Iron Oxide Nanoparticles in Photothermal Therapy
Abstract
:1. Introduction
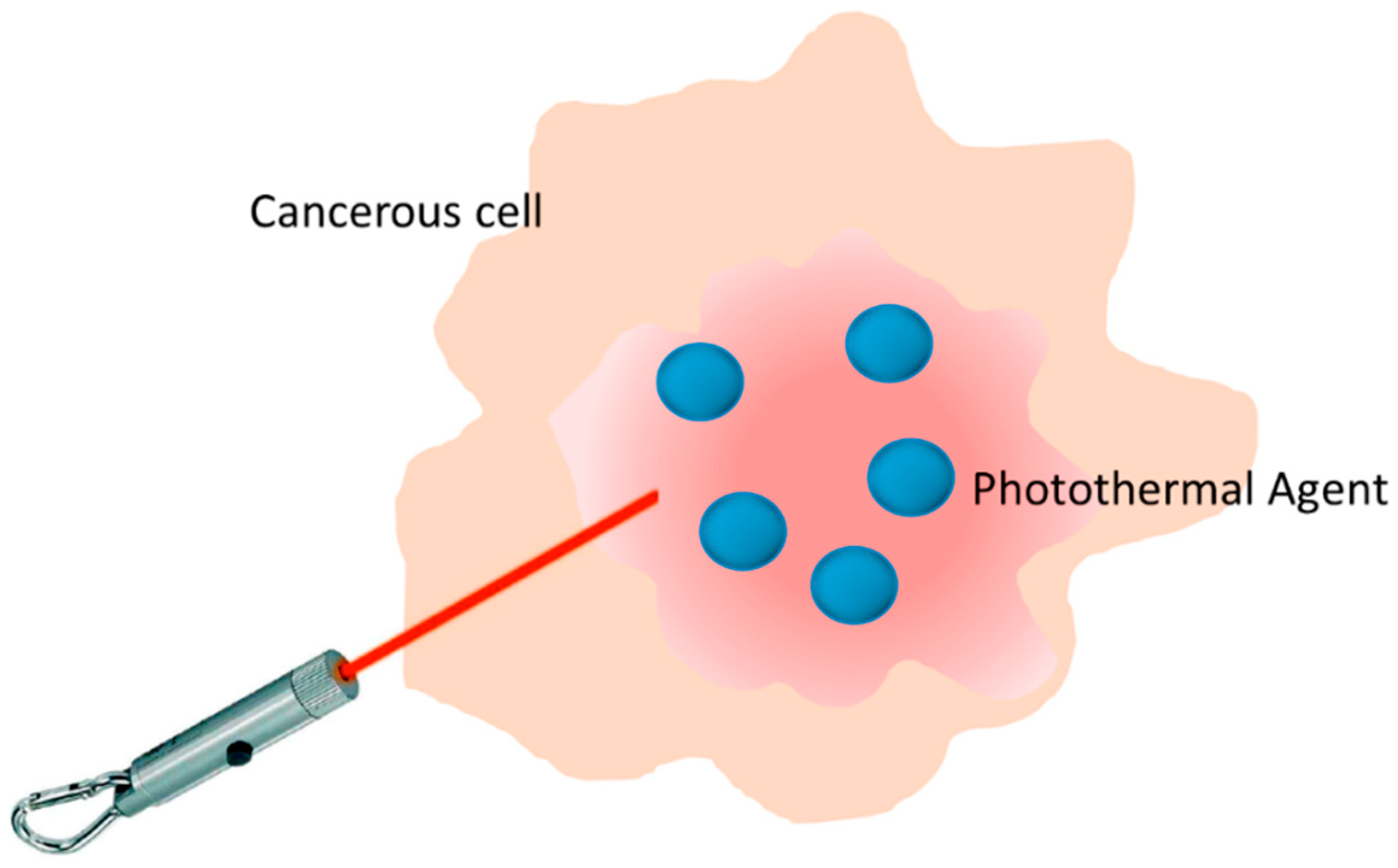
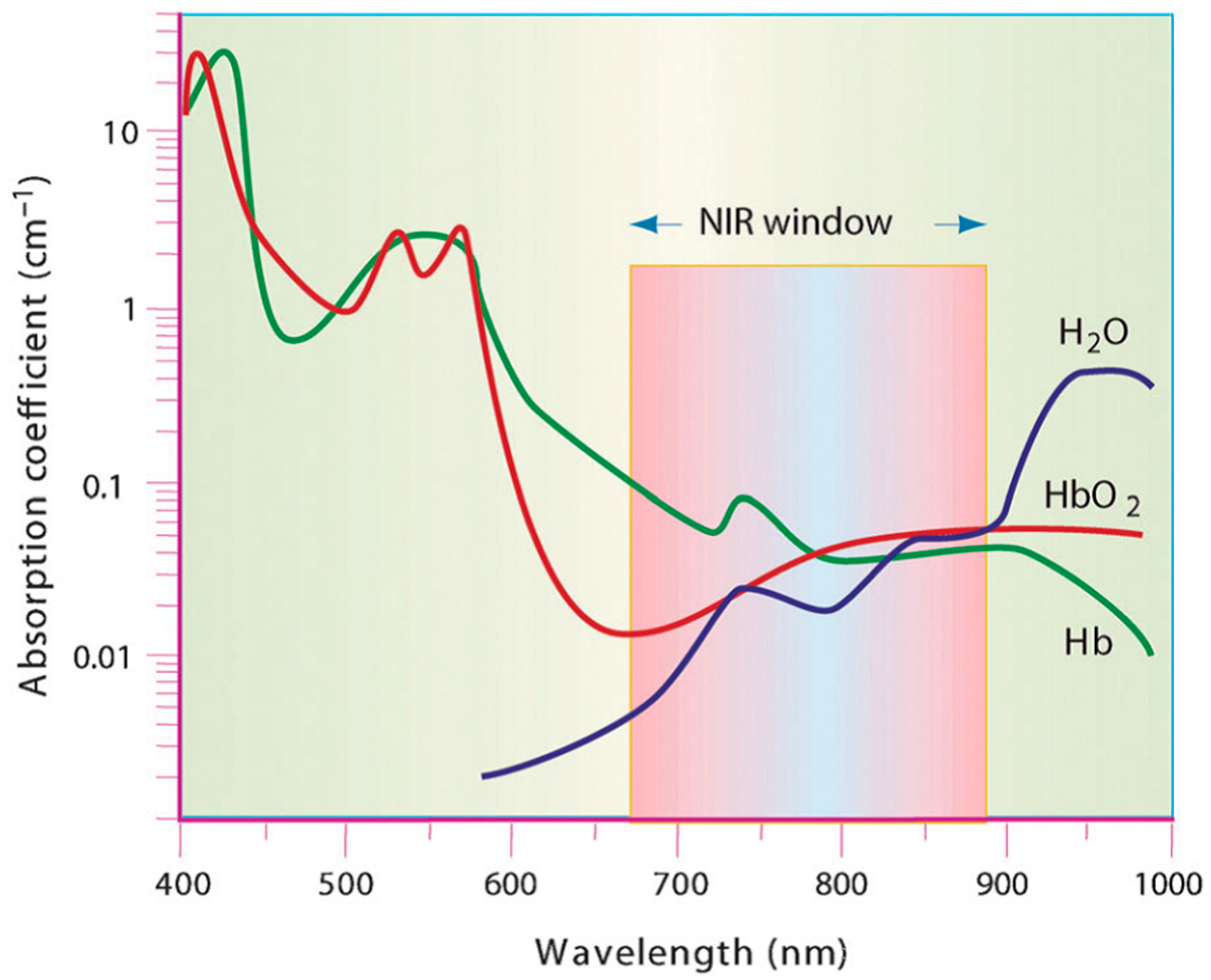
1.1. Photothermal Agents
1.2. Magnetic Nanoparticles: Iron Oxides
2. Iron Oxide Nanoparticles for Photothermal Therapy
2.1. Hybrid Nanoparticles Formed with Iron Oxide Nanoparticles
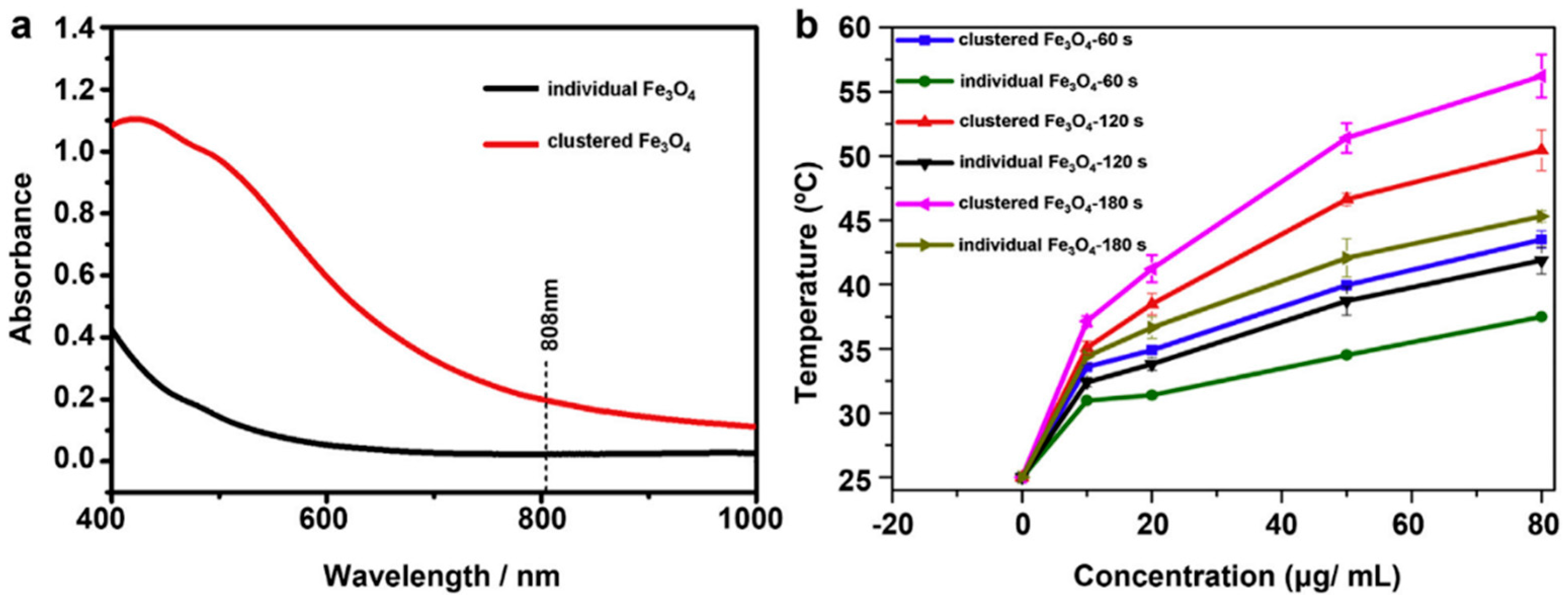
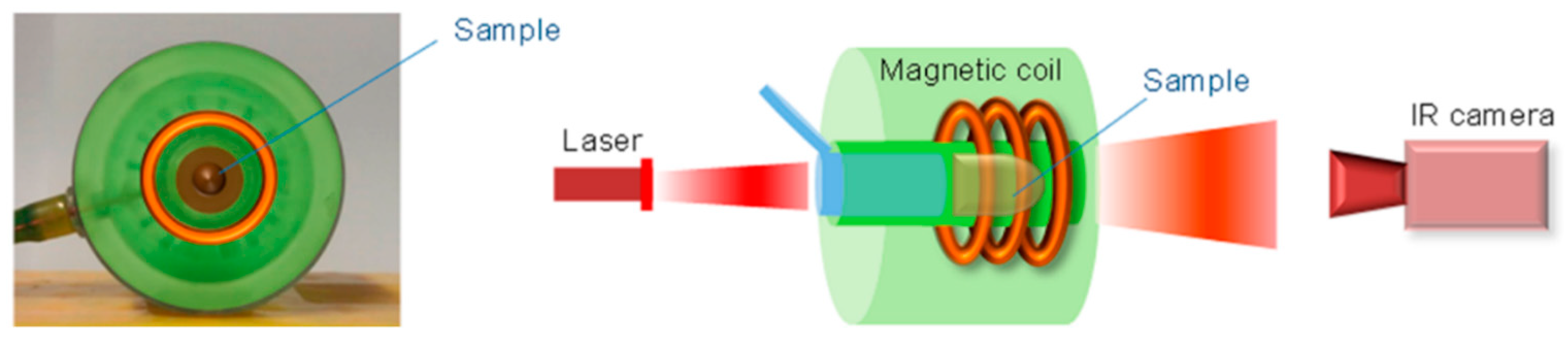
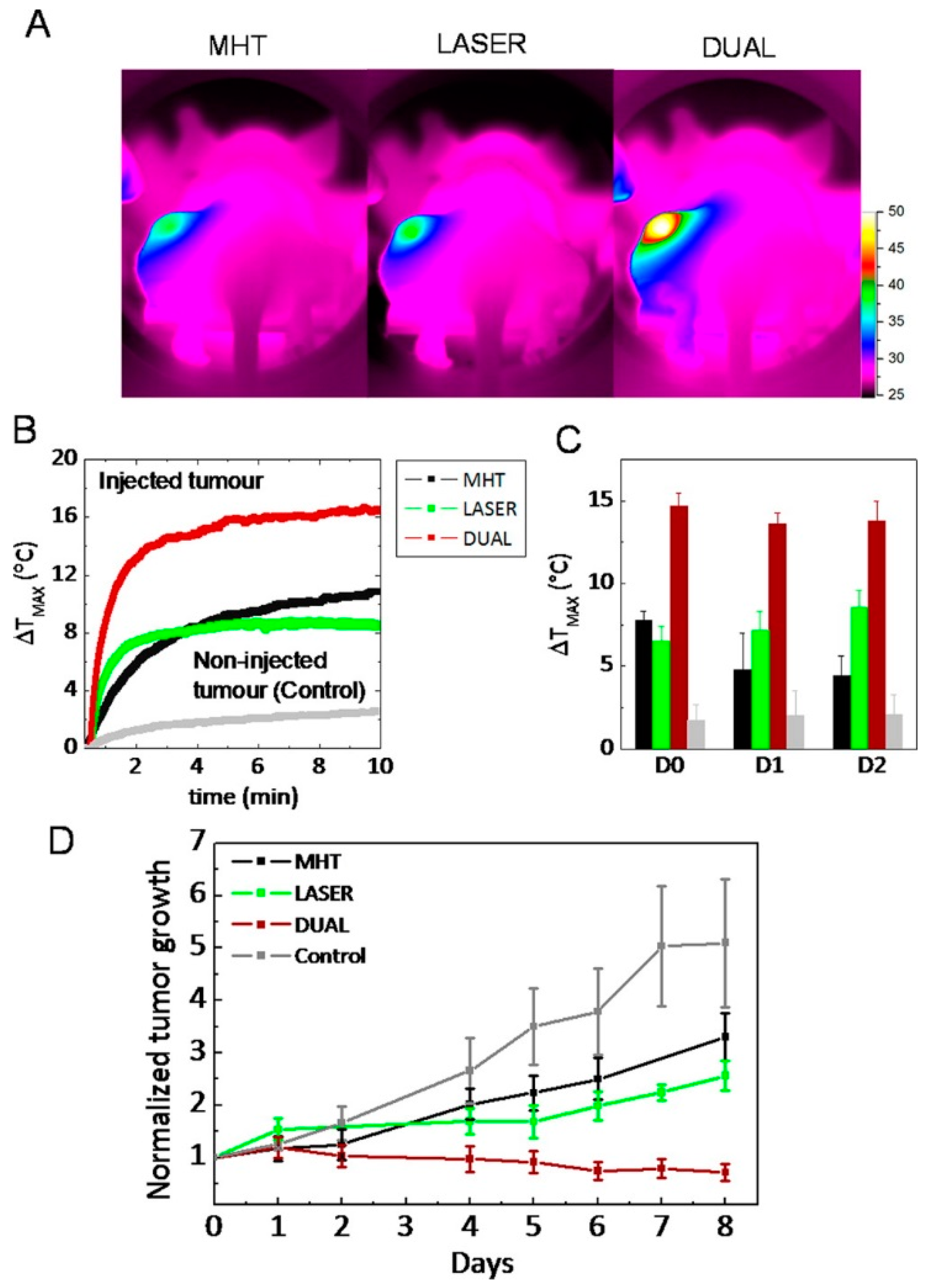
2.2. Iron Oxide Nanoparticles as Photothermal Agents Themselves
3. Conclusions and Perspectives
Author Contributions
Conflicts of Interest
Abbreviations
| ACB | accelerated blood clearance |
| AMF | alternating magnetic field |
| BSA | Bovine serum albumin |
| CNP | Cluster-structured nanoparticles |
| DMSA | meso-2,3-dimercaptosuccinic acid |
| DOX | doxorubicin |
| DSPE | distearoyl-sn-glycero-3-phosphoetanolamine |
| EGFR | epidermal growth factor receptor |
| EPR | enhanced permeability and retention |
| FDA | Food and Drug Administration |
| HSA | human serum albumin |
| ICG | indocyanine green |
| ION | iron oxide nanoparticle |
| LSPR | local surface plasmon resonance |
| MRI | magnetic resonance imaging |
| NIR | near-infrared |
| PA | photothermal agent |
| PDT | photodynamic therapy |
| PEG | poly(ethylene) glycol |
| PPY | polypyrrole |
| PTT | photothermal therapy |
| RBC | red blood cells |
References
- Saiyed, Z.; Telang, S.; Ramchand, C. Application of magnetic techniques in the field of drug discovery and biomedicine. Biomagn. Res. Technol. 2003, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 1: An introduction to thermal therapy. Crit. Rev. Biomed. Eng. 2006, 34, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Martinez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martin Rodriguez, E.; Garcia Sole, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.P.; Elliott, A.; Ji, X.; Shetty, A.; Yang, Z.; Tian, M.; Taylor, B.; Stafford, R.J.; Li, C. Theranostics with multifunctional magnetic gold nanoshells: Photothermal therapy and t2* magnetic resonance imaging. Investig. Radiol. 2011, 46, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, B.B.; Ritz, J.P.; Albrecht, T.; Valdeig, S.; Schenk, A.; Wolf, K.-J.; Lehmann, K. Influence of intrahepatic vessels on volume and shape of percutaneous thermal ablation zone: In vivo evaluation in a porcine model. Investig. Radiol. 2008, 43, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Melamed, J.R.; Edelstein, R.S.; Day, E.S. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 2015, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, M.; Del Pino, P.; Mitchell, S.G.; Moros, M.; Stepien, G.; Pelaz, B.; Parak, W.J.; Galvez, E.M.; Pardo, J.; de la Fuente, J.M. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano 2015, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, Y.; Yan, Y.; Zhang, Q.; Cheng, Y. Dendrimer-templated ultrasmall and multifunctional photothermal agents for efficient tumor ablation. ACS Nano 2016, 10, 4863–4872. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, S.; Ren, F.; Chen, L.; Zeng, J.; Zhu, M.; Cheng, Z.; Gao, M.; Li, Z. Ultrasmall magnetic CuFeSe2 ternary nanocrystals for multimodal imaging guided photothermal therapy of cancer. ACS Nano 2017, 11, 5633–5645. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis, mechanisms, characterization and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Pauli, J.; Vag, T.; Haag, R.; Spieles, M.; Wenzel, M.; Kaiser, W.A.; Resch-Genger, U.; Hilger, I. An in vitro characterization study of new near infrared dyes for molecular imaging. Eur. J. Med. Chem. 2009, 44, 3496–3503. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Stylianopoulos, T.; Cui, J.; Martin, J.; Chauhan, V.P.; Jiang, W.; Popovic, Z.; Jain, R.K.; Bawendi, M.G.; Fukumura, D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 2426–2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Sun, X.; Ma, X.; Zhou, Z.; Jin, E.; Zhang, B.; Shen, Y.; Van Kirk, E.A.; Murdoch, W.J.; Lott, J.R.; et al. Integration of nanoassembly functions for an effective delivery cascade for cancer drugs. Adv. Mater. 2014, 26, 7615–7621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Chen, C. Near-infrared light-mediated nanoplatforms for cancer thermo-chemotherapy and optical imaging. Adv. Mater. 2013, 25, 3869–3880. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Ji, C.; Shi, J.; Pridgen, E.M.; Frieder, J.; Wu, J.; Farokhzad, O.C. DNA self-assembly of targeted near-infrared-responsive gold nanoparticles for cancer thermo-chemotherapy. Angew. Chem. Int. Ed. Engl. 2012, 51, 11853–11857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, V.; Chien, Y.-H.; Cheng, Y.-S.; Liu, T.-Y.; Huang, C.-C.; Su, C.-H.; Chen, Y.-S.; Kumar, U.; Hsu, H.-F.; Yeh, C.-S. Oligonucleotides-assembled Au nanorod-assisted cancer photothermal ablation and combination chemotherapy with targeted dual-drug delivery of doxorubicin and cisplatin prodrug. ACS Appl. Mater. Interfaces 2014, 6, 4382–4393. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yan, D.D.; Yang, D.; Li, Y.; Wang, X.; Zalewski, O.; Yan, B.; Lu, W. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano 2014, 8, 5670–5681. [Google Scholar] [CrossRef] [PubMed]
- Rengan, A.K.; Bukhari, A.B.; Pradhan, A.; Malhotra, R.; Banerjee, R.; Srivastava, R.; De, A.I. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015, 15, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Riedinger, A.; Li, H.; Fu, C.; Liu, H.; Li, L.; Liu, T.; Tan, L.; Barthel, M.J.; Pugliese, G.; et al. Plasmonic copper sulfide nanocrystals exhibiting near-infrared photothermal and photodynamic therapeutic effects. ACS Nano 2015, 9, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Sanchez-Martin, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef]
- Armijo, L.M.; Brandt, Y.I.; Mathew, D.; Yadav, S.; Maestas, S.; Rivera, A.C.; Cook, N.C.; Withers, N.J.; Smolyakov, G.A.; Adolphi, N.L.; et al. Iron oxide nanocrystals for magnetic hyperthermia applications. Nanomaterials 2012, 2, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Deng, Z.S.; Liu, J. Areview of hyperthermia combined with radiotherapy/chemotherapy on malignant tumors. Crit. Rev. Biomed. Eng. 2010, 38, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Liao, B.J.; Chiang, C.S.; Chen, P.J.; Chen, I.W.; Chen, S.Y. Core-shell nanocapsules stabilized by single-component polymer and nanoparticles for magneto-chemotherapy/hyperthermia with multiple drugs. Adv. Mater. 2012, 24, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect for photoacoustic imaging and photothermal therapy. Angew. Chem. Int. Ed. Engl. 2013, 52, 13958–13964. [Google Scholar] [CrossRef] [PubMed]
- Larson, T.A.; Bankson, J.; Aaron, J.; Sokolov, K. Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells. Nanotechnology 2007, 18, 325101. [Google Scholar] [CrossRef]
- Ji, X.; Shao, R.; Elliott, A.M.; Stafford, R.J.; Esparza-Coss, E.; Bankson, J.A.; Liang, G.; Luo, Z.; Park, K.; Markert, J.T.; et al. Bifunctional gold nanoshells with a superparamagnetic iron oxide-silica core suitable for both MR imaging and photothermal therapy. J. Phys. Chem. C Nanomater. Interfaces 2007, 111, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Wang, X.; Liu, R.; Zhang, H.; Liu, X.; Zhang, Y. Facile synthesis of multifunctional Fe3O4@SiO2@Au magnet-plasmonic nanoparticles for MR/CT dual imaging and photothermal therapy. RSC Adv. 2017, 7, 18844–18850. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, M.; Wu, J.; Li, C.; Shen, D.; Yang, D.; Li, L.; Ge, M.; Chang, Z.; Dong, W. Core-shell magnetic gold nanoparticles for magnetic field-enhanced radio-photothermal therapy in cervical cancer. Nanomaterials 2017, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Pan, U.N.; Khandelia, R.; Sanpui, P.; Das, S.K.; Paul, A.; Chattopadhyay, A. Protein-based multifunctional nanocarriers for imaging, photothermal therapy, and anticancer drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 19495–19501. [Google Scholar] [CrossRef] [PubMed]
- Kirui, D.K.; Rey, D.A.; Batt, C.A. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging. Nanotechnology 2010, 21, 105105. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S.; Thompson, S.A.; Wark, A.W.; de la Rica, R. Gold suprashells: Enhanced photothermal nanoheaters with multiple localized surface plasmon resonances for broadband surface-enhanced Raman scattering. J. Phys. Chem. C Nanomater. Interfaces 2017, 121, 7404–7411. [Google Scholar] [CrossRef]
- Dong, W.; Li, Y.; Niu, D.; Ma, Z.; Gu, J.; Chen, Y.; Zhao, W.; Liu, X.; Liu, C.; Shi, J. Facile synthesis of monodisperse superparamagnetic Fe3O4 Core@hybrid@Au shell nanocomposite for bimodal imaging and photothermal therapy. Adv. Mater. 2011, 23, 5392–5397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ge, J.; Goebl, J.; Hu, Y.; Sun, Y.; Yin, Y. Tailored synthesis of superparamagnetic gold nanoshells with tunable optical properties. Adv. Mater. 2010, 22, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Meng, L.; Niu, L.; Lu, Q. Facile synthesis of superparamagnetic Fe3O4@polyphosphazene@Au shells for magnetic resonance imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2013, 5, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Young, J.K.; Nixon, A.V.; Drezek, R.A. Encapsulated Fe3O4/Ag complexed cores in hollow gold nanoshells for enhanced theranostic magnetic resonance imaging and photothermal therapy. Small 2014, 10, 3246–3251. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.F.; Hsu, C.; Yeh, C.S.; Hsiao, Y.J.; Su, C.H.; Wang, L.F. Tuning the distance of rattle-shaped IONP@shell-in-shell nanoparticles for magnetically-targeted photothermal Therapy in the Second Near-Infrared Window. ACS Appl. Mater. Interfaces 2018, 10, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, K.; Li, Y.; Zeng, X.; Shao, M.; Lee, S.T.; Liu, Z. Multifunctional nanoparticles for up conversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials 2012, 33, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Chen, D.; Meng, X.; Liu, T.; Fu, C.; Hao, N.; Zhang, Y.; Wu, X.; Ren, J.; et al. Multifunctional Fe3O4@P(St/MAA)@chitosan@Au core/shell nanoparticles for dual imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2013, 5, 4966–4971. [Google Scholar] [CrossRef] [PubMed]
- Azhdarzadeh, M.; Atyabi, F.; Saei, A.A.; Varnamkhasti, B.S.; Omidi, Y.; Fateh, M.; Ghavami, M.; Shanehsazzadeh, S.; Dinarvand, R. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloids Surf. B Biointerfaces 2016, 143, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Syu, W.J.; Huang, T.C.; Lee, Y.C.; Hsiao, J.K.; Huang, K.Y.; Yu, H.P.; Liao, M.Y.; Lai, P.S. Encapsulation of Au/Fe3O4 nanoparticles into a polymer nanoarchitecture with combined near infrared-triggered chemo-photothermal therapy based on intracellular secondary protein understanding. J. Mater. Chem. B 2017, 5, 5774–5782. [Google Scholar] [CrossRef]
- Nan, X.; Zhang, X.; Liu, Y.; Zhou, M.; Chen, X.; Zhang, X. Dual-targeted multifunctional nanoparticles for magnetic resonance imaging guided cancer diagnosis and therapy. ACS Appl. Mater. Interfaces 2017, 9, 9986–9995. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Cui, L.; Yan, F.; Zhang, Z.; Li, W.; Wang, L. Epigallocatechin gallate based magnetic gold nanoshells nanoplatform for cancer theranostic applications. J. Mater. Chem. B 2017, 5, 454–463. [Google Scholar] [CrossRef]
- Lin, L.S.; Yang, X.; Zhou, Z.; Yang, Z.; Jacobson, O.; Liu, Y.; Yang, A.; Niu, G.; Song, J.; Yang, H.H.; et al. Yolk-shell nanostructure: An ideal architecture to achieve harmonious integration of magnetic-plasmonic hybrid theranostic platform. Adv. Mater. 2017, 29, 1606681. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Ding, B.; Zhang, S.; Qi, X.; Wang, K.; Tian, J.; Yan, Y.; Ge, Y.; Wu, L. Near-infrared light-responsive nanoparticles with thermosensitive yolk-shell structure for multimodal imaging and chemo-photothermal therapy of tumor. Nanomedicine 2017, 13, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chen, H.; Chen, Y.; Wang, X.; Chen, F.; Cui, X.; Shi, J. Au capped magnetic core/mesoporous silica shell nanoparticles for combined photothermo-/chemo-therapy and multimodal imaging. Biomaterials 2012, 33, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wei, T.; Yu, J.; Hou, Y.; Cai, K.; Liang, X.J. Multifunctional metal rattle-type nanocarriers for MRI-guided photothermal cancer therapy. Mol. Pharm. 2014, 11, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Liu, H.L.; Li, M.L.; Hsi, I.W.; Fan, C.T.; Huang, C.Y.; Lu, Y.J.; Hua, M.Y.; Chou, H.Y.; Liaw, J.W.; et al. Magnetic gold-nanorod/ PNIPAAmMA nanoparticles for dual magnetic resonance and photoacoustic imaging and targeted photothermal therapy. Biomaterials 2013, 34, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhou, X.; Nie, W.; Chen, L.; Qiu, K.; Zhang, Y.; He, C. Au/polypyrrole@Fe3O4 nanocomposites for MR/CT dual-modal imaging guided-photothermal therapy: An in vitro study. ACS Appl. Mater. Interfaces 2015, 7, 4354–4367. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, Y.; Zhao, N.; Liu, F.; Xu, F.J. Multifunctional pDNA-conjugated polycationic Au nanorod-coated Fe3O4 hierarchical nanocomposites for trimodal imaging and combined photothermal/gene therapy. Small 2016, 12, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Redolfi, E.; Mattoli, V.; Pastoriza-Santos, I.; Pérez-Juste, J.; Lak, A.; Pellegrino, T. Plasmonic/magnetic nanocomposites: Gold-nanorods-functionalized silica coated magnetic nanoparticles. J. Int. Sci. 2017, 502, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Bhana, S.; Lin, G.; Wang, L.; Starring, H.; Mishra, S.R.; Liu, G.; Huang, X. Near-infrared-absorbing gold nanopopcorns with iron oxide cluster core for magnetically amplified photothermal and photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2015, 7, 11637–11647. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, R.; Wang, S.; Ding, L.; Li, J.; Luo, Y.; Wang, X.; Shen, M.; Shi, X. Multifunctional Fe3O4@Au core/shell nanostars: A unique platform for multimode imaging and photothermal therapy of tumors. Sci. Rep. 2016, 6, 28325. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Yang, J.; Wei, P.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials 2015, 38, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Y.; Yang, X.Q.; An, J.; Zhang, L.; Zhao, K.; Qin, M.Y.; Fang, B.Y.; Li, C.; Xuan, Y.; Zhang, X.S.; et al. Multifunctional magnetic-hollow gold nanospheres for bimodal cancer cell imaging and photothermal therapy. Nanotechnology 2015, 26, 315701. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, L.; Zhang, Y.; Petrenko, V.A.; Liu, A. An efficient strategy to synthesize a multifunctional ferroferric oxide core@dye/SiO2@Au shell nanocomposite and its targeted tumor theranostics. J. Mater. Chem. B 2017, 5, 8209–8218. [Google Scholar] [CrossRef]
- Peng, J.; Qi, T.; Liao, J.; Chu, B.; Yang, Q.; Qu, Y.; Li, W.; Li, H.; Luo, F.; Qian, Z. Mesoporous magnetic gold “nanoclusters” as theranostic carrier for chemo-photothermal co-therapy of breast cancer. Theranostics 2014, 4, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yin, W.; Zheng, X.; Tian, G.; Zhang, X.; Bao, T.; Dong, X.; Wang, Z.; Gu, Z.; Ma, X.; et al. Smart MoS2/Fe3O4 nanotheranostic for magnetically targeted photothermal therapy guided by magnetic resonance/photoacoustic imaging. Theranostics 2015, 5, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shi, S.; Liang, C.; Shen, S.; Cheng, L.; Wang, C.; Song, X.; Goel, S.; Barnhart, T.E.; Cai, W.; et al. Iron oxide decorated MoS2 nanosheets with double PEGylation for chelator-free radiolabeling and multimodal imaging guided photothermal therapy. ACS Nano 2015, 9, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, Y.; Wang, L.; Ma, Y.; Tu, X.; Zhang, Z. Manganese doped iron oxide theranostic nanoparticles for combined T1 magnetic resonance imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2015, 7, 4650–4658. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Hu, J.; Zhu, Y.; Zou, R.; Chen, Z.; Yang, S.; Li, R.; Su, Q.; Han, Y.; Liu, X. Sub-10 nm Fe3O4@Cu(2−x)S core-shell nanoparticles for dual-modal imaging and photothermal therapy. J. Am. Chem. Soc. 2013, 135, 8571–8577. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, X.; Li, C.; He, F.; Chen, Y.; Huang, S.; Jin, D.; Yang, P.; Cheng, Z.; Lin, J. Magnetically targeted delivery of DOX loaded Cu9S5@mSiO2@Fe3O4-PEG nanocomposites for combined MR imaging and chemo/photothermal synergistic therapy. Nanoscale 2016, 8, 12560–12569. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Hu, L.; Ma, X.; Ye, S.; Cheng, L.; Shi, X.; Li, C.; Li, Y.; Liu, Z. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 2012, 24, 1868–1872. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Q.; Luo, X.F.; Li, J.; He, H.; Yang, F.; Di, Y.; Jin, C.; Jiang, X.G.; Shen, S.; et al. Magnetic graphene-based nanotheranostic agent for dual-modality mapping guided photothermal therapy in regional lymph nodal metastasis of pancreatic cancer. Biomaterials 2014, 35, 9473–9483. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, H.; Wang, Y.; Shi, B.; Guo, L.; Wu, D.; Yang, S.; Wu, H. Graphene oxide/manganese ferrite nanohybrids for magnetic resonance imaging, photothermal therapy and drug delivery. J. Biomater. Appl. 2016, 30, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Liu, W.; Li, Y.; Jin, Y.; Jiang, L.; Liang, X.; Feng, S.; Dai, Z. Magnetic Prussian blue nanoparticles for targeted photothermal therapy under magnetic resonance imaging guidance. Bioconjug. Chem. 2014, 25, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.S.; Burga, R.A.; Sweeney, E.E.; Zun, Z.; Sze, R.W.; Tuesca, A.; Subramony, J.A.; Fernandes, R. Composite iron oxide-Prussian blue nanoparticles for magnetically guided T1-weighted magnetic resonance imaging and photothermal therapy of tumors. Int. J. Nanomed. 2017, 12, 6413–6424. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huang, H.; Zhang, Y.; Zhang, H.; Hou, L.; Zhang, Z. Cit/CuS@Fe3O4-based and enzyme-responsive magnetic nanoparticles for tumor chemotherapy, photothermal, and photodynamic therapy. J. Biomater. Appl. 2017, 31, 1010–1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Gai, Z.; Hong, K.; Banerjee, P.; Zhou, S. Magnetic/NIR-responsive drug carrier, multicolor cell imaging, and enhanced photothermal therapy of gold capped magnetite-fluorescent carbon hybrid nanoparticles. Nanoscale 2015, 7, 7885–7895. [Google Scholar] [CrossRef] [PubMed]
- Santha Moorthy, M.; Subramanian, B.; Panchanathan, M.; Mondal, S.; Kim, H.C.; Lee, K.D.; Oh, J. Fucoidan-coated core-shell magnetic mesoporous silica nanoparticles for chemotherapy and magnetic hyperthermia-based thermal therapy applications. New J. Chem. 2017, 41, 15334–15346. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, Y.; Zhang, W.; Hao, Y.; Wang, Y.; Zhang, H.; Hou, L.; Zhang, Z. Programmed near-infrared light-responsive drug delivery system for combined magnetic tumor-targeting magnetic resonance imaging and chemo-phototherapy. Acta Biomater. 2017, 49, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yang, K.; Chen, Q.; Liu, Z. Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer. ACS Nano 2012, 6, 5605–5613. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tong, S.; Bao, G.; Gao, C.; Dai, Z. Indocyanine green loaded SPIO nanoparticles with phospholipid-PEG coating for dual-modal imaging and photothermal therapy. Biomaterials 2013, 34, 7706–7714. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gong, H.; Liu, T.; Cheng, L.; Wang, C.; Sun, X.; Liang, C.; Liu, Z. J-aggregates of organic dye molecules complexed with iron oxide nanoparticles for imaging-guided photothermal therapy under 915-nm light. Small 2014, 10, 4362–4370. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xu, H.; Cheng, L.; Sun, C.; Wang, J.; Liu, Z. In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv. Mater. 2012, 24, 5586–5592. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wang, Q.; Yao, K.X.; Teng, B.; Zhang, J.; Yang, S.; Han, Y. Multifunctional polypyrrole@Fe3O4 nanoparticles for dual-modal imaging and in vivo photothermal cancer therapy. Small 2014, 10, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, H.; Liang, C.; Liu, Y.; Li, Z.; Yang, G.; Cheng, L.; Li, Y.; Liu, Z. Iron oxide@polypyrrole nanoparticles as a multifunctional drug carrier for remotely controlled cancer therapy with synergistic antitumor effect. ACS Nano 2013, 7, 6782–6795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, X.; Li, T.; Lin, M.; Lin, X.; Zhang, H.; Sun, H.; Yang, B. Composite photothermal platform of polypyrrole-enveloped Fe3O4 nanoparticle self-assembled superstructures. ACS Appl. Mater. Interfaces 2014, 6, 14552–14561. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Y.; Zhang, Y.; Shu, Y.; Chen, X.W.; Wang, J.H. A magnetic polypyrrole/iron oxide core/gold shell nanocomposite for multimodal imaging and photothermal cancer therapy. Talanta 2017, 171, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ren, X.; Paholak, H.J.; Burnett, J.; Ni, F.; Fang, X.; Sun, D. Facile Fabrication of Near-Infrared-Resonant and Magnetic Resonance Imaging-Capable Nanomediators for Photothermal Therapy. ACS Appl. Mater. Interfaces 2015, 7, 12814–12823. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Li, X.; Kong, X.; Li, Y.; Liu, X.; Zhang, Y.; Tu, L.; Xue, B.; Wu, F.; Cao, D.; et al. A highly effective in vivo photothermal nanoplatform with dual imaging-guide therapy of cancer based on the charge reversal complex of dye and iron oxide. J. Mater. Chem. B 2015, 3, 8321–8327. [Google Scholar] [CrossRef]
- Lin, L.S.; Cong, Z.X.; Cao, J.B.; Ke, K.M.; Peng, Q.L.; Gao, J.; Yang, H.H.; Liu, G.; Chen, X. Multifunctional Fe3O4@polydopamine core-shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, D.; Zeng, Y.; Wu, L.; Liu, X.; Liu, J. Nanocluster of superparamagnetic iron oxide nanoparticles coated with poly(dopamine) for magnetic field-targeting, highly sensitive MRI and photothermal cancer therapy. Nanotechnology 2015, 26, 115102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Wang, S.; Tian, Y.; Jiang, X.; Fu, D.; Shen, S.; Yang, W. Polydopamine-coated magnetic composite particles with an enhanced photothermal effect. ACS Appl. Mater. Interfaces 2015, 7, 15876–15884. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Li, X.; Lin, M.; Wang, D.; Li, S.; Liu, S.; Tang, Q.; Liu, Y.; Jiang, J.; Liu, L.; et al. Fe3O4@ polydopamine composite theranostic superparticles employing preassembled Fe3O4 nanoparticles as the core. ACS Appl. Mater. Interfaces 2016, 8, 22942–22952. [Google Scholar] [CrossRef] [PubMed]
- Mrówczyński, R. Polydopamine-Based Multifunctional (Nano) materials for Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 7541–7561. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, Q.; Zhang, D.; Liao, N.; Wu, L.; Huang, A.; Liu, X. Magnetite nanocluster@poly(dopamine)-PEG@indocyanine green nanobead with magnetic field-targeting enhanced MR imaging and photothermal therapy in vivo. Colloids Surf. B 2016, 141, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Tang, J.; Zheng, R.; Guo, G.; Dong, A.; Wang, Y.; Yang, W. Nuclear-targeted multifunctional magnetic nanoparticles for photothermal therapy. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, Q.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. PEGylated polydopamine nanoparticles incorporated with indocyanine green and doxorubicin for magnetically guided multimodal cancer therapy triggered by near-infrared light. ACS Appl. Nano Mater. 2018, 1, 325–336. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Fu, Y.; Li, X.; Chen, Y.; Zhang, H.; Wang, Z. Fabrication of multifunctional ferric oxide nanoparticles for tumor5-targeted magnetic resonance imaging and precise photothermal therapy with magnetic field enhancement. J. Mater. Chem. B 2017, 5, 8554–8562. [Google Scholar] [CrossRef]
- Xue, P.; Sun, L.; Li, Q.; Zhang, L.; Guo, J.; Xu, Z.; Kang, Y. PEGylated polydopamine-coated magnetic nanoparticles for combined targeted chemotherapy and photothermal ablation of tumour cells. Colloids Surf. B Biointerfaces 2017, 160, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhang, F.; Kong, C.; Zhang, H.; Zhang, W.; Ge, R.; Liu, Y.; Jiang, J.E. GFR-targeted delivery of DOX-loaded Fe3O4@ polydopamine multifunctional nanocomposites for MRI and antitumor chemo-photothermal therapy. Int. J. Nanomed. 2017, 12, 2899–2911. [Google Scholar] [CrossRef] [PubMed]
- Mrówczyński, R.; Jedrzak, A.; Szutkowski, K.; Grzeskowiak, B.F.; Coy, E.; Markiewicz, R.; Jesionowski, T.; Jurga, S. Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy. Nanomaterials (Basel) 2018, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Shen, H.; Yang, T.; Wang, L.; Fu, H.; Chen, H.; Zhang, Z. Indocyanine green loaded magnetic carbon nanoparticles for near infrared fluorescence/magnetic resonance dual-modal imaging and photothermal therapy of tumor. ACS Appl. Mater. Interfaces 2017, 9, 9484–9495. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Je, J.Y.; Moorthy, M.S.; Seo, H.; Cho, W.H. pH and NIR-light-responsive magnetic iron oxide nanoparticles for mitochondria-mediated apoptotic cell death induced by chemo-photothermal therapy. Int. J. Pharm. 2017, 531, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lin, G.; Zhu, C.H.; Pang, X.; Zhang, Y.; Wang, X.; Li, X.; Wang, B.; Xia, H.; Liu, G. Metalla-aromatic loaded magnetic nanoparticles for MRI/photoacoustic imaging-guided cancer phototherapy. J. Mater. Chem. B 2018, 6, 2528–2535. [Google Scholar] [CrossRef]
- Johnson, R.J.G.; Schultz, J.D.; Lear, B.J. Photothermal effectiveness of magnetite nanoparticles: Dependence upon particle size probed by experiment and simulation. Molecules 2018, 23, 1234. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Institute, A.N.S. American National Standard for the Save Use of Lasers Inc.; Laser Institute of America: New York, NY, USA, 2000. [Google Scholar]
- Guo, X.; Wu, Z.; Li, W.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Zhu, X.; Du, Y.P.; Jin, Y.; et al. Appropriate size of magnetic nanoparticles for various bioapplications in cancer diagnostics and therapy. ACS Appl. Mater. Interfaces 2016, 8, 3092–3106. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, W.; Luo, L.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Yang, J.; Zhu, C.; Du, Y.; et al. External magnetic field-enhanced chemo-photothermal combination tumor therapy via iron oxide nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 16581–16593. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Deng, Z.; Yang, Z.; Wang, Y.; Zhu, H.; Chen, B.; Cui, Z.; Ewing, R.C.; Shi, D. Biomarkerless targeting and photothermal cancer cell killing by surface-electrically-charged superparamagnetic Fe3O4 composite nanoparticles. Nanoscale 2017, 9, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.Y.; Lai, P.S.; Yu, H.P.; Lin, H.P.; Huang, C.C. Innovative ligand-assisted synthesis of NIR-activated iron oxide for cancer theranostics. Chem. Commun. (Camb.) 2012, 48, 5319–5321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.R.; Li, S.H.; Dai, J.; Song, L.; Huang, G.; Lin, R.; Li, J.; Liu, G.; Yang, H.H. Polyphenol-inspired facile construction of smart assemblies for ATP- and pH-responsive tumor MR/optical imaging and photothermal therapy. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Alegret, N.; Criado, A.; Prato, M. Recent Advances of Graphene-based Hybrids with Magnetic Nanoparticles for biomedical applications. Curr. Med. Chem. 2017, 24, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.M.; Fu, C.P.; Fang, J.Z.; Xu, X.D.; Wei, X.H.; Tang, W.J.; Jiang, X.Q.; Zhang, L.M. Hyaluronan-modified superparamagnetic iron oxide nanoparticles for bimodal breast cancer imaging and photothermal therapy. Int. J. Nanomed. 2017, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Hai, J.; Piraux, H.; Mazario, E.; Volatron, J.; Ha-Duong, N.; Decorse, P.; Lomas, J.; Verbeke, P.; Ammar, S.; Wilhelm, C.; et al. Maghemite nanoparticles coated with human serum albumin: Combining targeting by the iron-acquisition pathway and potential in photothermal therapies. J. Mater. Chem. B 2017, 5, 3154–3162. [Google Scholar] [CrossRef]
- Chen, H.; Burnett, J.; Zhang, F.; Zhang, J.; Paholak, H.; Sun, D. Highly crystallized iron oxide nanoparticles as effective and biodegradable mediators for photothermal cancer therapy. J. Mater. Chem. B 2014, 2, 757–765. [Google Scholar] [CrossRef]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Kong, F.; Guo, X.; Wu, L.; Shen, H.; Xie, M.; Wang, X.; Jin, Y.; Ge, Y.C. MCTS stabilized Fe3O4 particles with extremely low toxicity as highly efficient near-infrared photothermal agents for in vivo tumor ablation. Nanoscale 2013, 5, 8056–8066. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.W.; Ehsan, S.M.; Mast, D.; Pauletti, G.M.; Xu, H.; Zhang, J.; Ewing, R.C.; Shi, D. Photothermal effects and toxicity of Fe3O4 nanoparticles via near infrared laser irradiation for cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Yuan, Y.; Xu, K.; Luo, Q. Biocompatible PEGylated Fe3O4 nanoparticles as photothermal agents for near-infrared light modulated cancer therapy. Int. J. Mol. Sci. 2014, 15, 18776–18788. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, H.; Zhou, Z.; Zhou, P.; Yan, Y.; Wang, M.; Yang, H.; Zhang, Y.; Yang, S.M. R/SPECT imaging guided photothermal therapy of tumor-targeting Fe@Fe3O4 nanoparticles in vivo with low mononuclear phagocyte uptake. ACS Appl. Mater. Interfaces 2016, 8, 19872–19882. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zheng, R.; Fang, X.; Wang, X.; Zhang, X.; Yang, W.; Sha, X. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials 2016, 92, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Maeda, R.; Ichihara, M.; Irimura, K.; Kiwada, H. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J. Control. Release 2003, 88, 35–42. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A.; Morán, M.C. Effect of PEGylation on ligand-targeted magnetoliposomes: A missed goal. ACS Omega 2017, 2, 6544–6555. [Google Scholar] [CrossRef]
- Rao, L.; Bu, L.L.; Xu, J.H.; Cai, B.; Yu, G.T.; Yu, X.; He, Z.; Huang, Q.; Li, A.; Guo, S.S.; et al. Red blood cell membrane as a biomimetic Nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small 2015, 11, 6225–6236. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Bu, L.L.; Cai, B.; Xu, J.H.; Li, A.; Zhang, W.F.; Sun, Z.J.; Guo, S.S.; Liu, W.; Wang, T.H.; et al. Cancer cell membrane-coated up conversion nanoprobes for highly specific tumor imaging. Adv. Mater. 2016, 28, 3460–3466. [Google Scholar] [CrossRef] [PubMed]
- Aihara, H.; Miyazaki, J.-I. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 1998, 16, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Cai, B.; Bu, L.L.; Liao, Q.Q.; Guo, S.S.; Zhao, X.Z.; Dong, W.F.; Liu, W. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Bu, L.L.; Meng, Q.-F.; Cai, B.; Deng, W.-W.; Li, A.; Li, K.; Guo, S.-S.; Zhang, W.-F.; Liu, W.; et al. Antitumor platelet-mimicking magnetic nanoparticles. Adv. Func. Mater. 2017, 27, 1604774. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Cheng, L.; Gong, H.; Yin, S.; Gong, Q.; Li, Y.; Liu, Z. PEG-functionalized iron oxide nanoclusters loaded with chlorin e6 for targeted, NIR light induced, photodynamic therapy. Biomaterials 2013, 34, 9160–9170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of iron oxide nanoparticles in cancer therapy: Amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, L.; Liu, F.; Qi, X.; Ge, Y.; Shen, S. Remotely controlled drug release based on iron oxide nanoparticles for specific therapy of cancer. Colloids Surf. B Biointerfaces 2017, 152, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Zheng, S.; Han, J.; Le, V.H.; Park, J.O.; Park, S. Nanohybrid magnetic liposome functionalized with hyaluronic acid for enhanced cellular uptake and near-infrared-triggered drug release. Colloids Surf. B Biointerfaces 2017, 154, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Chang, P.Y.; Liu, C.L.; Xu, J.P.; Wu, S.P.; Kuo, W.C. New insight on optical and magnetic Fe3O4 nanoclusters promising for near infrared theranostic applications. Nanoscale 2015, 7, 12689–12697. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Bombelli, F.B.; Dawson, K.A. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, B.C.; Park, J.; Kim, H.D.; Kim, N.H.; Suh, Y.D.; Kim, Y.K. White-light-emitting magnetite nanoparticle-polymer composites: Photonic reactions of magnetic multi-granule nanoclusters as photothermal agents. Nanoscale 2016, 8, 17136–17140. [Google Scholar] [CrossRef] [PubMed]
- Park, B.C.; Kim, H.D.; Park, J.; Kim, Y.J.; Kim, Y.K. Photonic reactions leading to fluorescence in a polymeric system induced by the photothermal effect of magnetite nanoparticles using a 780 nm multiphoton laser. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estelrich, J.; Busquets, M.A. Iron Oxide Nanoparticles in Photothermal Therapy. Molecules 2018, 23, 1567. https://doi.org/10.3390/molecules23071567
Estelrich J, Busquets MA. Iron Oxide Nanoparticles in Photothermal Therapy. Molecules. 2018; 23(7):1567. https://doi.org/10.3390/molecules23071567
Chicago/Turabian StyleEstelrich, Joan, and Maria Antònia Busquets. 2018. "Iron Oxide Nanoparticles in Photothermal Therapy" Molecules 23, no. 7: 1567. https://doi.org/10.3390/molecules23071567





