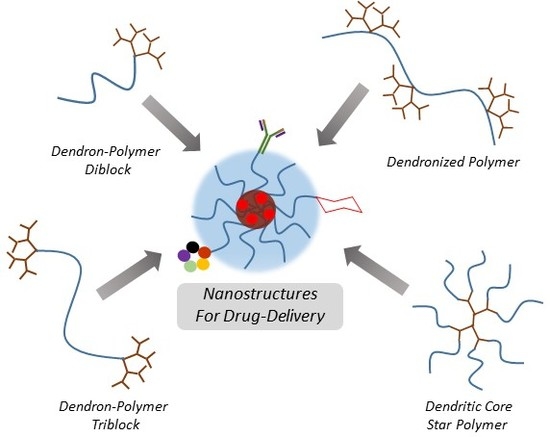
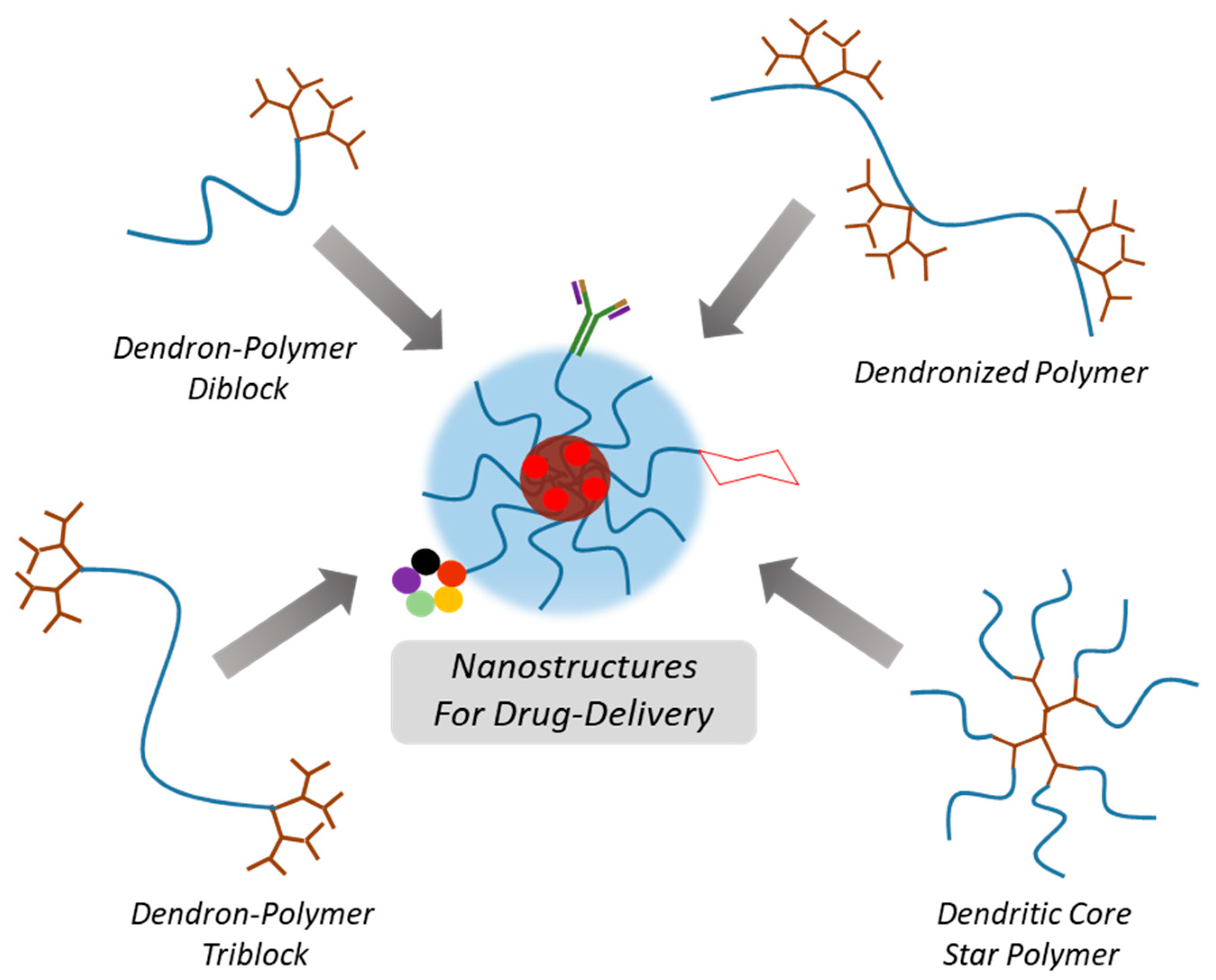
Drug Delivery Systems from Self-Assembly of Dendron-Polymer Conjugates †
Abstract
:1. Introduction
1.1. Nanosized Polymeric Materials as Drug Delivery Vehicles
1.2. Dendritic Architecture-Based Drug Delivery Vehicles
2. Nano-Sized Aggregates from Polymer-Dendron Diblock Conjugates
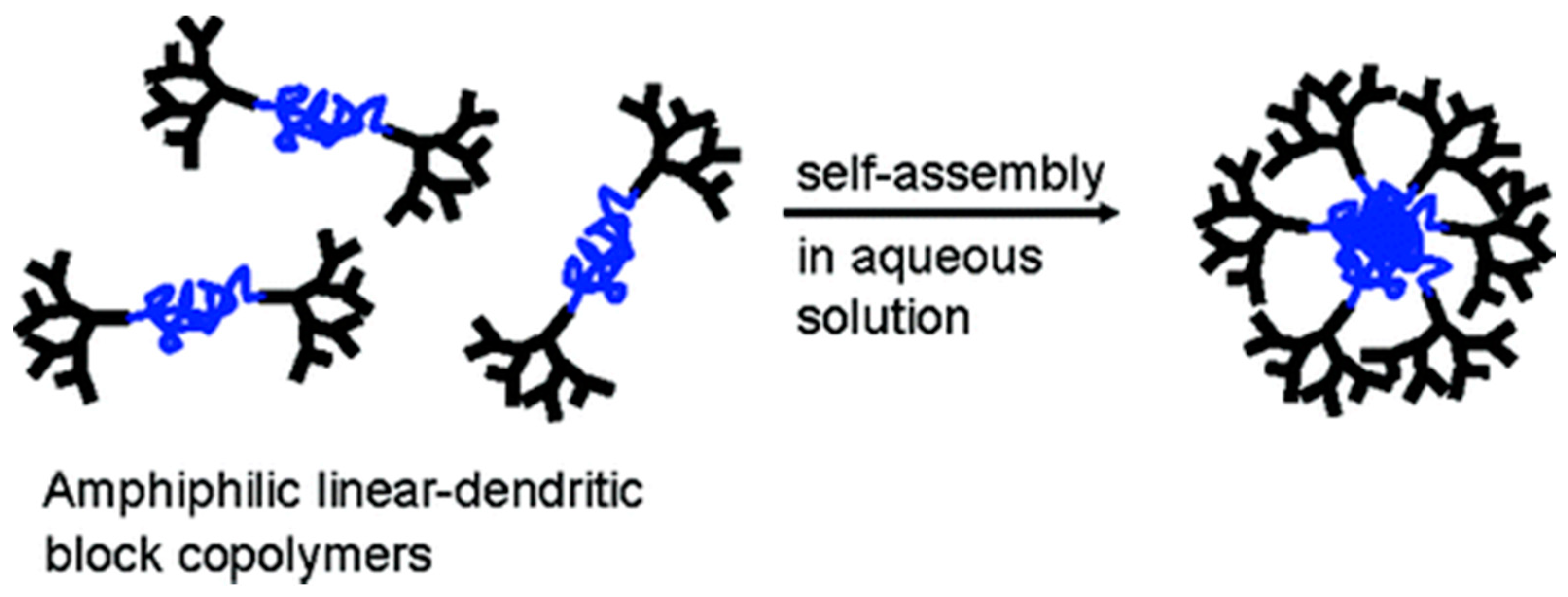
2.1. Nanoparticles Obtained with Hydrophilic Dendron and Hydrophobic Polymer Based Conjugates
2.2. Nanoparticles Obtained with Hydrophobic Dendron and Hydrophilic Polymer Based Conjugates
2.3. Nanoparticles Obtained with Hydrophilic Dendron and Hydrophilic Polymer Based Conjugates
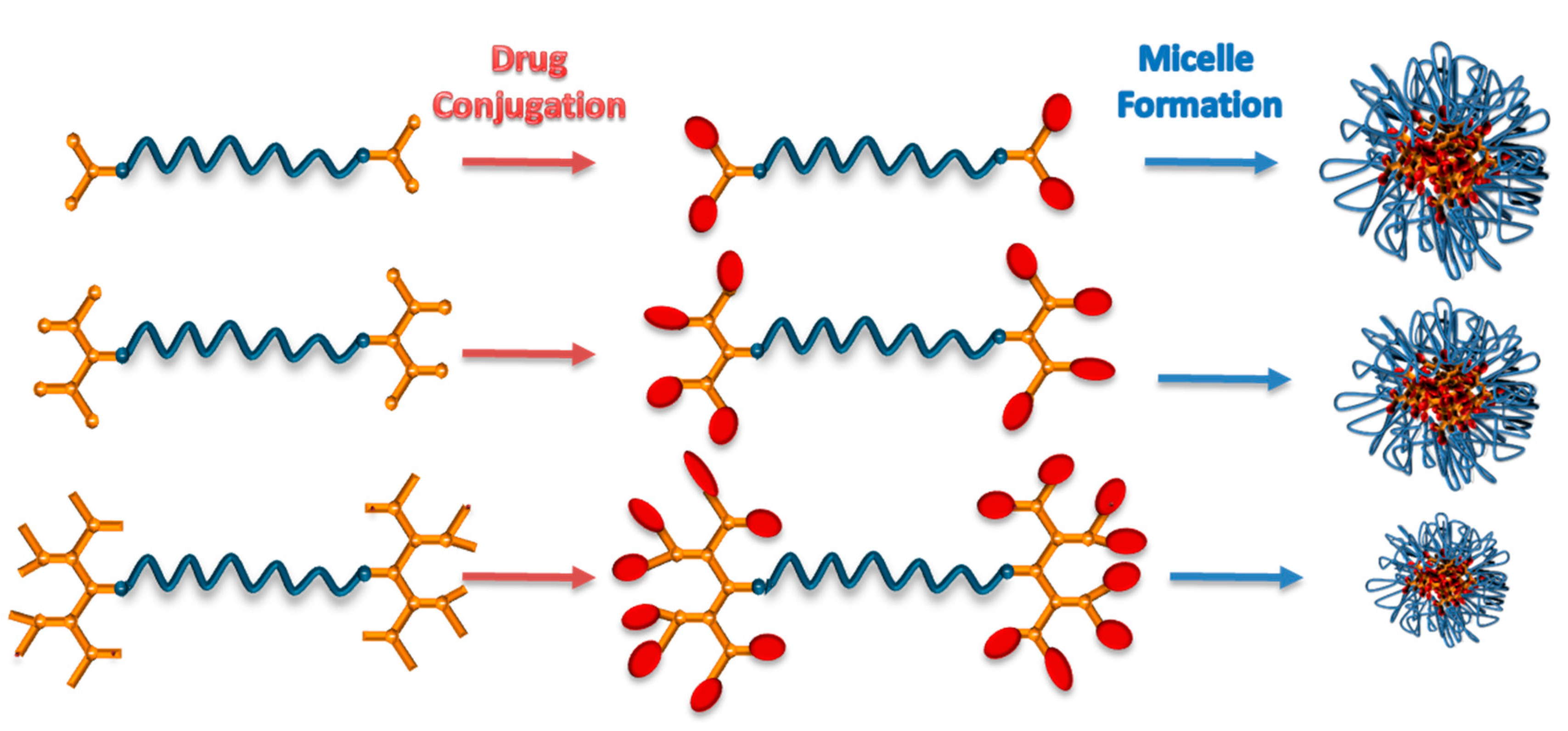
3. Nano-Sized Aggregates from Polymer-Dendron Triblock Conjugates
4. Nano-Sized Aggregates from Star Type Architectures
5. Nano-Sized Aggregates from Miscellaneous Dendron-Polymer Architectures
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Sirova, M.; Mrkvan, T.; Etrych, T.; Chytil, P.; Rossmann, P.; Ibrahimova, M.; Kovar, L.; Ulbrich, K.; Rihova, B. Preclinical evaluation of linear HPMA-doxorubicin conjugates with pH-sensitive drug release: Efficacy, safety, and immunomodulating activity in murine model. Pharm. Res. 2010, 27, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.C.; Weiner, L.M.; Shuptrine, C.W. Antibody-Based Immunotherapy of Cancer. Cell 2012, 148, 1081–1084. [Google Scholar]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Song, H.; Yang, Q.; Cai, H.; Qi, R.; Yan, L.; Liu, S.; Zheng, Y.; Huang, Y.; Liu, T.; et al. A prodrug strategy to deliver cisplatin(IV) and paclitaxel in nanomicelles to improve efficacy and tolerance. Biomaterials 2012, 33, 6507–6519. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. Development of HPMA copolymer-anticancer conjugates: Clinical experience and lessons learnt. Adv. Drug Deliv. Rev. 2009, 61, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Kopecek, J.; Kopecková, P. HPMA copolymers: Origins, early developments, present, and future. Adv. Drug Deliv. Rev. 2010, 62, 122–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Van, S.; Shu, Y.; Liu, X.; Ge, Y.; Jiang, X.; Jin, Y.; Yu, L. Synthesis, characterization, and in vivo efficacy evaluation of PGG-docetaxel conjugate for potential cancer chemotherapy. Int. J. Nanomed. 2012, 7, 581–589. [Google Scholar] [CrossRef]
- Sharma, A.; Kakkar, A. Designing Dendrimer and Miktoarm Polymer Based Multi-Tasking Nanocarriers for Efficient Medical Therapy. Molecules 2015, 20, 16987–17015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Torchilin, V.P. Nanopreparations for organelle-specific delivery in cancer. Adv. Drug Deliv. Rev. 2014, 66, 26–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkar, A.; Traverso, G.; Farokhzad, O.C.; Weissleder, R.; Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 2017, 1, 0063. [Google Scholar] [CrossRef]
- Zhao, Z.; Harris, B.; Hu, Y.; Harmon, T.; Pentel, P.R.; Ehrich, M.; Zhang, C. Rational incorporation of molecular adjuvants into a hybrid nanoparticle-based nicotine vaccine for immunotherapy against nicotine addiction. Biomaterials 2018, 155, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hu, Y.; Hoerle, R.; Devine, M.; Raleigh, M.; Pentel, P.; Zhang, C. A nanoparticle-based nicotine vaccine and the influence of particle size on its immunogenicity and efficacy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Powers, K.; Hu, Y.; Raleigh, M.; Pentel, P.; Zhang, C. Engineering of a hybrid nanoparticle-based nicotine nanovaccine as a next-generation immunotherapeutic strategy against nicotine addiction: A focus on hapten density. Biomaterials 2017, 123, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Hu, Y.; Harmon, T.; Pentel, P.; Ehrich, M.; Zhang, C. Rationalization of a nanoparticle-based nicotine nanovaccine as an effective next-generation nicotine vaccine: A focus on hapten localization. Biomaterials 2017, 138, 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eetezadi, S.; Ekdawi, S.N.; Allen, C. The challenges facing block copolymer micelles for cancer therapy: In vivo barriers and clinical translation. Adv. Drug Deliv. Rev. 2015, 91, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.-D.; Huang, L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol. Pharm. 2008, 5, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.M.; Fries, L.F. The role of complement in inflammation and phagocytosis. Immunol. Today 1991, 12, 322–326. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- van Vlerken, L.E.; Vyas, T.K.; Amiji, M.M. Poly(ethylene glycol)-modified Nanocarriers for Tumor-targeted and Intracellular Delivery. Pharm. Res. 2007, 24, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. VI. Branched Polymers Containing A—R—Bf−1 Type Units. J. Am. Chem. Soc. 1952, 74, 2718–2723. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vögtle, F. “Cascade”- and “Nonskid-Chain-like” Syntheses of Molecular Cavity Topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- Denkewalter, R.G.; Kolc, J.; Lukasavage, W.J. Macromolecular Highly Branched Homogeneous Compound Based on Lysine Units. U.S. Patent 4289872, 6 April 1979. [Google Scholar]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Tomalia, D.A.; Dewald, J.R. Dense Star Polymers Having Core, Core Branches, Terminal Groups. U.S. Patent 4507466A, 7 January 1983. [Google Scholar]
- Newkome, G.R.; Yao, Z.; Baker, G.R.; Gupta, V.K. Micelles. Part 1. Cascade molecules: A new approach to micelles. A [27]-arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Ambade, A.V.; Savariar, E.N.; Thayumanavan, S. Dendrimeric Micelles for Controlled Drug Release and Targeted Delivery. Mol. Pharm. 2005, 2, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillies, E.R.; Frechet, J.M.J. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Medina, S.H.; El-Sayed, M.E.H. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem. Rev. 2009, 109, 3141–3157. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Zimmerman, S.C. Dendrimers in Supramolecular Chemistry: From Molecular Recognition to Self-Assembly. Chem. Rev. 1997, 97, 1681–1712. [Google Scholar] [CrossRef] [PubMed]
- Patri, A.K.; Majoros, I.J.; Baker, J.R. Dendritic polymer macromolecular carriers for drug delivery. Curr. Opin. Chem. Biol. 2002, 6, 466–471. [Google Scholar] [CrossRef]
- Newkome, G.R.; Moorefield, C.N.; Baker, G.R.; Saunders, M.J.; Grossman, S.H. Unimolecular Micelles. Angew. Chem. Int. Ed. Engl. 1991, 30, 1178–1180. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Sideratou, Z.; Tsiourvas, D.; Paleos, C.M. Quaternized Poly(propylene imine) Dendrimers as Novel pH-Sensitive Controlled-Release Systems. Langmuir 2000, 16, 1766–1769. [Google Scholar] [CrossRef]
- Sideratou, Z.; Tsiourvas, D.; Paleos, C.M. Solubilization and Release Properties of PEGylated Diaminobutane Poly(propylene imine) Dendrimers. J. Colloid Interface Sci. 2001, 242, 272–276. [Google Scholar] [CrossRef]
- Grayson, S.M.; Jayaraman, M.; Fréchet, J.M.J.; Verkade, J.G.; Gianasi, E.; Strohalm, J.; Duncan, R. Convergent synthesis and ‘surface’ functionalization of a dendritic analog of poly(ethylene glycol). Chem. Commun. 1999, 112, 1329–1330. [Google Scholar] [CrossRef]
- Liu, M.; Kono, K.; Frechet, J.M.J. Water-soluble dendrimer-poly(ethylene glycol) starlike conjugates as potential drug carriers. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3492–3503. [Google Scholar] [CrossRef]
- Carlmark, A.; Malmström, E.; Malkoch, M. Dendritic architectures based on bis-MPA: Functional polymeric scaffolds for application-driven research. Chem. Soc. Rev. 2013, 42, 5858–5879. [Google Scholar] [CrossRef] [PubMed]
- Ihre, H.R.; de Jesús, O.L.P.; Szoka, F.C.; Fréchet, J.M.J. Polyester Dendritic Systems for Drug Delivery Applications: Design, Synthesis, and Characterization. Bioconjug. Chem. 2002. [Google Scholar] [CrossRef]
- Grinstaff, M.W. Biodendrimers: New Polymeric Biomaterials for Tissue Engineering. Chem. A Eur. J. 2002, 8, 2838. [Google Scholar] [CrossRef]
- Feliu, N.; Walter, M.V.; Montañez, M.I.; Kunzmann, A.; Hult, A.; Nyström, A.; Malkoch, M.; Fadeel, B. Stability and biocompatibility of a library of polyester dendrimers in comparison to polyamidoamine dendrimers. Biomaterials 2012, 33, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Oelker, A.M.; Berlin, J.A.; Wathier, M.; Grinstaff, M.W. Synthesis and characterization of dendron cross-linked PEG hydrogels as corneal adhesives. Biomacromolecules 2011, 12, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, P.; Walter, M.V.; Montañez, M.I.; Hult, D.; Hult, A.; Nyström, A.; Malkoch, M. Linear dendritic polymeric amphiphiles with intrinsic biocompatibility: Synthesis and characterization to fabrication of micelles and honeycomb membranes. Polym. Chem. 2011, 2, 394–402. [Google Scholar] [CrossRef]
- Padilla De Jesús, O.L.; Ihre, H.R.; Gagne, L.; Fréchet, J.M.J.; Szoka, F.C. Polyester Dendritic Systems for Drug Delivery Applications: In Vitro and In Vivo Evaluation. Bioconjug. Chem. 2002, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Gitsov, I.; Wooley, K.L.; Fréchet, J.M.J. Novel Polyether Copolymers Consisting of Linear and Dendritic Blocks. Angew. Chem. Int. Ed. Engl. 1992, 31, 1200–1202. [Google Scholar] [CrossRef]
- Han, R.; Sun, Y.; Kang, C.; Sun, H.; Wei, W. Amphiphilic dendritic nanomicelle-mediated co-delivery of 5-fluorouracil and doxorubicin for enhanced therapeutic efficacy. J. Drug Target. 2017, 25, 140–148. [Google Scholar] [CrossRef] [PubMed]
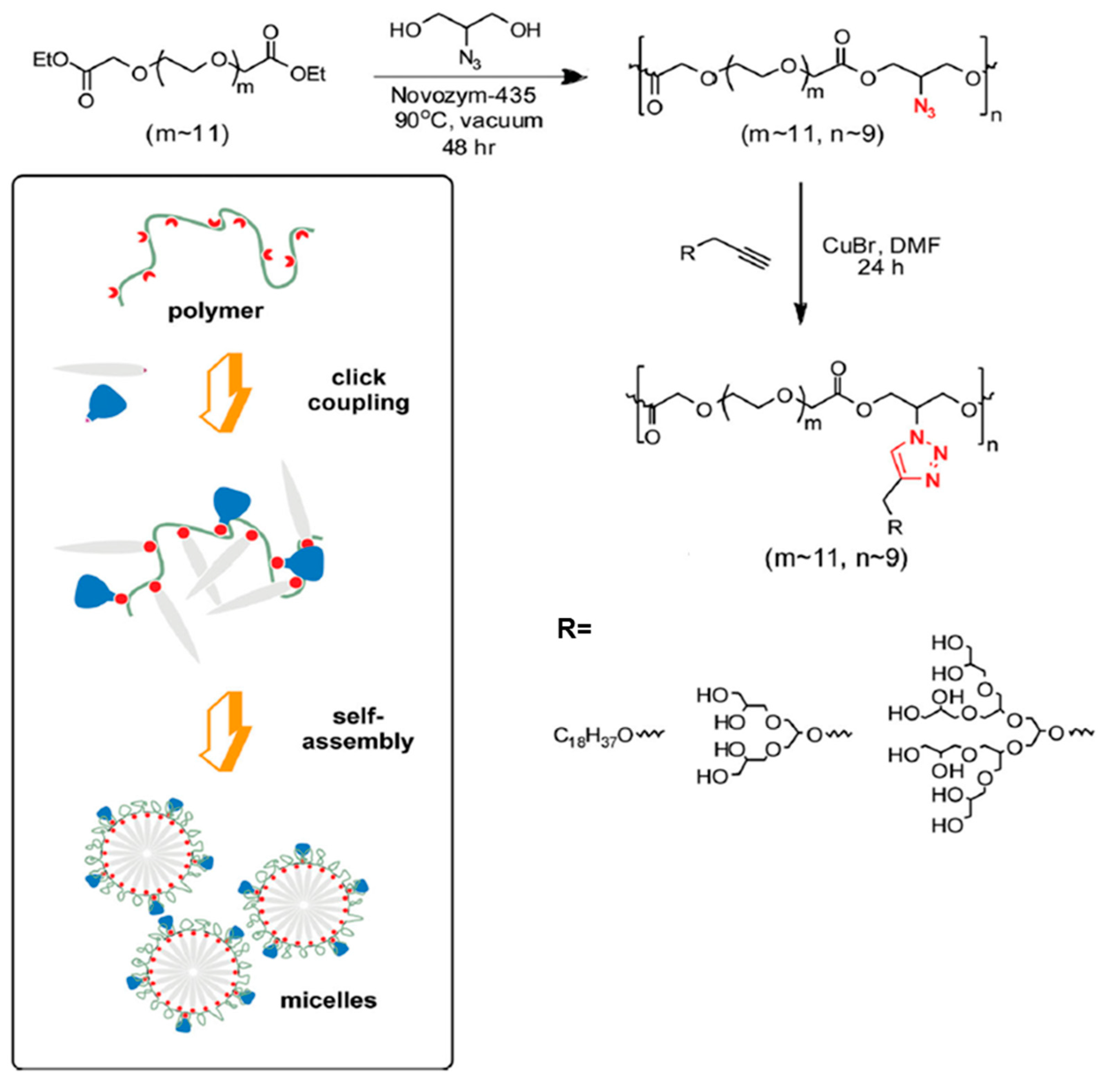
- Bae, J.W.; Pearson, R.M.; Patra, N.; Sunoqrot, S.; Vuković, L.; Král, P.; Hong, S. Dendron-mediated self-assembly of highly PEGylated block copolymers: A modular nanocarrier platform. Chem. Commun. 2011, 47, 10302–10304. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.M.; Patra, N.; Hsu, H.J.; Uddin, S.; Král, P.; Hong, S. Positively charged dendron micelles display negligible cellular interactions. ACS Macro Lett. 2013, 2, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pearson, R.M.; Lee, O.; Lee, C.W.; Chatterton, R.T.; Khan, S.A.; Hong, S. Dendron-based micelles for topical delivery of endoxifen: A potential chemo-preventive medicine for breast cancer. Adv. Funct. Mater. 2014, 24, 2442–2449. [Google Scholar] [CrossRef]
- Pearson, R.M.; Sen, S.; Hsu, H.; Pasko, M.; Gaske, M.; Král, P.; Hong, S. Tuning the Selectivity of Dendron Micelles Through Variations of the Poly(ethylene glycol) Corona. ACS Nano 2016, 10, 6905–6914. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Sousa-Herves, A.; Riguera, R.; Fernandez-Megia, E. PEG-dendritic block copolymers for biomedical applications. New J. Chem. 2012, 36, 205. [Google Scholar] [CrossRef]
- Hak, S.; Helgesen, E.; Hektoen, H.H.; Huuse, E.M.; Jarzyna, P.A.; Mulder, W.J.M.; Haraldseth, O.; Davies, C.D.L. The Effect of Nanoparticle Polyethylene Glycol Surface Density on Ligand-Directed Tumor Targeting Studied in Vivo by Dual Modality Imaging. ACS Nano 2012, 6, 5648–5658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poon, Z.; Chen, S.; Engler, A.C.; Lee, H. Il; Atas, E.; Von Maltzahn, G.; Bhatia, S.N.; Hammond, P.T. Ligand-clustered “patchy” nanoparticles for modulated cellular uptake and in vivo tumor targeting. Angew. Chem. Int. Ed. 2010, 49, 7266–7270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Malkoch, M.; Hunt, J.N.; Vestberg, R.; Kaltgrad, E.; Finn, M.G.; Fokin, V.V.; Sharpless, K.B.; Hawker, C.J. Multivalent, bifunctional dendrimers prepared by click chemistry. Chem. Commun. 2005, 5775. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-M.; Liu, G. Linear–dendritic biodegradable block copolymers: From synthesis to application in bionanotechnology. Polym. Chem. 2013, 46–52. [Google Scholar] [CrossRef]
- del Barrio, J.; Oriol, L.; Alcalá, R.; Sánchez, C. Azobenzene-Containing Linear−Dendritic Diblock Copolymers by Click Chemistry: Synthesis, Characterization, Morphological Study, and Photoinduction of Optical Anisotropy. Macromolecules 2009, 42, 5752–5760. [Google Scholar] [CrossRef]
- Gillies, E.R.; Jonsson, T.B.; Fréchet, J.M.J. Stimuli-responsive supramolecular assemblies of linear-dendritic copolymers. J. Am. Chem. Soc. 2004, 126, 11936–11943. [Google Scholar] [CrossRef] [PubMed]
- Kalva, N.; Parekh, N.; Ambade, A.V. Controlled micellar disassembly of photo- and pH-cleavable linear-dendritic block copolymers. Polym. Chem. 2015, 6, 6826–6835. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, B.; Su, Y.; Dong, C.-M. Light-responsive linear-dendritic amphiphiles and their nanomedicines for NIR-triggered drug release. Polym. Chem. 2014, 5, 1605–1613. [Google Scholar] [CrossRef]
- Andrén, O.C.J.; Zhang, Y.; Lundberg, P.; Hawker, C.J.; Nyström, A.M.; Malkoch, M. Therapeutic Nanocarriers via Cholesterol Directed Self-Assembly of Well-Defined Linear-Dendritic Polymeric Amphiphiles. Chem. Mater. 2017, 29, 3891–3898. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Liu, G.; Du, Z.; Cheng, X. A Biodegradable and Amphiphilic Linear-Dendritic Copolymer as a Drug Carrier Platform for Intracellular Drug Delivery. Macromol. Chem. Phys. 2016, 217, 2004–2012. [Google Scholar] [CrossRef]
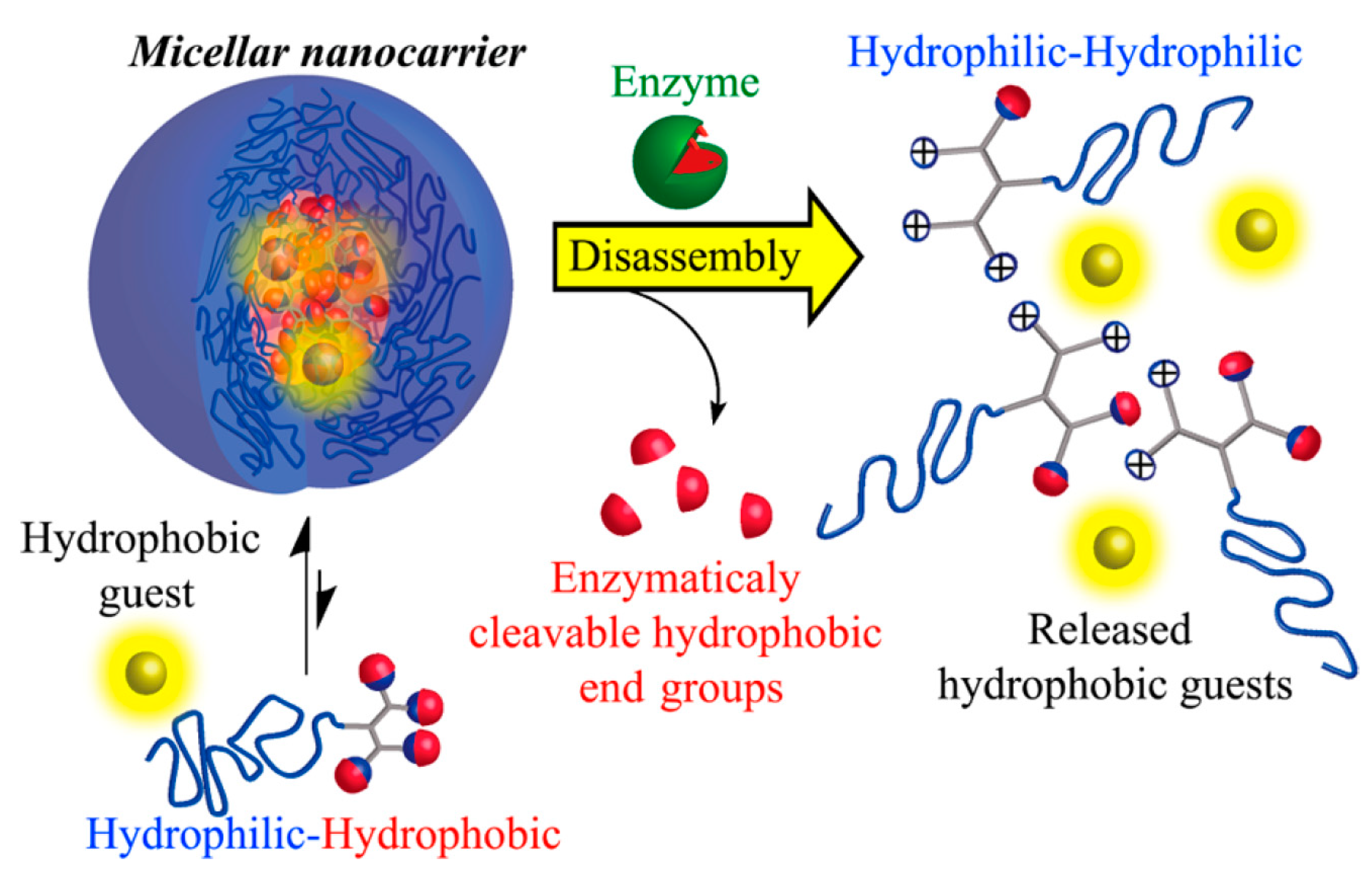
- Harnoy, A.J.; Rosenbaum, I.; Tirosh, E.; Ebenstein, Y.; Shaharabani, R.; Beck, R.; Amir, R.J. Enzyme-Responsive Amphiphilic PEG-Dendron Hybrids and Their Assembly into Smart Micellar Nanocarriers. J. Am. Chem. Soc. 2014, 136, 7531–7534. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, I.; Avinery, R.; Harnoy, A.J.; Slor, G.; Tirosh, E.; Hananel, U.; Beck, R.; Amir, R.J. Reversible Dimerization of Polymeric Amphiphiles Acts as a Molecular Switch of Enzymatic Degradability. Biomacromolecules 2017, 18, 3457–3468. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Luo, J.; Fowler, W.L.; Li, Y.; Lee, J.S.; Xing, L.; Cheng, R.H.; Wang, L.; Lam, K.S. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials 2009, 30, 6006–6016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
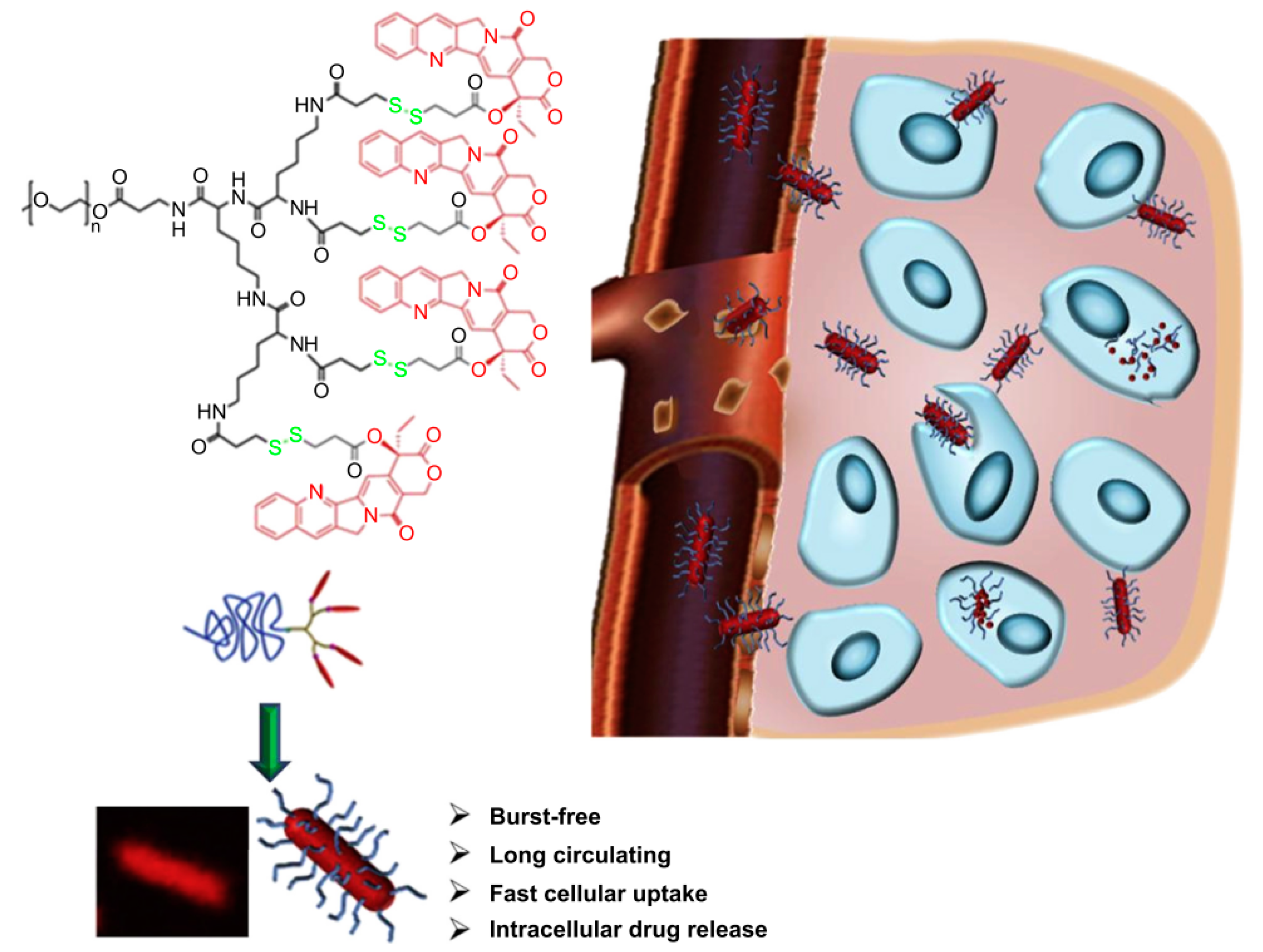
- Zhou, Z.; Ma, X.; Jin, E.; Tang, J.; Sui, M.; Shen, Y.; Van Kirk, E.A.; Murdoch, W.J.; Radosz, M. Linear-dendritic drug conjugates forming long-circulating nanorods for cancer-drug delivery. Biomaterials 2013, 34, 5722–5735. [Google Scholar] [CrossRef] [PubMed]
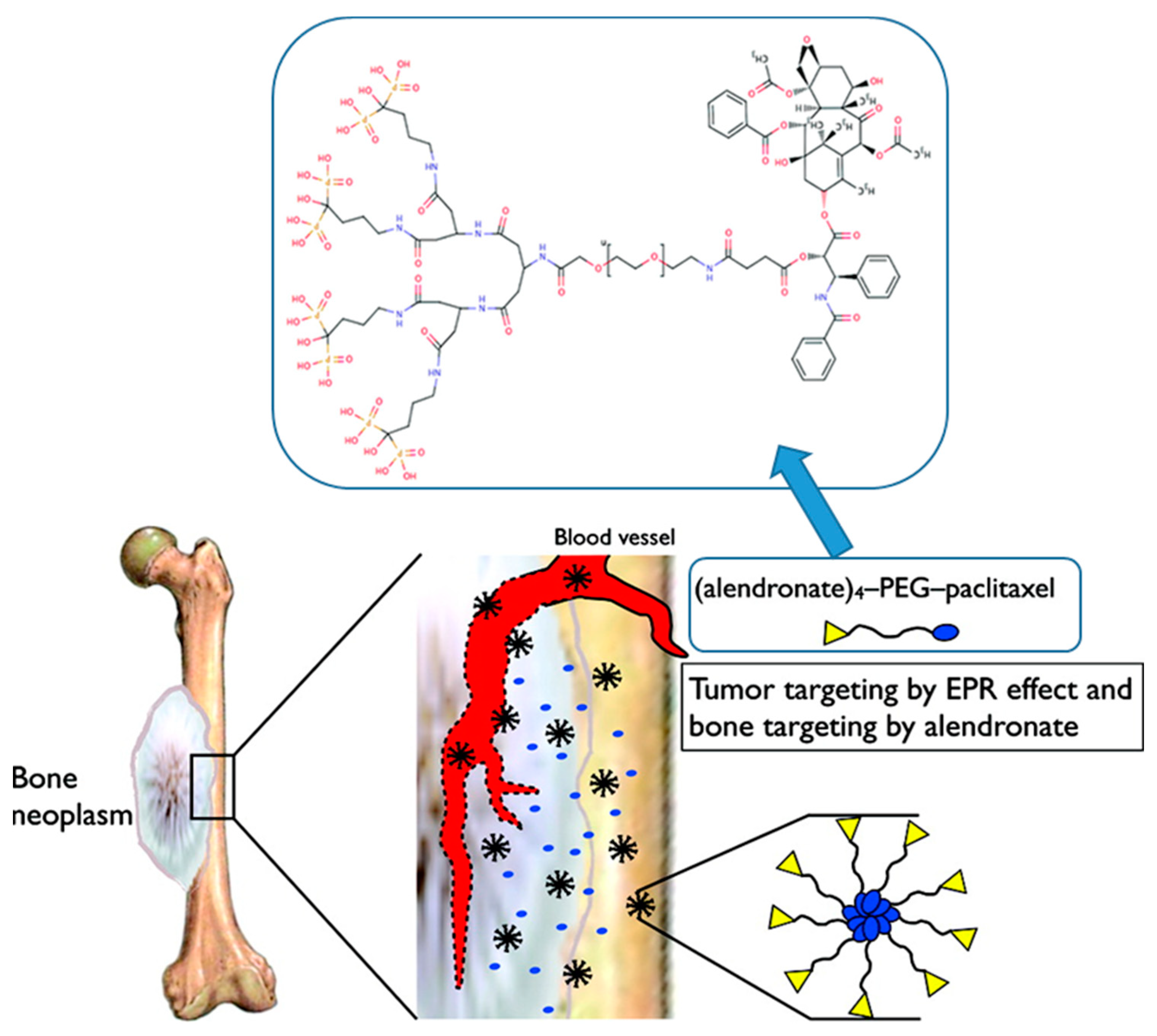
- Clementi, C.; Miller, K.; Mero, A.; Satchi-Fainaro, R.; Pasut, G. Dendritic Poly(ethylene glycol) Bearing Paclitaxel and Alendronate for Targeting Bone Neoplasms. Mol. Pharm. 2011, 8, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Clementi, C.; Polyak, D.; Eldar-Boock, A.; Benayoun, L.; Barshack, I.; Shaked, Y.; Pasut, G.; Satchi-Fainaro, R. Poly(ethylene glycol)-paclitaxel-alendronate self-assembled micelles for the targeted treatment of breast cancer bone metastases. Biomaterials 2013, 34, 3795–3806. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Hu, M.; He, F.; You, D.; Qian, Y.; Bi, Y. Dendritic poly(benzyl ether)-b-poly(N-vinylcaprolactam) block copolymers: Self-organization in aqueous media, thermoresponsiveness and biocompatibility. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 300–308. [Google Scholar] [CrossRef]
- Kempe, K.; Onbulak, S.; Schubert, U.S.; Sanyal, A.; Hoogenboom, R. pH degradable dendron-functionalized poly(2-ethyl-2-oxazoline) prepared by a cascade “double-click” reaction. Polym. Chem. 2013, 4, 3236. [Google Scholar] [CrossRef]
- Tomalia, D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Verma, I.M.; Somia, N. Gene therapy—Promises, problems and prospects. Nature 1997, 389, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Dufes, C.; Uchegbu, I.F.; Schatzlein, A.G. Dendrimers in Gene Delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Hu, K.; Cheng, Y. Tailoring the dendrimer core for efficient gene delivery. Acta Biomater. 2016, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.C.; Little, S.R.; Langer, R.; Hammond, P.T. A Family of Hierarchically Self-Assembling Linear-Dendritic Hybrid Polymers for Highly Efficient Targeted Gene Delivery. Angew. Chem. Int. Ed. 2005, 44, 6704–6708. [Google Scholar] [CrossRef] [PubMed]
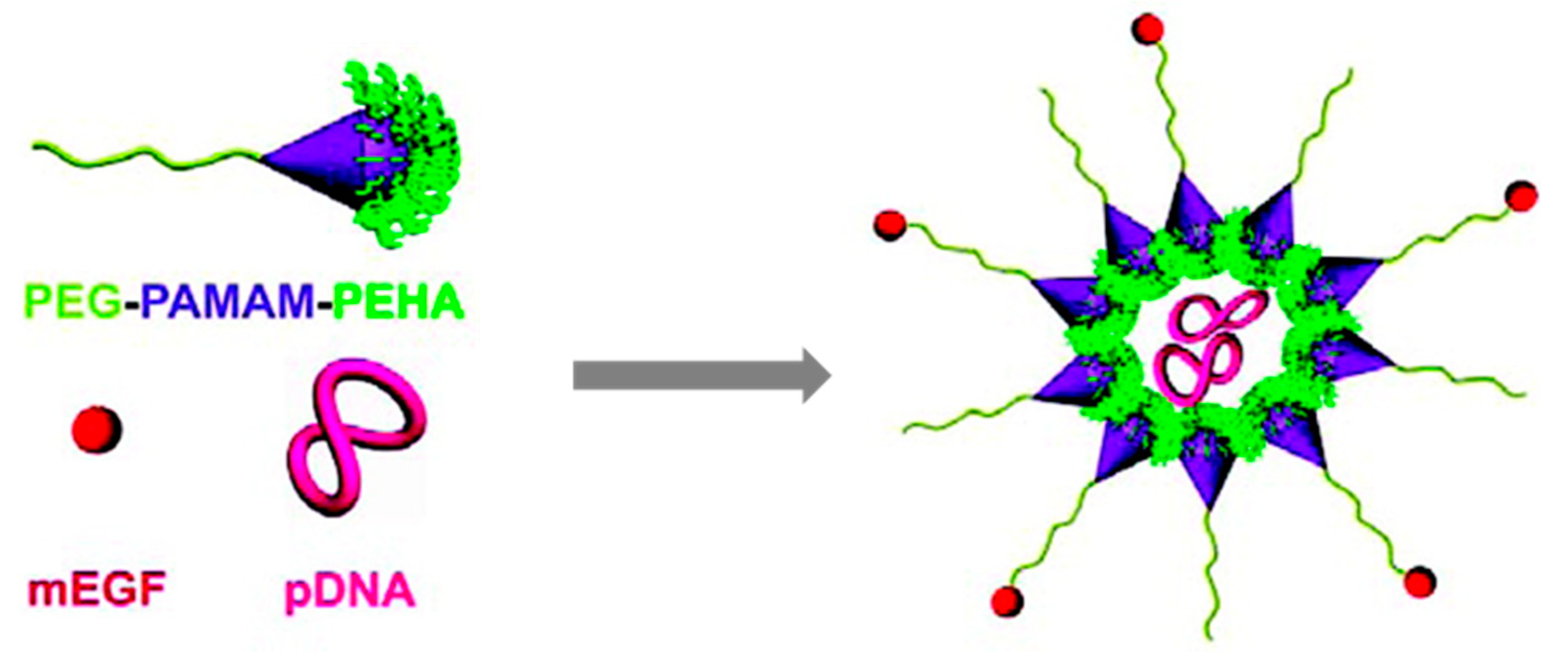
- Yu, H.; Nie, Y.; Dohmen, C.; Li, Y.; Wagner, E. Epidermal Growth Factor–PEG Functionalized PAMAM-Pentaethylenehexamine Dendron for Targeted Gene Delivery Produced by Click Chemistry. Biomacromolecules 2011, 12, 2039–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demattei, C.R.; Huang, B.; Tomalia, D.A. Designed Dendrimer Syntheses by Self-Assembly of Single-Site, ssDNA Functionalized Dendrons. Nano Lett. 2004, 4, 771–777. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Xie, Y.; An, Y.; Huang, Y.; Zhu, Z.; Yang, C.J. A Controllable Aptamer-Based Self-Assembled DNA Dendrimer for High Affinity Targeting, Bioimaging and Drug Delivery. Sci. Rep. 2015, 5, 10099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, H.-M.; Zhang, X.; Lv, Y.; Zhao, Z.; Wang, N.-N.; Fu, T.; Fan, H.; Liang, H.; Qiu, L.; Zhu, G.; et al. DNA Dendrimer: An Efficient Nanocarrier of Functional Nucleic Acids for Intracellular Molecular Sensing. ACS Nano 2014, 8, 6171–6181. [Google Scholar] [CrossRef] [PubMed]
- Lambrych, K.R.; Gitsov, I. Linear−Dendritic Poly(ester)-block-poly(ether)-b lock-poly(ester) ABA Copolymers Constructed by a Divergent Growth Method 1. Macromolecules 2003, 36, 1068–1074. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Hammond, P.T. Amphiphilic Linear-Dendritic Triblock Copolymers Composed of Poly(amidoamine) and Poly(propylene oxide) and Their Micellar-Phase and Encapsulation Properties. Langmuir 2006, 22, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
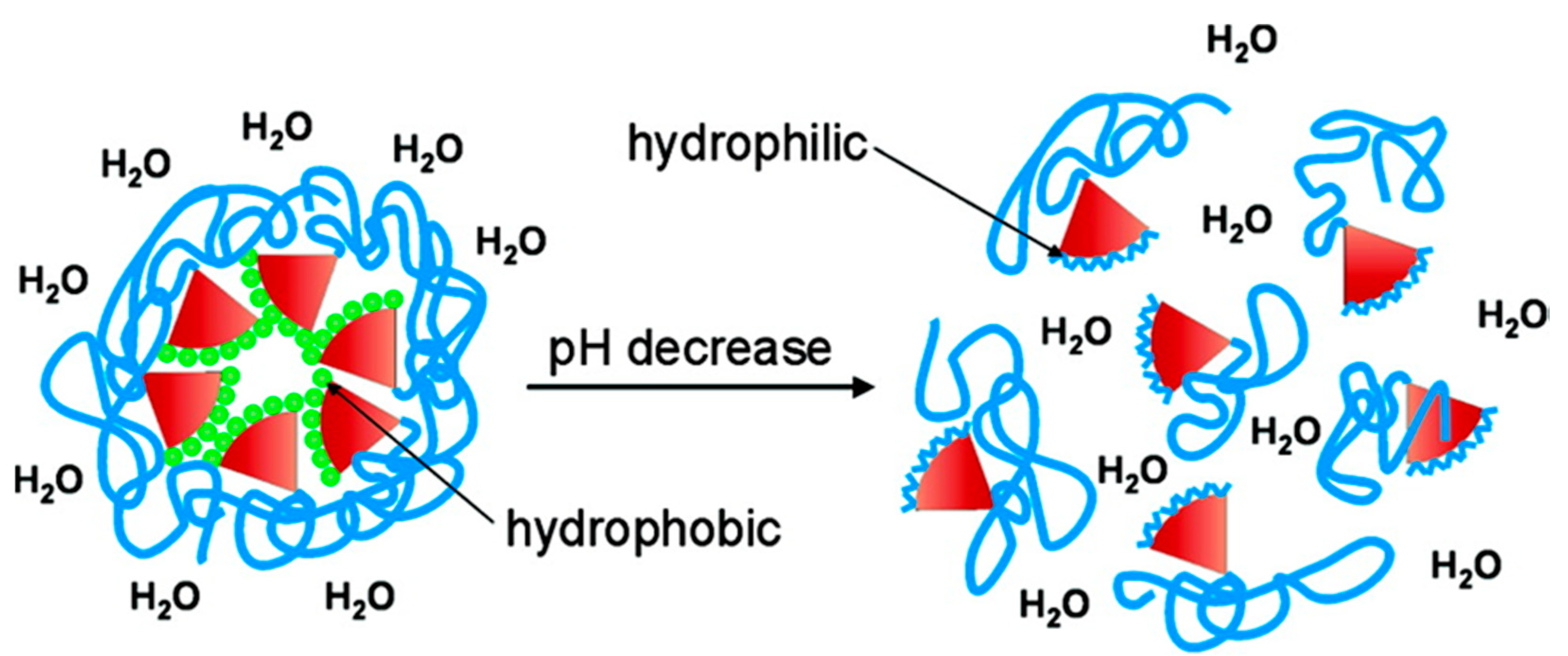
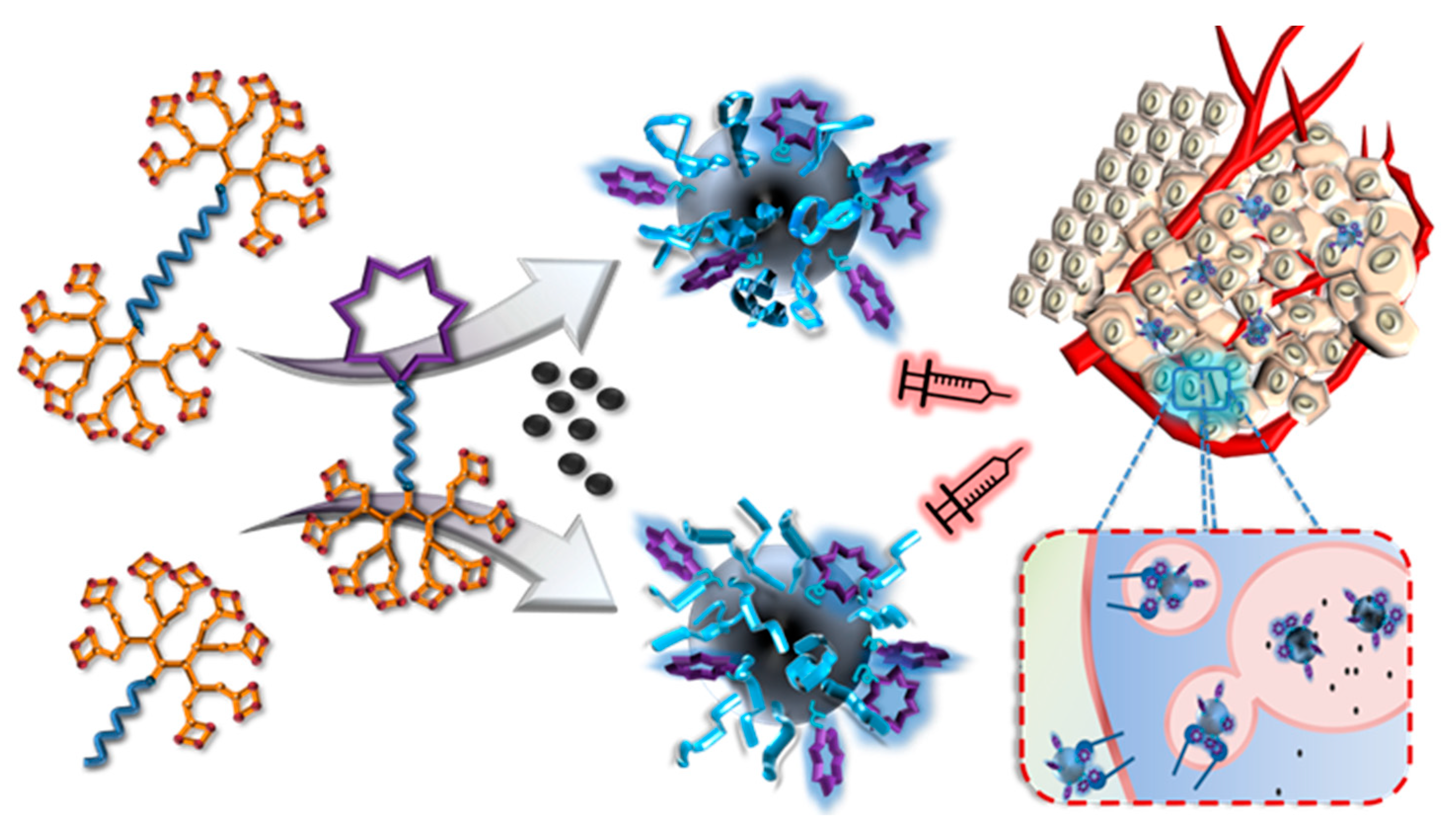
- Sumer Bolu, B.; Manavoglu Gecici, E.; Sanyal, R. Combretastatin A-4 Conjugated Antiangiogenic Micellar Drug Delivery Systems Using Dendron–Polymer Conjugates. Mol. Pharm. 2016, 13, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
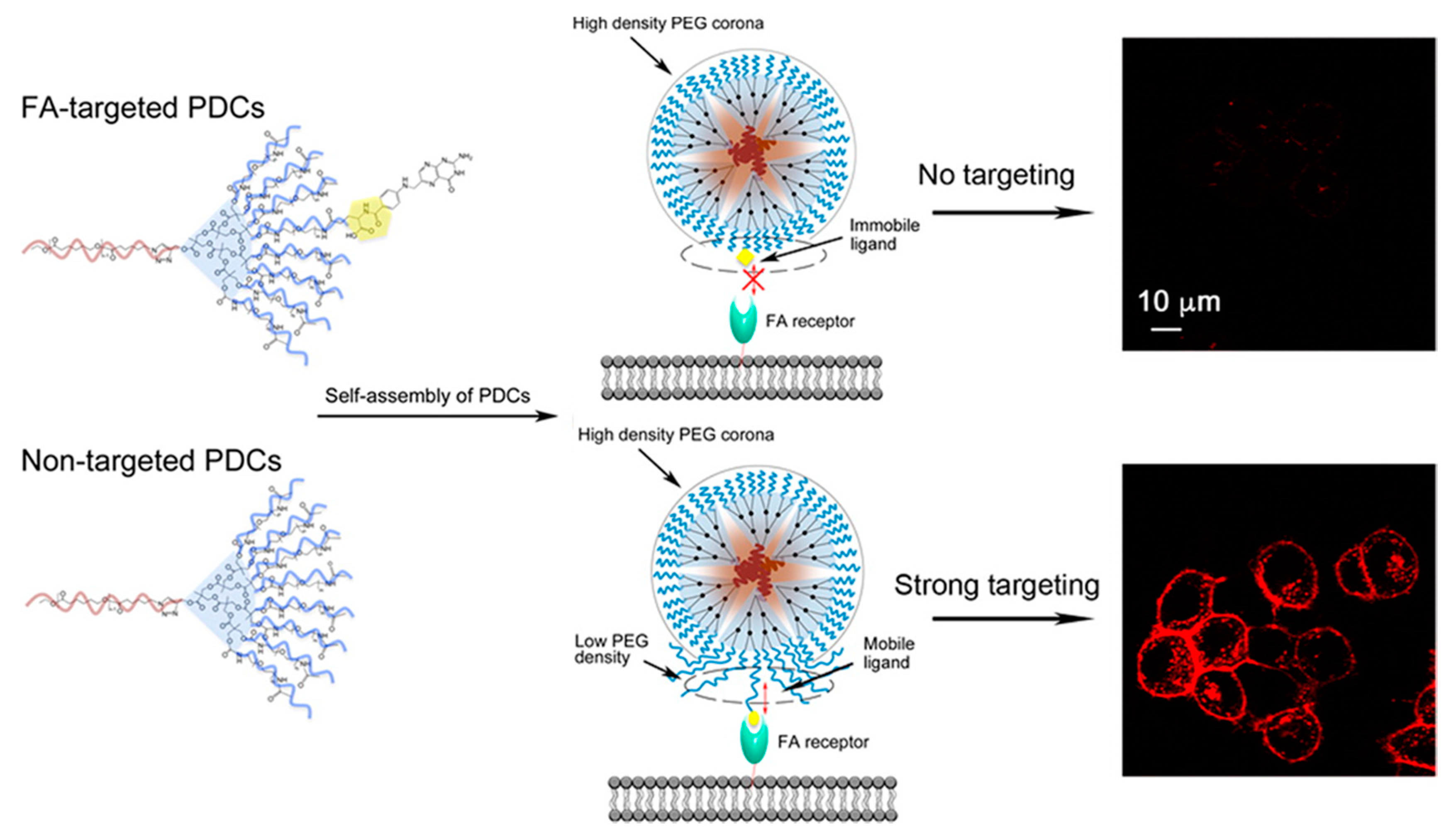
- Bolu, B.S.; Golba, B.; Boke, N.; Sanyal, A.; Sanyal, R. Designing Dendron-Polymer Conjugate Based Targeted Drug Delivery Platforms with a “mix-and-Match” Modularity. Bioconjug. Chem. 2017, 28, 2962–2975. [Google Scholar] [CrossRef] [PubMed]
- Movellan, J.; Urbán, P.; Moles, E.; de la Fuente, J.M.; Sierra, T.; Serrano, J.L.; Fernàndez-Busquets, X. Amphiphilic dendritic derivatives as nanocarriers for the targeted delivery of antimalarial drugs. Biomaterials 2014, 35, 7940–7950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svenson, S.; Chauhan, A.S. Dendrimers for enhanced drug solubilization. Nanomedicine 2008, 3, 679–702. [Google Scholar] [CrossRef] [PubMed]
- Esfand, R.; Beezer, A.E.; Mitchell, J.C.; Twyman, L.J. Synthesis, Complexation and Pharmaceutical Applications of Tetra-directional Cascade Dendrimers. Pharm. Pharmacol. Commun. 1996, 2, 157–159. [Google Scholar] [CrossRef]
- Morgan, M.T.; Nakanishi, Y.; Kroll, D.J.; Griset, A.P.; Carnahan, M.A.; Wathier, M.; Oberlies, N.H.; Manikumar, G.; Wani, M.C.; Grinstaff, M.W. Dendrimer-Encapsulated Camptothecins: Increased Solubility, Cellular Uptake, and Cellular Retention Affords Enhanced Anticancer Activity In vitro. Cancer Res. 2006, 66, 11913–11921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patri, A.K.; Kukowskalatallo, J.; Baker, J. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 2005, 57, 2203–2214. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, L.M.; McLeod, V.M.; Porter, C.J.H.; Boyd, B.J. Association of Chemotherapeutic Drugs with Dendrimer Nanocarriers: An Assessment of the Merits of Covalent Conjugation Compared to Noncovalent Encapsulation. Mol. Pharm. 2012, 9, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Kono, K.; Fréchet, J.M. Water-soluble dendritic unimolecular micelles: Their potential as drug delivery agents. J. Control. Release 2000, 65, 121–131. [Google Scholar] [CrossRef]
- Kojima, C.; Kono, K.; Maruyama, K.; Takagishi, T. Synthesis of Polyamidoamine Dendrimers Having Poly(ethylene glycol) Grafts and Their Ability To Encapsulate Anticancer Drugs. Bioconjug. Chem. 2000, 11, 910–917. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.; Ginski, M.; Rhodes, C.; Ghandehari, H. Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J. Control. Release 2002, 81, 355–365. [Google Scholar] [CrossRef]
- Luo, D.; Haverstick, K.; Belcheva, N.; Han, E.; Saltzman, W.M. Poly(ethylene glycol)-Conjugated PAMAM Dendrimer for Biocompatible, High-Efficiency DNA Delivery. Macromolecules 2002, 35, 3456–3462. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, U.; Asthana, A.; Jain, N.K. Folate and Folate−PEG−PAMAM Dendrimers: Synthesis, Characterization, and Targeted Anticancer Drug Delivery Potential in Tumor Bearing Mice. Bioconjug. Chem. 2008, 19, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bronich, T.K.; Kabanov, A.V.; Rauh, R.D.; Roovers, J. Synthesis and Evaluation of a Star Amphiphilic Block Copolymer from Poly(ε-caprolactone) and Poly(ethylene glycol) as a Potential Drug Delivery Carrier. Bioconjug. Chem. 2005, 16, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Bronich, T.K.; Kabanov, A.V.; Rauh, R.D.; Roovers, J. Synthesis and Characterization of Star Poly(ε-caprolactone)-b-Poly(ethylene glycol) and Poly(l-lactide)-b-Poly(ethylene glycol) Copolymers: Evaluation as Drug Delivery Carriers. Bioconjug. Chem. 2008, 19, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
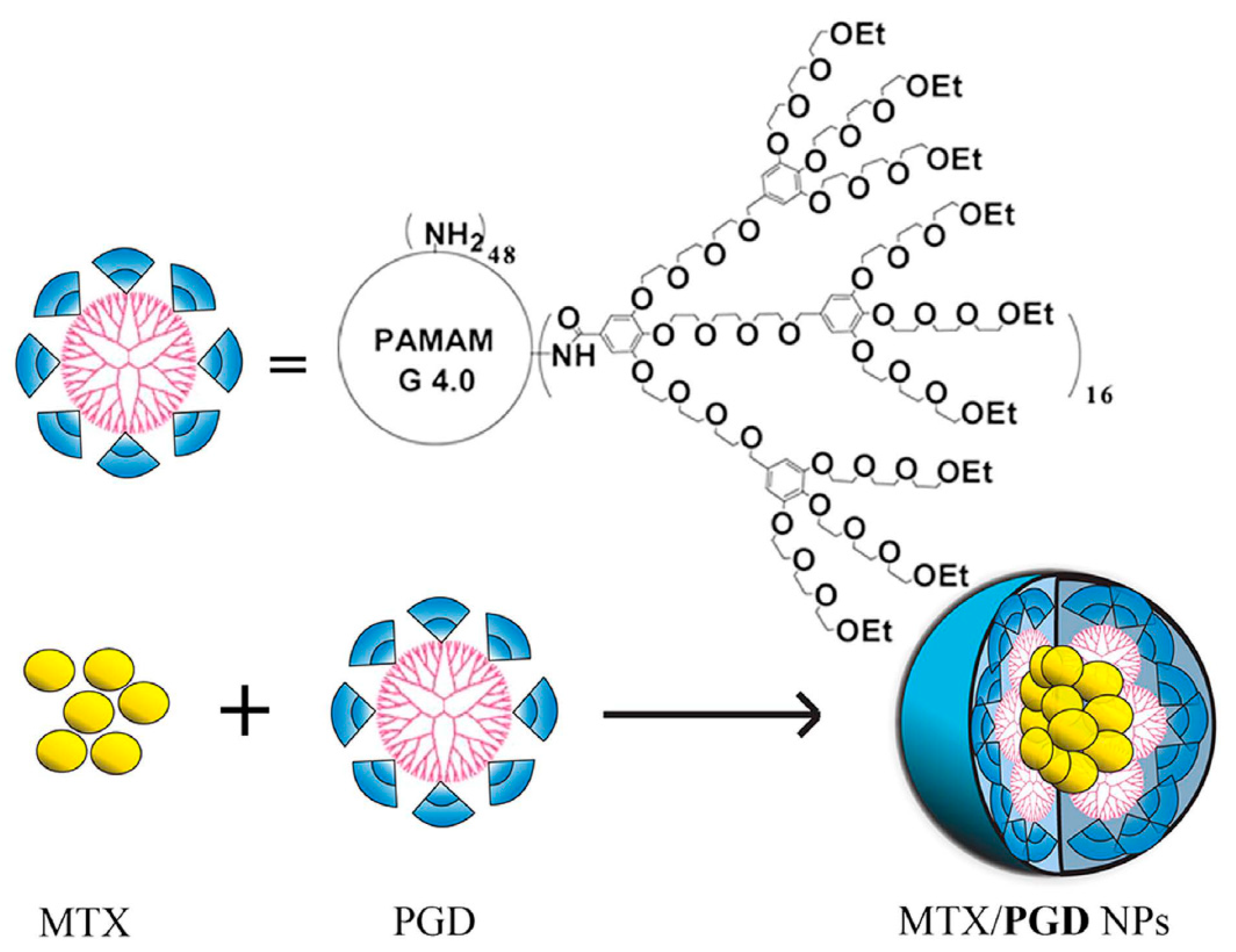
- Guo, Y.; Zhao, Y.; Zhao, J.; Han, M.; Zhang, A.; Wang, X. Codendrimer from polyamidoamine (PAMAM) and oligoethylene dendron as a thermosensitive drug carrier. Bioconjug. Chem. 2014, 25, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, J.; Li, R.; Han, M.; Zhu, C.; Wang, M.; Guo, Y.; Wang, X. A series of codendrimers from polyamidoamine (PAMAM) and oligoethylene glycols (OEG) dendrons as drug carriers: The effect of OEG dendron decoration degree. RSC Adv. 2015, 5, 85547–85555. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Y.; Li, R.; Wang, T.; Han, M.; Zhu, C.; Wang, X. Methotrexate Nanoparticles Prepared with Codendrimer from Polyamidoamine (PAMAM) and Oligoethylene Glycols (OEG) Dendrons: Antitumor Efficacy in Vitro and in Vivo. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
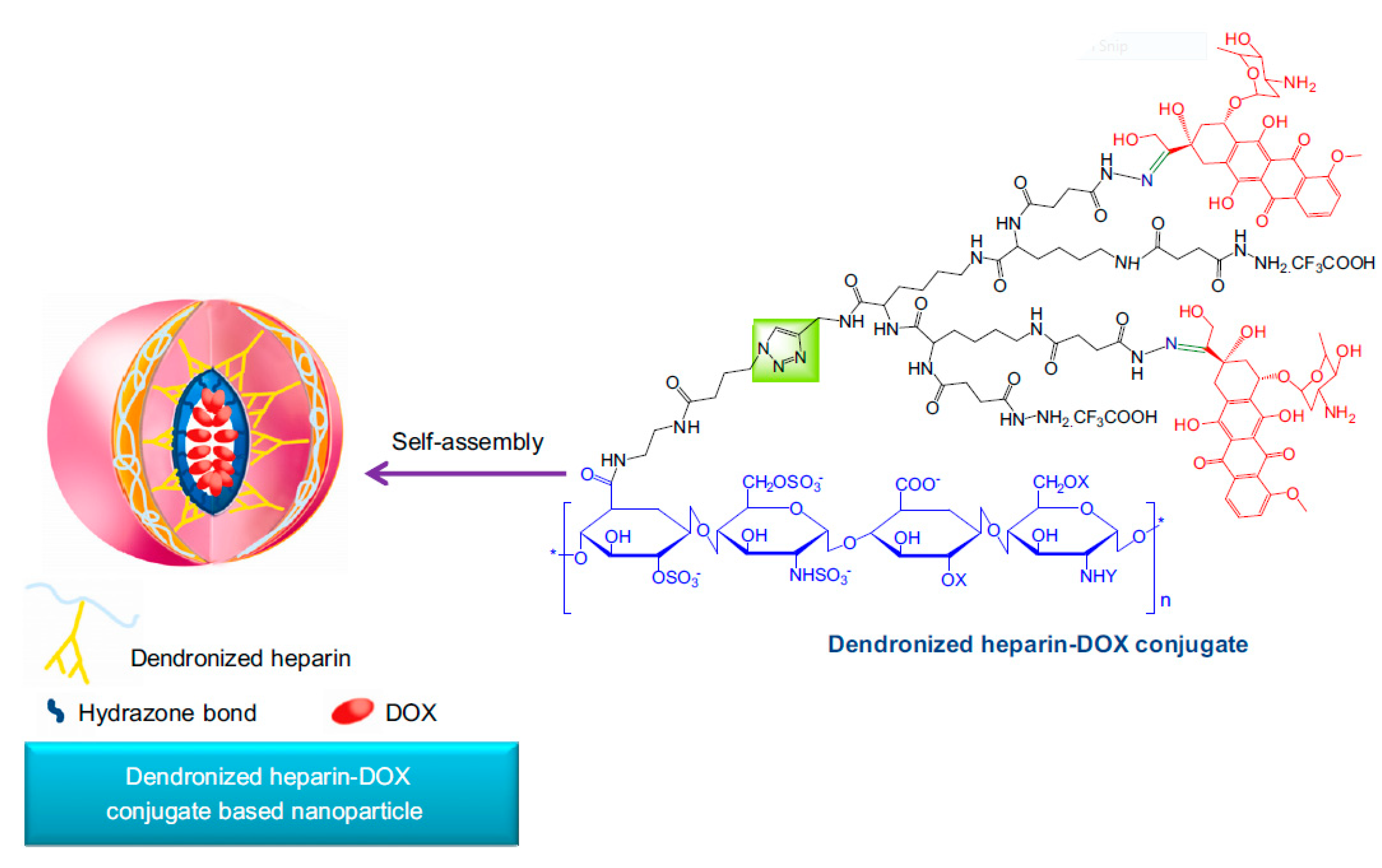
- She, W.; Li, N.; Luo, K.; Guo, C.; Wang, G.; Geng, Y.; Gu, Z. Dendronized heparin-doxorubicin conjugate based nanoparticle as pH-responsive drug delivery system for cancer therapy. Biomaterials 2013, 34, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhou, J.; Wali, A.R.M.; He, Y.; Xu, X.; Tang, J.Z.; Gu, Z. Self-assembly of pH-sensitive fluorinated peptide dendron functionalized dextran nanoparticles for on-demand intracellular drug delivery. J. Mater. Sci. Mater. Med. 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- She, W.; Luo, K.; Zhang, C.; Wang, G.; Geng, Y.; Li, L.; He, B.; Gu, Z. The potential of self-assembled, pH-responsive nanoparticles of mPEGylated peptide dendron-doxorubicin conjugates for cancer therapy. Biomaterials 2013, 34, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
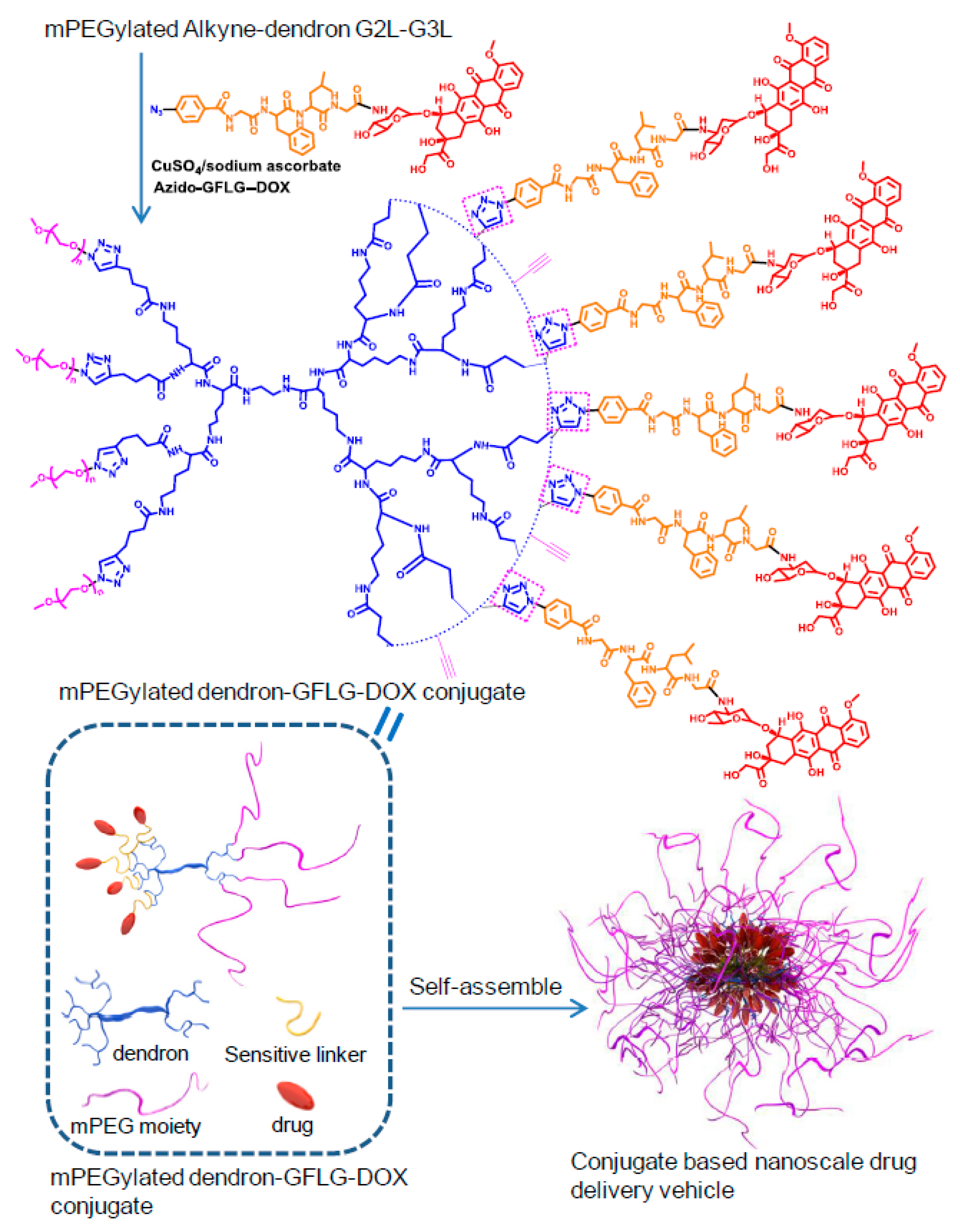
- Li, N.; Li, N.; Yi, Q.; Luo, K.; Guo, C.; Pan, D.; Gu, Z. Amphiphilic peptide dendritic copolymer-doxorubicin nanoscale conjugate self-assembled to enzyme-responsive anti-cancer agent. Biomaterials 2014, 35, 9529–9545. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.M.; Sloane, B.F. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer 2006, 6, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Little, H.C.; Tiambeng, T.N.; Williams, G.A.; Guan, Z. Multifunctional Dendronized Peptide Polymer Platform for Safe and Effective siRNA Delivery. J. Am. Chem. Soc. 2013, 135, 4962–4965. [Google Scholar] [CrossRef] [PubMed]
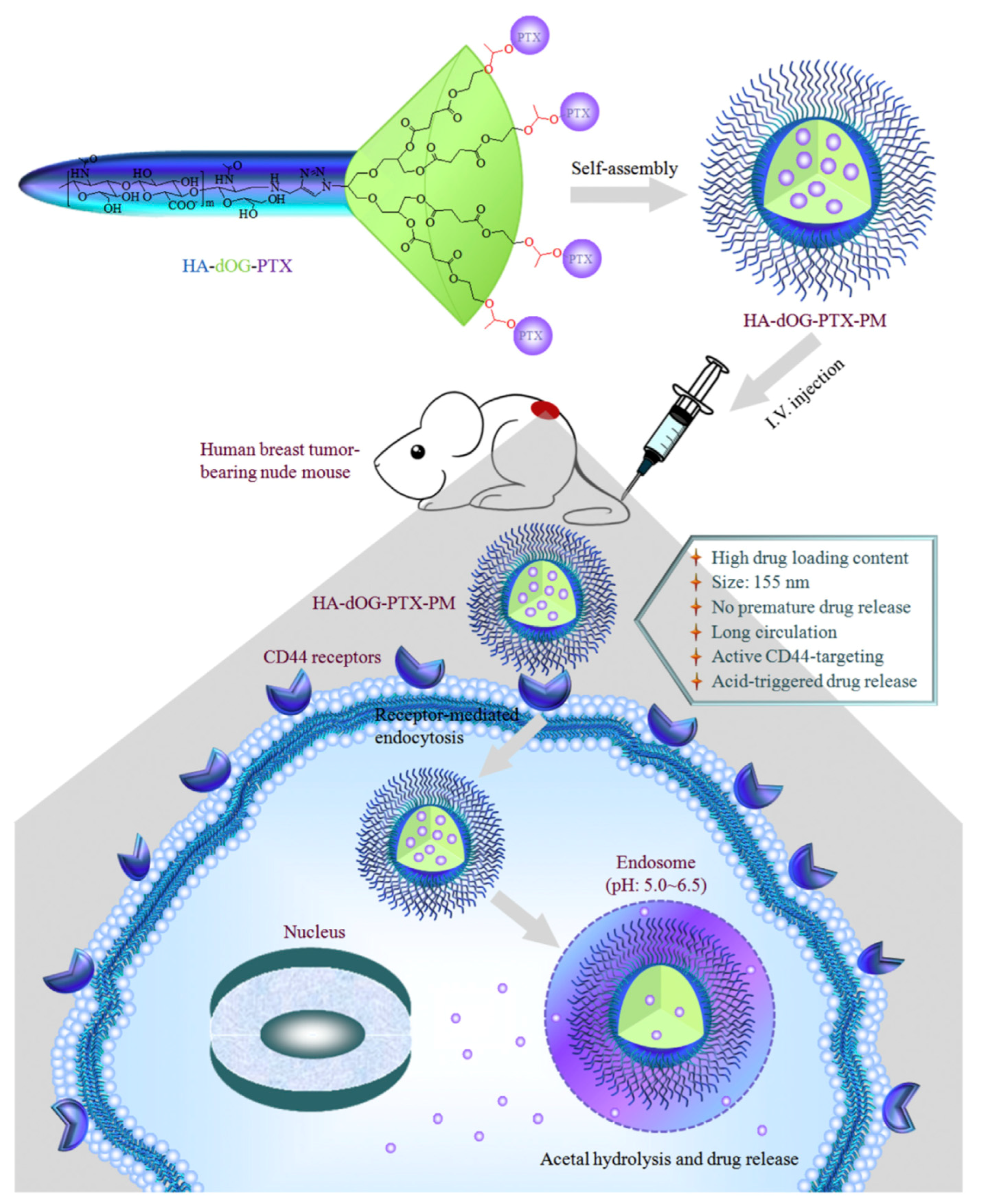
- Zhong, Y.; Goltsche, K.; Cheng, L.; Xie, F.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials 2016, 84, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Schade, B.; Kumar, S.; Böttcher, C.; Sharma, S.K.; Haag, R. Non-ionic Dendronized Multiamphiphilic Polymers as Nanocarriers for Biomedical Applications. Small 2013, 9, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Gupta, S.; Achazi, K.; Böttcher, C.; Khandare, J.; Sharma, S.K.; Haag, R. Dendronized Multifunctional Amphiphilic Polymers as Efficient Nanocarriers for Biomedical Applications. Macromol. Rapid Commun. 2015, 36, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Parshad, B.; Kumari, M.; Achazi, K.; Bӧttcher, C.; Haag, R.; Sharma, S. Chemo-Enzymatic Synthesis of Perfluoroalkyl-Functionalized Dendronized Polymers as Cyto-Compatible Nanocarriers for Drug Delivery Applications. Polymers 2016, 8, 311. [Google Scholar] [CrossRef]
- Viswanathan, G.; Hsu, Y.-H.; Voon, S.H.; Imae, T.; Siriviriyanun, A.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Yusa, S. A Comparative Study of Cellular Uptake and Subcellular Localization of Doxorubicin Loaded in Self-Assemblies of Amphiphilic Copolymers with Pendant Dendron by MDA-MB-231 Human Breast Cancer Cells. Macromol. Biosci. 2016, 16, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Voon, S.H.; Kue, C.S.; Imae, T.; Saw, W.S.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Yusa, S. ichi Doxorubicin-loaded micelles of amphiphilic diblock copolymer with pendant dendron improve antitumor efficacy: In vitro and in vivo studies. Int. J. Pharm. 2017, 534, 136–143. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolu, B.S.; Sanyal, R.; Sanyal, A. Drug Delivery Systems from Self-Assembly of Dendron-Polymer Conjugates †. Molecules 2018, 23, 1570. https://doi.org/10.3390/molecules23071570
Bolu BS, Sanyal R, Sanyal A. Drug Delivery Systems from Self-Assembly of Dendron-Polymer Conjugates †. Molecules. 2018; 23(7):1570. https://doi.org/10.3390/molecules23071570
Chicago/Turabian StyleBolu, Burcu Sumer, Rana Sanyal, and Amitav Sanyal. 2018. "Drug Delivery Systems from Self-Assembly of Dendron-Polymer Conjugates †" Molecules 23, no. 7: 1570. https://doi.org/10.3390/molecules23071570