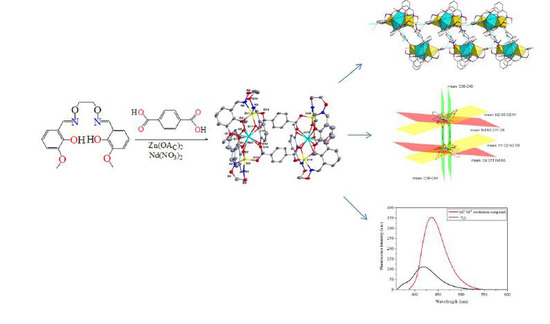
A Self-Assembled ZnII-NdIII Heterohexanuclear Dimer Based on a Hexadentate N2O4-Type Ligand and Terephthalic Acid: Synthesis, Structure, and Fluorescence Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Physical Measurements
2.2. Synthesis of the Ligand H2L
2.3. Synthesis of the ZnII-NdIII Coordination Compound
2.4. X-ray Structure Determination of the ZnII-NdIII Coordination Compound
3. Results and Discussion
3.1. Description of Crystal Structure of the ZnII-NdIII Coordination Compound
3.2. Supramolecular Interaction of the ZnII-NdIII Coordination Compound
3.3. IR Spectra of H2L and Its ZnII-NdIII Coordination Compound
3.4. UV/Vis Absorption Spectra of H2L and Its ZnII-NdIII Coordination Compound
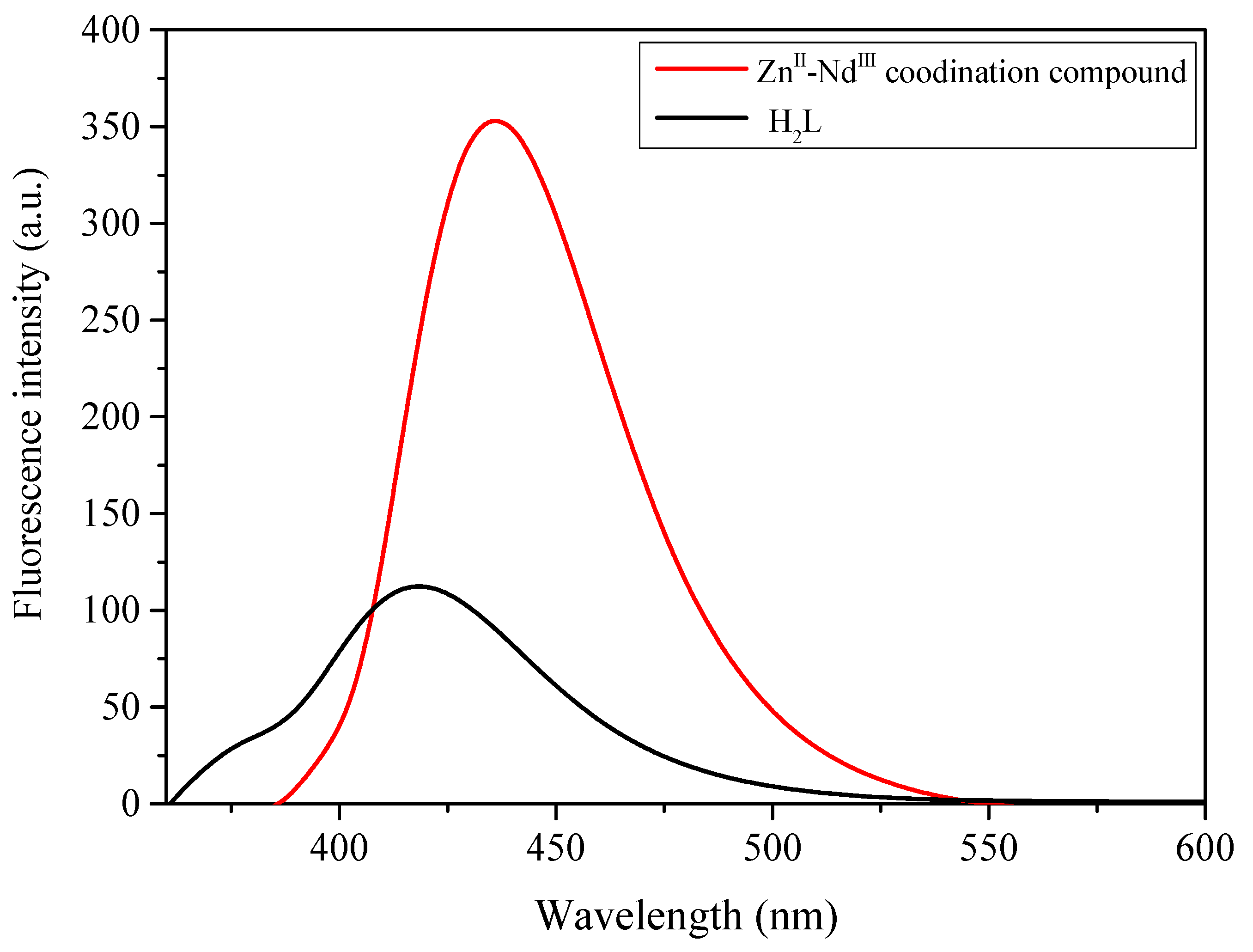
3.5. Fluorescence Properties of H2L and Its ZnII-NdIII Coordination Compound
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Akine, S.; Varadi, Z.; Nabeshima, T. Synthesis of planar metal complexes and the stacking abilities of naphthalenediol-based acyclic and macrocyclic salen-type ligands. Eur. J. Inorg. Chem. 2013, 2013, 5987–5998. [Google Scholar] [CrossRef]
- Sun, S.S.; Stern, C.L.; Nguyen, S.T.; Hupp, J.T. Directed assembly of transition-metal-coordinated molecular loops and square from Salen-type components. Examples of metalation controlled structural conversion. J. Am. Chem. Soc. 2004, 126, 6314–6326. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Q.; Huang, J.J.; Zhang, H.S. An unexpected cobalt(III) complex containing a Schiff base ligand: Synthesis, crystal structure, spectroscopic behavior, electrochemical property and SOD-like activity. Spectrochim. Acta Part A 2014, 131, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J.; Shi, F.R.; Zhang, Y.H.; Wang, X.L. Synthesis, structure, antioxidation, and DNA-bindingstudies of a binuclear ytterbium(III) complex with bis(N-salicylidene)-3-oxapentane-1,5-diamine. Res. Chem. Intermed. 2015, 41, 3375–3388. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Liu, Y.A.; Zhou, J.J.; Wang, X.L. Two dodecanuclear heterometallic [Zn6Ln6] clusters constructed by a multidentate salicylamide Salen-like ligand: Synthesis, structure, luminescence and magnetic properties. Dalton Trans. 2016, 45, 8154–8163. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Q.; Peng, Y.J.; Chen, G.Q.; Wang, X.R.; Liu, P.P.; Xu, W.Y. Substituted group-directed assembly of Zn(II) coordination complexes based on two new structural related pyrazolone based Salen ligands: Syntheses, structures and fluorescence properties. Inorg. Chim. Acta 2015, 427, 13–21. [Google Scholar] [CrossRef]
- Chen, L.; Dong, W.K.; Zhang, H.; Zhang, Y.; Sun, Y.X. Structural variation and luminescence properties of tri- and dinuclear CuII and ZnII complexes constructed from a naphthalenediol-based bis(Salamo)-type ligand. Cryst. Growth Des. 2017, 17, 3636–3648. [Google Scholar] [CrossRef]
- Di Bella, S.; Fragala, I. Synthesis and second-order nonlinear optical properties of bis(salicylaldiminato)M(II) metalloorganic materials. Synth. Met. 2000, 115, 191–196. [Google Scholar] [CrossRef]
- Zheng, S.S.; Dong, W.K.; Zhang, Y.; Chen, L.; Ding, Y.J. Four Salamo-type 3d–4f hetero-bimetallic [ZnIILnIII] complexes: Syntheses, crystal structures, and luminescent and magnetic properties. New J. Chem. 2017, 41, 4966–4973. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, J.W.; Zhang, Y.H.; Yang, Z.H.; Wu, H.L. Gadolinium(III) and dysprosium(III) complexes with a Schiff base bis(N-salicylidene)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidation, and DNA-binding studies. J. Coord. Chem. 2015, 68, 1054–1071. [Google Scholar] [CrossRef]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Novel synthetic approach to trinuclear 3d-4f complexes: Specific exchange of the central metal of a trinuclear zinc(II) complex of a tetraoxime ligand with a lanthanide(III) ion. Angew. Chem. 2002, 41, 4670–4673. [Google Scholar] [CrossRef] [PubMed]
- Cahill, C.L.; de Lill, D.T.; Frisch, M. Homo- and heterometallic coordination polymers from the f elements. CrystEngComm 2007, 9, 15–26. [Google Scholar] [CrossRef]
- Huang, Y.G.; Jiang, F.L.; Hong, M.C. Magnetic lanthanide–transition-metal organic–inorganic hybrid materials: From discrete clusters to extended frameworks. Coord. Chem. Rev. 2009, 253, 2814–2834. [Google Scholar] [CrossRef]
- Gupta, S.; Kirillova, M.V.; Fátima, M.; Guedes da Silva, C.; Pombeiro, A.J.L.; Kirillov, A.M. Alkali metal directed assembly of heterometallic Vv/M (M = Na, K, Cs) coordination polymers: Structures, topological analysis, and oxidation catalytic properties. Inorg. Chem. 2013, 52, 8601–8611. [Google Scholar] [CrossRef] [PubMed]
- Sotnik, S.A.; Polunin, R.A.; Kiskin, M.A.; Kirillov, A.M.; Dorofeeva, V.N.; Gavrilenko, K.S.; Eremenko, I.L.; Novotortsev, V.M.; Kolotilov, S.V. Heterometallic coordination polymers assembled from trigonal trinuclear Fe2Ni-pivalate blocks and polypyridine spacers: Topological diversity, sorption, and catalytic properties. Inorg. Chem. 2015, 54, 5169–5181. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Synthesis and characterization of novel ligands 1,2-bis(salicylideneaminooxy)ethanes. Chem. Lett. 2001, 30, 682–683. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y. Nine self-assembled nickel(II)–lanthanide(III) heterometallic complexes constructed from a Salamo-type bisoxime and bearing a N- or O-donor auxiliary ligand: Syntheses, structures and magnetic properties. New J. Chem. 2016, 40, 6998–7010. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.C.; Dong, W.K.; Zhu, L.C.; Zhang, Y. A novel self–assembled nickel(II)–cerium(III) heterotetranuclear dimer constructed from N2O2-type bisoxime and terephthalic acid: Synthesis, structure and photophysical properties. Z. Anorg. Allg. Chem. 2016, 642, 834–839. [Google Scholar] [CrossRef]
- Dong, X.Y.; Zhao, Q.; Kang, Q.P.; Li, X.Y.; Dong, W.K. Self-assembly of 3d-4f ZnII-LnIII (Ln = Ho and Er) bis(salamo)-based complexes: Controlled syntheses, structures and fluorescence properties. Crystals 2018, 8, 230. [Google Scholar] [CrossRef]
- Dong, W.K.; Zhang, J.; Zhang, Y.; Li, N. Novel multinuclear transition metal(II) complexes based on an asymmetric Salamo-type ligand: Syntheses, structure characterizations and fluorescent properties. Inorg. Chim. Acta 2016, 444, 95–102. [Google Scholar] [CrossRef]
- Dong, X.Y.; Zhao, Q.; Wei, Z.L.; Mo, H.R.; Zhang, H.; Dong, W.K. Synthesis and fluorescence properties of structurally characterized heterobimetalic Cu(II)–Na(I) bis(salamo)-based complex bearing square planar, square pyramid and triangular prism geometries of metal centers. Crystals 2018, 23, 1006. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



| Coordination Compound | ZnII-NdIII |
|---|---|
| Empirical Formula | C88H80N10Nd2Zn4O38 |
| Molecular Weight | 2435.58 |
| Color | colorless |
| Crystal Size, mm | 0.270 × 0.250 × 0.220 |
| Habit | Block-shaped |
| Crystal System | Triclinic |
| Space Group | P-1 |
| Unit Cell Dimension | |
| a, Å | 12.5088(9) |
| b, Å | 16.0858(12) |
| c, Å | 16.2996(12) |
| α, ° | 69.0298(19) |
| β, ° | 83.1411(18) |
| γ, ° | 87.649(2) |
| Volume, Å3 | 3040.6(4) |
| Z | 1 |
| Calculated Density, mg·m–3 | 1.330 |
| Absorption Coefficient, mm–1 | 1.688 |
| F(000) | 1222 |
| θ Range for Data Collection, ° | 1.346 to 27.756 |
| h/k/l (min, max) | −16, 16/−14, 21/−20, 21 |
| Reflections Collected | 25,768 |
| Completeness | 98.5% |
| Data/Restraints/Parameters | 13,926/3/644 |
| Final R Indices [I > 2σ(I)] | R1 = 0.0422, wR2 = 0.1254 |
| R Indices (All Data) | R1 = 0.0606, wR2 = 0.1294 |
| Largest Diff. Peak and Hole (e·Å–3) | 1.033, −0.898 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, L.-J.; Gao, L.; Guo, W.-T.; Ma, J.-C.; Dong, W.-K. A Self-Assembled ZnII-NdIII Heterohexanuclear Dimer Based on a Hexadentate N2O4-Type Ligand and Terephthalic Acid: Synthesis, Structure, and Fluorescence Properties. Molecules 2018, 23, 1609. https://doi.org/10.3390/molecules23071609
Ru L-J, Gao L, Guo W-T, Ma J-C, Dong W-K. A Self-Assembled ZnII-NdIII Heterohexanuclear Dimer Based on a Hexadentate N2O4-Type Ligand and Terephthalic Acid: Synthesis, Structure, and Fluorescence Properties. Molecules. 2018; 23(7):1609. https://doi.org/10.3390/molecules23071609
Chicago/Turabian StyleRu, Li-Jun, Lei Gao, Wen-Ting Guo, Jian-Chun Ma, and Wen-Kui Dong. 2018. "A Self-Assembled ZnII-NdIII Heterohexanuclear Dimer Based on a Hexadentate N2O4-Type Ligand and Terephthalic Acid: Synthesis, Structure, and Fluorescence Properties" Molecules 23, no. 7: 1609. https://doi.org/10.3390/molecules23071609