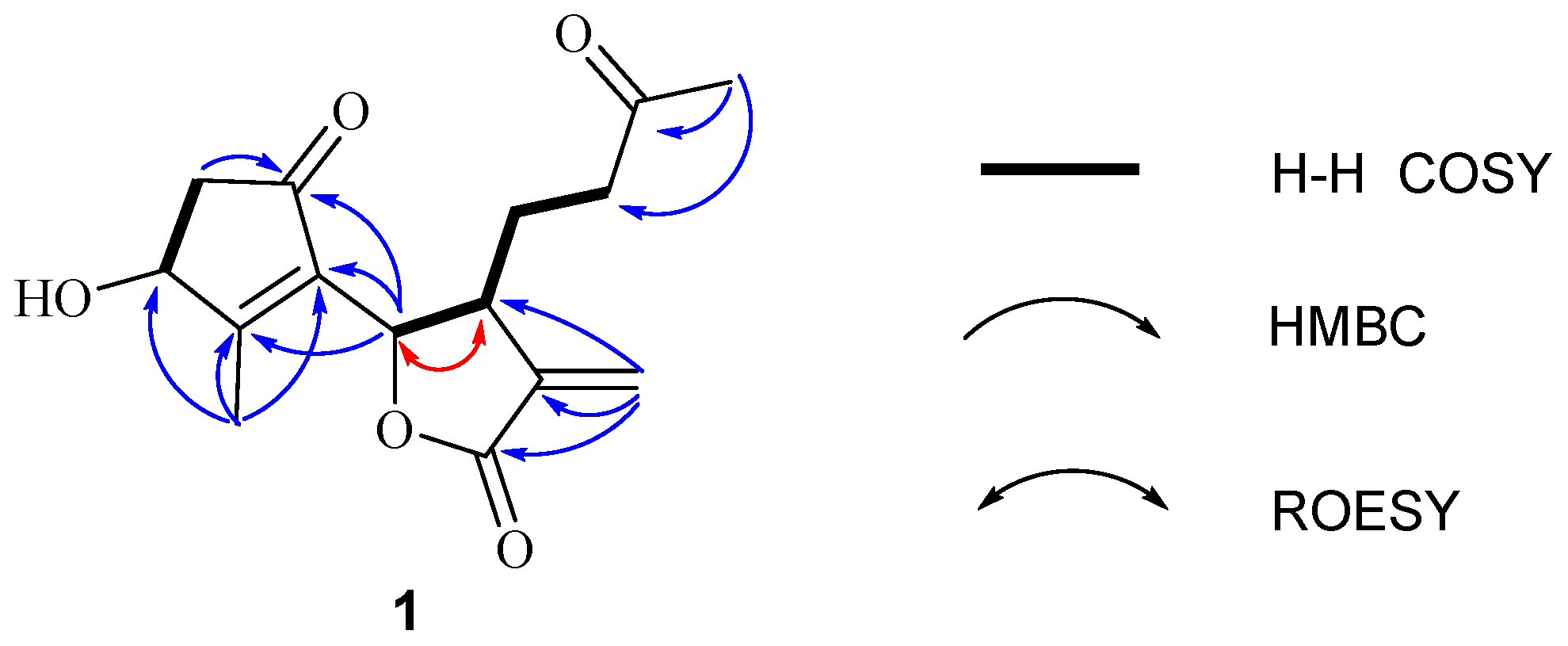
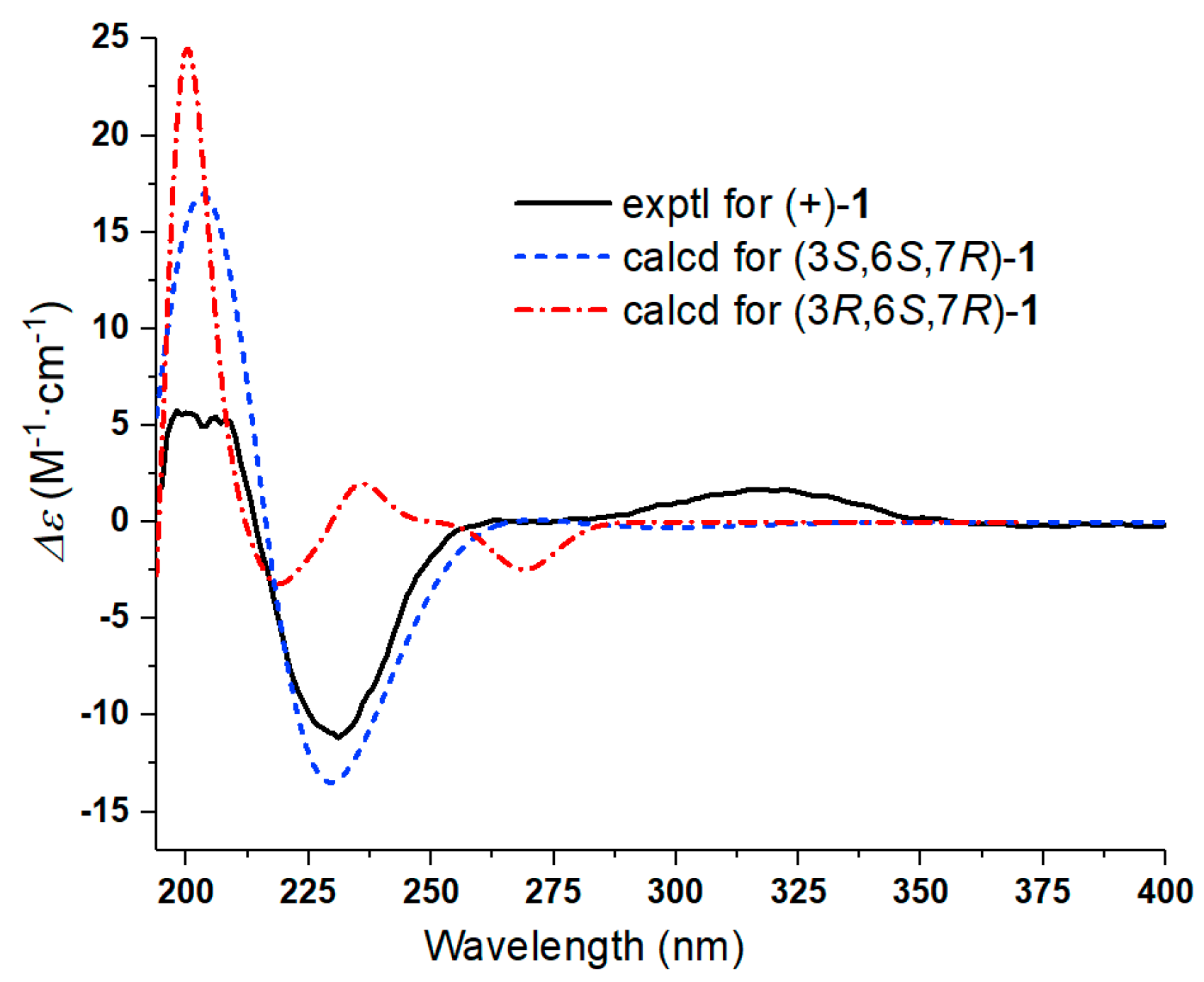
2.1. Structural Identification of Compounds
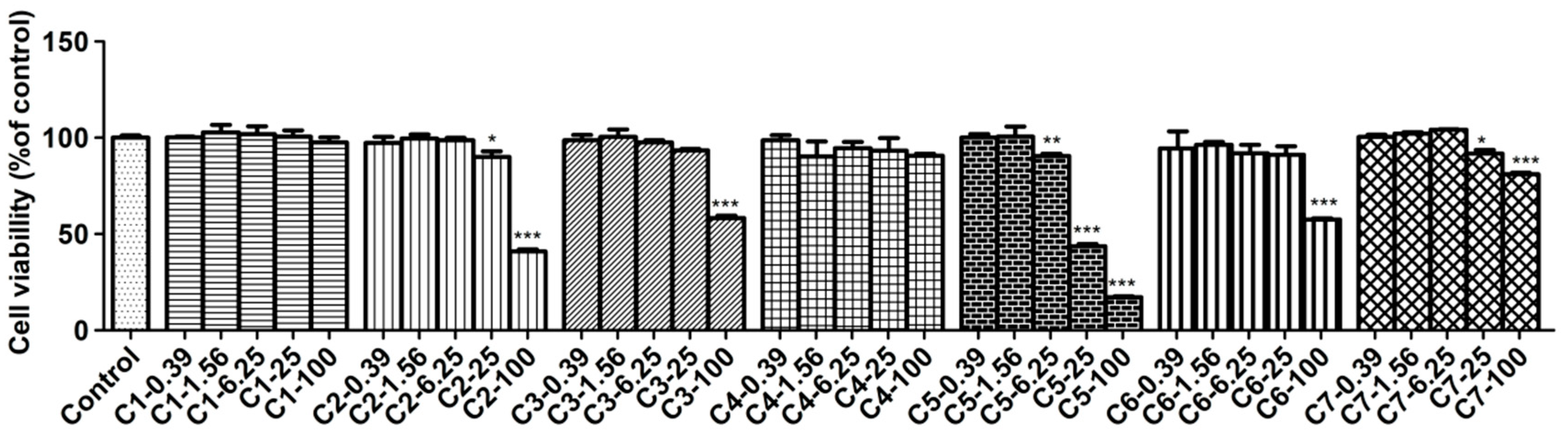
2.2. Cytotoxicity Assay by MTT Method
2.3. Effects of Compounds on Inhibition against Nitric Oxide (NO) Production
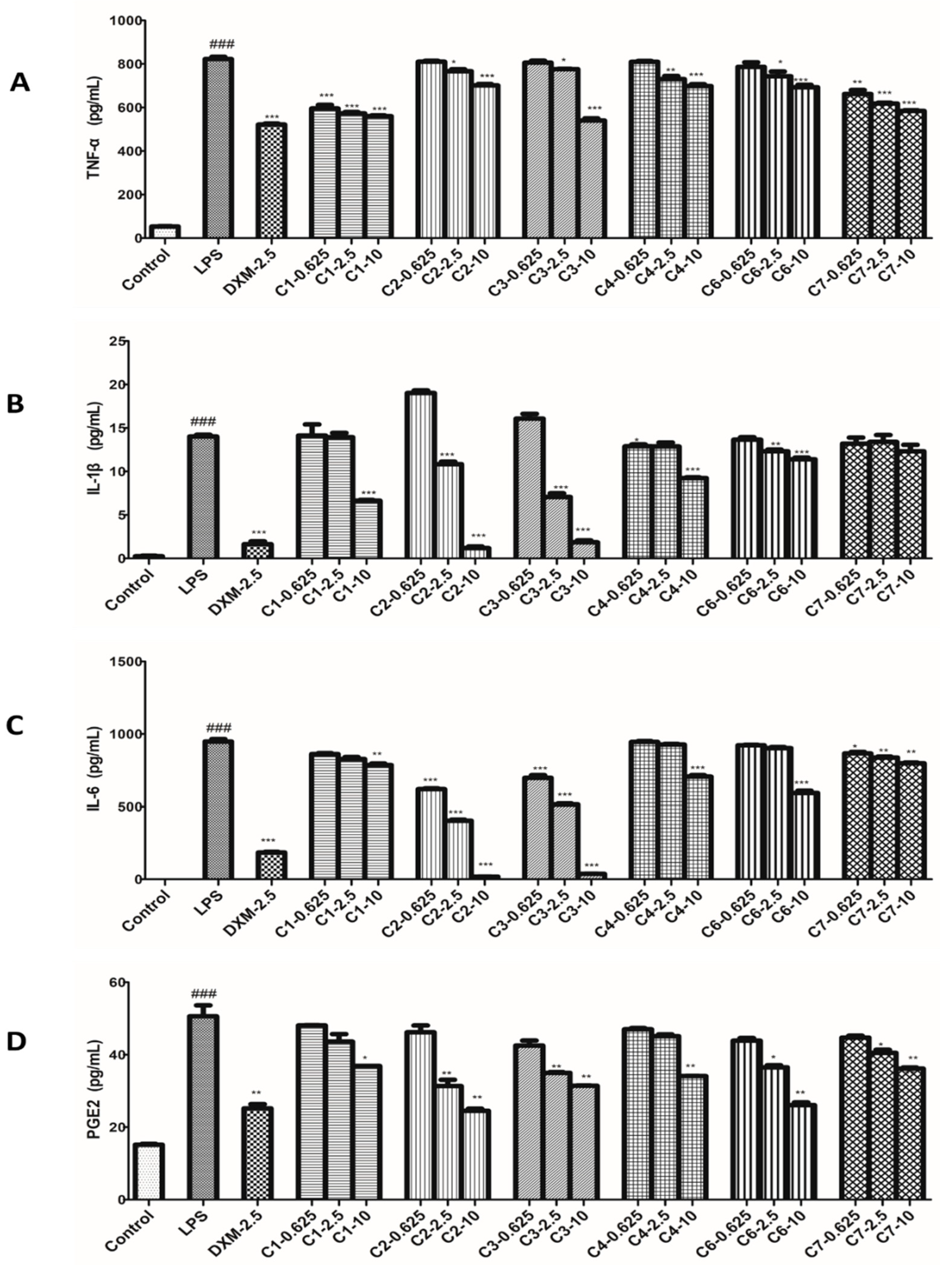
2.4. Effects of Compounds on Levels of TNF-α, IL-1β, IL-6 and PGE2 in RAW264.7 Cells
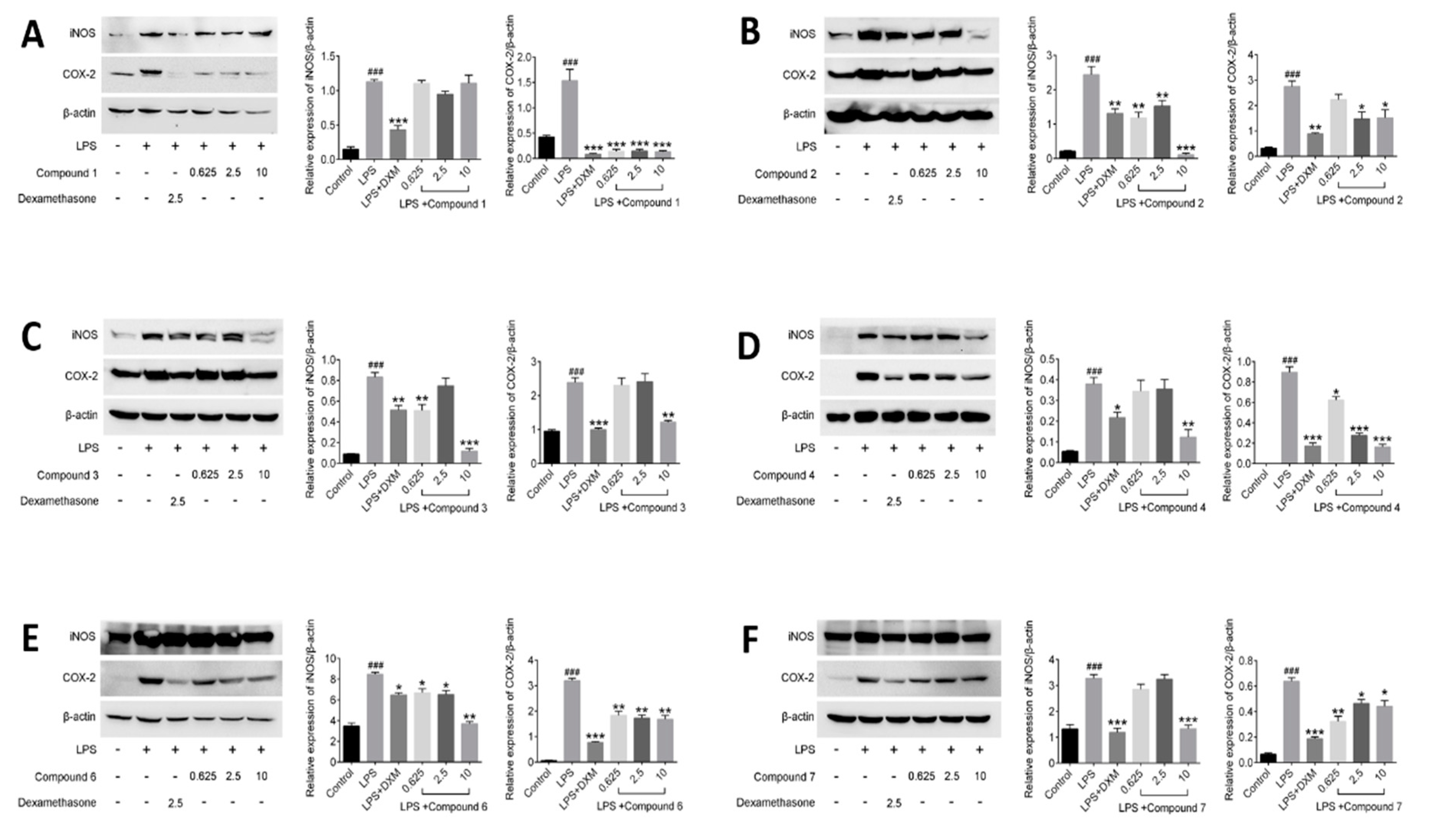
2.5. Effects of Compounds on LPS-Induced iNOS and COX-2 Proteins in RAW264.7 Cells
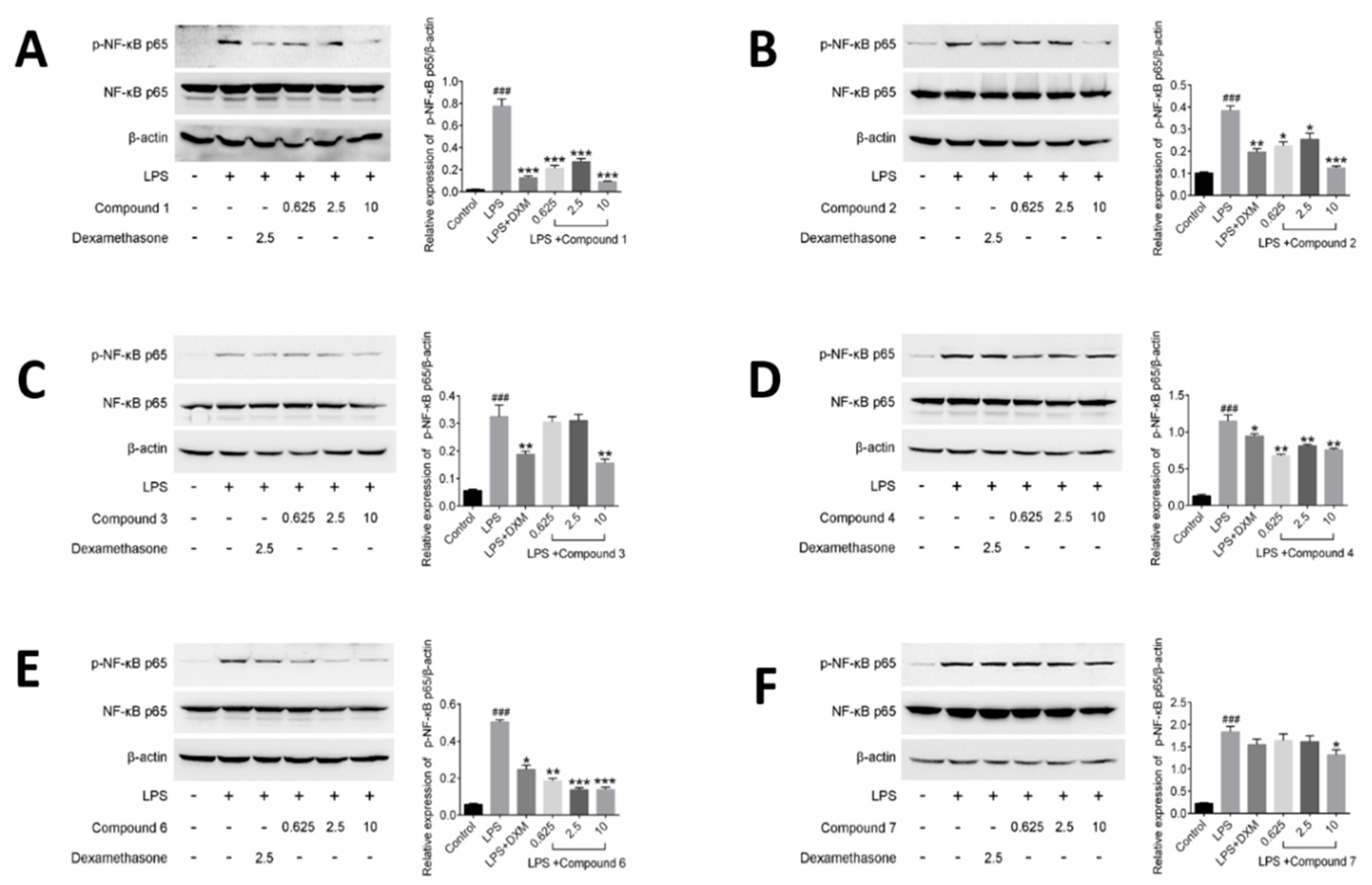
2.6. Effects of Compounds on Regulating NF-κB Activation in LPS-induced RAW264.7 Cells
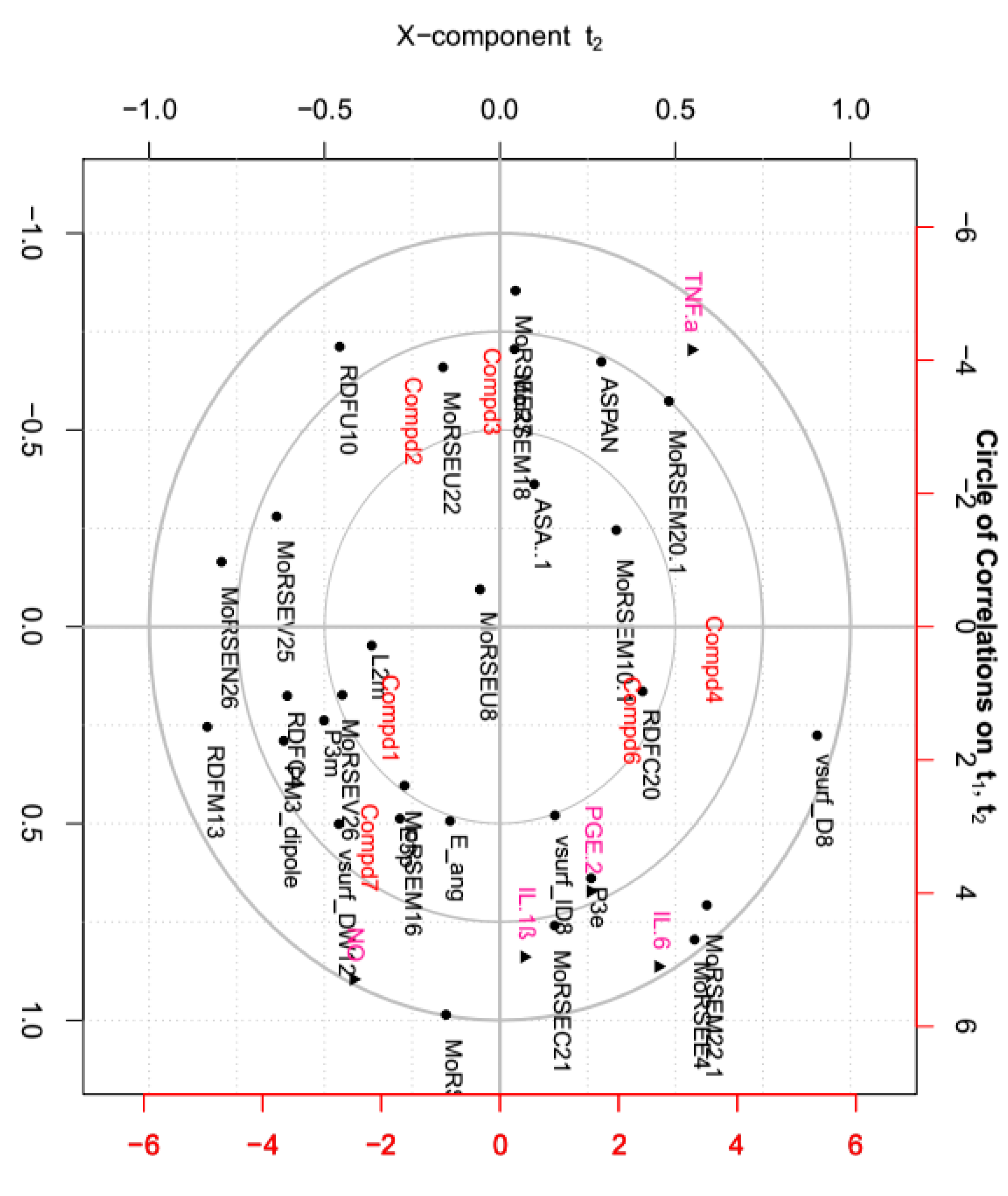
2.7. Correlations between Tested Inflammatory Factors and Calculated Molecular Descriptors of Compounds 1–4, 6 and 7
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization of New Compound 1
3.5. Samples and Reagents
3.6. Cell Culture
3.7. Cell Viability by the MTT Assay
3.8. Assay for NO Production
3.9. Assay for TNF-α, IL-1β, IL-6 and PGE2 Levels
3.10. Western Blot Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HRESIMS | high resolution electrospray mass spectroscopy |
| NMR | nuclear magnetic resonance spectrometer |
| 1H-1H COSY | 1H-1H chemical-shift correlation spectroscopy |
| HSQC | heteronuclear single quantum coherence |
| HMBC | heteronuclear multiple-bond correlation |
| ROESY | rotating-frame overhauser effect spectroscopy |
| ECD | electronic circular dichroism |
| LPS | lipopolysaccharide |
| l-NMMA | l-NG-monomethyl arginine citrate |
| DXM | dexamethasone |
| NO | nitric oxide |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| TNF-α | tumour necrosis factor-α |
| iNOS | inducible NO synthase |
| COX-2 | cyclooxygenase-2 |
| NF-κB | nuclear factor-κB |
| PGE2 | prostaglandin E2 |
| ELISA | enzyme-linked immunosorbent assay |
| WB | western blot |
| SDS-PAGE | sodium dodecyl sulfate-polyacylamide gel electrophoresis |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide |
| DMSO | dimethyl sulfoxide |
References
- Yan, L.; Feng, Z.; Hao, H.; Peng, K.F.; Zhou, X.H.; Chen, L.X.; Feng, Q. Guaiane-type sesquiterpenes from Curcuma wenyujin and their inhibitory effects on nitric oxide production. J. Nat. Prod. 2009, 11, 737–747. [Google Scholar]
- Wender, P.A.; Mucciaro, T.P. ChemInform abstract: A new and practical approach to the synthesis of taxol and taxol analogues. J. Am. Chem. Soc. 1992, 23, 5878–5879. [Google Scholar] [CrossRef]
- Jiang, H.L.; Chen, J.; Jin, X.J.; Yang, J.L.; Li, Y.; Yao, X.J.; Wu, Q.X. Sesquiterpenoids, alantolactone analogues, and -guaiene from the roots of Inula helenium. Tetrahedron 2011, 67, 9193–9198. [Google Scholar] [CrossRef]
- Hurst, J.J.; Whitham, G.H. The photochemistry of verbenone. J. Chem. Soc. 1960, 579, 2864–2869. [Google Scholar] [CrossRef]
- Chidambaram, N.; Chandrasekaran, S. Tert-Butyl hydroperoxide-pyridinium dichromate: A convenient reagent system for allylic and benzylic oxidations. J. Org. Chem. 1987, 52, 5048–5051. [Google Scholar] [CrossRef]
- Zidorn, C.; Ellmerermuller, E.P.; Stuppner, H. Eudesmanolides and inositol derivatives from Taraxacum linearisquameum. Phytochemistry 1999, 51, 991–994. [Google Scholar] [CrossRef]
- Tomanová, P.; Rimpelová, S.; Jurášek, M.; Buděšínský, M.; Vejvodová, L.; Ruml, T.; Kmoníčková, E.; Drašar, P.B. Trilobolide-porphyrin conjugates: On synthesis and biological effects evaluation. Steroids 2015, 97, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.Z.; Lee, J.H.; Lee, D.; Hong, Y.S.; Kim, Y.H.; Lee, J.J. Inhibitors of the LPS-induced NF-kappaB activation from Artemisia sylvatica. Phytochemistry 2004, 65, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Wendel, G.H.; Maria, A.O.; Mohamed, F.; Dominguez, S.; Scardapane, L.; Giordano, O.S.; Guerreiro, E.; Guzman, J.A. Effect of dehydroleucodine in experimental colitis in rats and mice. Pharmacol. Res. 1999, 40, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Giordano, O.S.; Guerreiro, E.; Pestchanker, M.J.; Guzman, J.; Pastor, D.; Guardia, T. The gastric cytoprotective effect of several sesquiterpene lactones. J. Nat. Prod. 1990, 53, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Saleh-E-In, M.M.; Staden, J.V. Ethnobotany, phytochemistry and pharmacology of Arctotis arctotoides (L.f.) O. Hoffm.: A review. J. Ethnopharmacol. 2018, 220, 294–320. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Li, B.C.; Zhang, M. Chemical constituents from Artemisia Austro-yunnanensis. J. Kunming Univ. Sci. Tech. (Nat. Sci. Ed.) 2015, 40, 93–96. [Google Scholar]
- Chi, J.; Li, B.C.; Dai, W.F.; Liu, L.; Zhang, M. Highly oxidized sesquiterpenes from Artemisia austro-yunnanensis. Fitoterapia 2016, 115, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Li, B.C.; Yang, B.T.; Zhang, M. Chemical components and their bioactivities of Artemisia austro-yunnanensis. J. Chem. Soc. Park. 2016, 38, 533–537. [Google Scholar]
- Makiyi, E.F.; Frade, R.F.M.; Lebl, T.; Jaffray, E.G.; Cobb, S.E.; Harvey, A.L.; Slawin, A.M.Z.; Hay, R.T.; Westwood, N.J. Iso-seco-tanapartholides: Isolation, synthesis and biological evaluation. Eur. J. Org. Chem. 2009, 2009, 5711–5715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olagnier, D.; Peri, S.; Steel, C.; Montfoort, N.V.; Chiang, C.; Beljanski, V.; Slifker, M.; He, Z.; Nichols, C.N. Cellular oxidative stress response controls the antiviral and apoptotic programs in dengue virus-infected dendritic cells. PLoS Pathog. 2014, 10, e1004566. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Cao, X.J.; Wei, W. Melatonin decreases TLR3-mediated inflammatory factor expression via inhibition of NF-kappa B activation in respiratory syncytial virus-infected RAW264.7 macrophages. J. Pineal. Res. 2008, 45, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Berova, N.; Polavarapu, P.L.; Nakanishi, K.; Woody, R.W. Comprehensive Chiroptical Spectroscopy: Instrumentation, Methodologies, and Theoretical Simulations; John Wiley & Sons Inc.: Hoboken, NL, USA, 2012; pp. 65–90. [Google Scholar]
- Stephens, P.J.; Pan, J.J.; Devlin, F.J.; Urbanová, M.; Hájícek, J. Determination of the absolute configurations of natural products via density functional theory calculations of vibrational circular dichroism, electronic circular dichroism and optical rotation: The schizozygane alkaloid schizozygine. J. Org. Chem. 2007, 72, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Louzao, I.; Seco, J.M.; Quiñoá, E.; Riguera, R. The use of a single derivative in the configurational assignment of ketone cyanohydrins. Chem. Commun. 2010, 2010, 6520–6524. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.C.; Wang, K.L.; Liao, X.J.; Deng, Z.; Xu, S.H. Pentacyclic hemiacetal sterol with antifouling and cytotoxic activities from the soft coral Nephthea sp. Bioorg. Med. Chem. Lett. 2013, 23, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Zan, K.; Chen, X.Q.; Fu, Q.; Shi, S.P.; Zhou, S.X.; Xiao, M.T.; Tu, P.F. 1, 10-Secoguaianolides from Artemisia anomala (Asteraceae). Biochem. Syst. Ecol. 2010, 38, 431–434. [Google Scholar] [CrossRef]
- Jakupovic, J.; Tan, R.X.; Bohlmann, F.; Jia, Z.J.; Huneck, S. Sesquiterpene lactones from Artemisia rutifolia. Phytochemistry 1991, 30, 1714–1716. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Gati, T.; Hussein, T.A.; Ali, A.T.; Tzakou, O.A.; Couladis, M.A.; Mabry, T.J.; Toth, G. Ligustolide A and B, two novel sesquiterpenes with rare skeletons and three 1,10-seco-guaianolide derivatives from Achillea ligustica. Tetrahedron 2003, 59, 3729–3735. [Google Scholar] [CrossRef]
- Yadav, S.; Pathak, S.; Sarikhani, M.; Majumdar, S.; Ray, S.; Chandrasekar, B.S.; Adiga, V.; Sundaresan, N.R.; Nandi, D. Nitric oxide synthase 2 enhances the survival of mice during Salmonella Typhimurium infection-induced sepsis by increasing reactive oxygen species, inflammatory cytokines and recruitment of neutrophils to the peritoneal cavity. Free Radic. Bio. Med. 2018, 116, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, P. Differential biological effects of products of nitric oxide (NO) synthase: It is not enough to say NO. Life Sci. 2003, 73, 2137–2149. [Google Scholar] [CrossRef]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, J.S.; Kong, L.Y. Anti-inflammatory effects of Huang-Lian-Jie-Du decoction, its two fractions and four typical compounds. J. Ethnopharmacol. 2011, 134, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.H.; Zhao, L.; Xu, Y.K.; Bao, J.M.; Liu, X.; Zhang, J.S.; Li, W.; Ahmed, A.; Yin, S.; Tang, G.H. Anti-inflammatory sesquiterpenoids from the Traditional Chinese Medicine Salvia plebeia: Regulates pro-inflammatory mediators through inhibition of NF-κB and Erk1/2 signaling pathways in LPS-induced Raw264.7 cells. J. Ethnopharmacol. 2018, 210, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ahn, C.B.; Je, J.Y. Anti-inflammatory action of high molecular weight Mytilus edulis hydrolysates fraction in LPS-induced RAW264.7 macrophage via NF-κB and MAPK pathways. Food Chem. 2016, 202, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tian, X.; Wang, Y.; Wang, D.; Li, W.; Chen, L.; Pan, W.; Mehmood, S.; Chen, Y. Immunomodulating activity of the polysaccharide TLH-3 from Tricholoma lobayense in RAW264.7 macrophages. Int. J. Biol. Macromol. 2017, 107, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Sadhukhan, P.; Saha, S.; Pal, P.B.; Sil, P.C. Morin protects gastric mucosa from nonsteroidal anti-inflammatory drug, indomethacin induced inflammatory damage and apoptosis by modulating NF-κB pathway. Biochim. Biophys. Acta 2015, 1850, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Biradar, S.; Veeresh, B. Protective effect of lawsone on L-Arginine induced acute pancreatitis in rats. Indian J. Exp. Biol. 2013, 51, 256–261. [Google Scholar] [PubMed]
- Naglah, A.M.; Ahmed, A.F.; Wen, Z.H.; Al-Omar, M.A.; Ael-G, A.; Kalmouch, A. New inducible nitric oxide synthase and cyclooxygenase-2 inhibitors, nalidixic acid linked to isatin schiff bases via certain l-amino acid bridges. Molecules 2016, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.M.; Lin, H.Y.; Shen, S.C.; Wu, M.S.; Lin, C.W.; Chiu, W.T.; Lin, C.H.; Chen, Y.C. Zinc protoporphyrin inhibition of lipopolysaccharide-, lipoteichoic acid-, and peptidoglycan-induced nitric oxide production through stimulating iNOS protein ubiquitination. Toxicol. Appl. Pharmacol. 2009, 237, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.N.; Jensen, J.; Asay, K.; West, B.; Kreuter, M.H.; Rawson, B.; Palu, A.K. Morinda citrifolia Based Compositions for Treatment of Anti-Inflamatory Diseases through Inhibition of COX-1, COX-2, Interleukin-1β, Interleukin-6, TNF-α, HLE, and iNOS. U.S. Patent Application 11/613,820, 11 October 2007. [Google Scholar]
- Sugita, Y.; Komatani, H.; Ohshima, K.; Shigemori, M.; Nakashima, A. Expression of cyclooxygenase-2 and vascular endothelial growth factor in primary central nervous system lymphomas. Oncol. Rep. 2007, 18, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, B.; Berenbaum, F.; Humbert, L.; Bian, H.; Béréziat, G.; Crofford, L.; Olivier, J.L. Critical role of C/EBPδ and C/EBPβ factors in the stimulation of the cyclooxygenase-2 gene transcription by interleukin-1β in articular chondrocytes. FEBS J. 2000, 267, 6798–6809. [Google Scholar] [CrossRef]
- Brasier, A.R. The NF-κB regulatory network. Cardiovasc. Toxicol. 2006, 6, 111–130. [Google Scholar] [CrossRef] [PubMed]
- May, M.J.; Ghosh, S. NF-kappaB and Rel proteins: Evolutionarily conserved medators of immune responses. Immunol. Today 1998, 19, 80–88. [Google Scholar] [CrossRef]
- Arsuaga, J.M.; López-Muñoz, M.J.; Sotto, A. Correlation between retention and adsorption of phenolic compounds in nanofiltration membranes. Desalination 2010, 250, 829–832. [Google Scholar] [CrossRef]
- De Matteis, C.I.; Simpson, D.A.; Doughty, S.W.; Euerby, M.R.; Shaw, P.N.; Barrett, D.A. Chromatographic retention behaviour of n-alkylbenzenes and pentylbenzene structural isomers on porous graphitic carbon and octadecyl-bonded silica studied using molecular modelling and QSRR. J. Chromatogr. A 2010, 1217, 6987–6993. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, X.; Cheng, W.; Xie, Q.; Liu, Y.; Zhao, C. Quantitative structure activity relationship (QSAR) approach to multiple drug resistance (MDR) modulators based on combined hybrid system. Mol. Inform. 2010, 28, 969–978. [Google Scholar] [CrossRef]
- Devinyak, O.; Havrylyuk, D.; Lesyk, R. 3D-MoRSE descriptors explained. J. Mol. Graph. Model. 2014, 54, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yamaguchi, K.; Mizuno, A.; Unno, Y.; Asai, A.; Sone, T.; Yokosawa, H.; Matsuda, A.; Arisawa, A.; Shuto, S. Three-dimensional structure-activity relationship study of belactosin A and its stereo- and regioisomers: Development of potent proteasome inhibitors by a stereochemical diversity-oriented strategy. Org. Biomol. Chem. 2009, 7, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Duclos, R.I.; O’Doherty, G.A. Stereochemical structure activity relationship studies (S-SAR) of tetrahydrolipstatin. ACS Med. Chem. Lett. 2018, 9, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Matoba, S.; Yamazaki, Y.M.; Shimasaki, R.; Miyanaga, S.; Igarashi, Y. complete stereochemistry and preliminary structure-activity relationship of rakicidin A, a hypoxia-selective cytotoxin from Micromonospora sp. J. Nat. Prod. 2014, 77, 2561–2565. [Google Scholar] [CrossRef] [PubMed]
- Boshkow, J.; Fischer, S.; Bailey, A.; Wolfrum, S.; Carreira, E.M. Stereochemistry and biological activity of chlorinated lipids: A study of danicalipin A and selected diastereomers. Chem. Sci. 2017, 8, 6904–6910. [Google Scholar] [CrossRef] [PubMed]
- Wybenga, G.G.; Szymanski, W.; Wu, B.; Feringa, B.L.; Janssen, D.B.; Dijkstra, B.W. Structural investigations into the stereochemistry and activity of a phenylalanine-2,3-aminomutase from Taxus chinensis. Biochemistry 2014, 53, 3187–3198. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.P. Monoterpene and Sesquiterpene Chemistry; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Shie, P.H.; Wang, S.Y.; Lay, H.L.; Huang, G.J. 4,7-Dimethoxy-5-methyl-1,3-benzodioxole from Antrodia camphorata inhibits LPS-induced inflammation via suppression of NF-κB and induction HO-1 in RAW264.7 cells. Int. Immunopharmacol. 2016, 31, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Sevaljević, L.; Dobrić, S.; Bogojević, D.; Petrović, M.; Koricanać, G.; Vulović, M.; Kanazir, D.; Ribarac-Stepić, N. The radioprotective activities of turpentine-induced inflammation and alpha2-macroglobulin: The effect of dexamethasone on the radioprotective efficacy of the inflammation. J. Radiat. Res. 2003, 44, 59–67. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–7 are available from the authors. |
| No. | 1H NMR a | 13C NMR a |
|---|---|---|
| 1 | 203.8 | |
| 2 | 2.34, m | 44.2 |
| 2.84, ddd (18.8, 6.7, 3.1) | ||
| 3 | 4.71, brs | 72.4 |
| 4 | 173.4 | |
| 5 | 135.5 | |
| 6 | 5.45, d (7.2) | 75.9 |
| 7 | 3.29, m | 42.0 |
| 8 | 1.62, m | 23.8 |
| 9 | 2.32, m | 40.2 |
| 2.48, m | ||
| 10 | 208.3 | |
| 11 | 138.2 | |
| 12 | 169.8 | |
| 13 | 5.66, s | 123.2 |
| 6.36, s | ||
| 14 | 2.12, s | 30.3 |
| 15 | 2.25, s | 14.9 |
| Compound | IC50 (μM) | SD |
|---|---|---|
| 1 | 15.39 | 3.16 |
| 2 | 1.62 | 0.20 |
| 3 | 0.79 | 0.13 |
| 4 | 7.63 | 1.99 |
| 6 | 19.32 | 0.22 |
| 7 | 31.53 | 5.07 |
| l-NMMA | 24.95 | 1.46 |
| DXM | 0.20 | 0.98 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Dai, W.; Xiang, C.; Chi, J.; Zhang, M. 1,10-Secoguaianolides from Artemisia austro-yunnanensis and Their Anti-Inflammatory Effects. Molecules 2018, 23, 1639. https://doi.org/10.3390/molecules23071639
Liu L, Dai W, Xiang C, Chi J, Zhang M. 1,10-Secoguaianolides from Artemisia austro-yunnanensis and Their Anti-Inflammatory Effects. Molecules. 2018; 23(7):1639. https://doi.org/10.3390/molecules23071639
Chicago/Turabian StyleLiu, Lan, Weifeng Dai, Cheng Xiang, Jun Chi, and Mi Zhang. 2018. "1,10-Secoguaianolides from Artemisia austro-yunnanensis and Their Anti-Inflammatory Effects" Molecules 23, no. 7: 1639. https://doi.org/10.3390/molecules23071639