In-Vitro Activity of Silybin and Related Flavonolignans against Leishmania infantum and L. donovani
Abstract
:1. Introduction
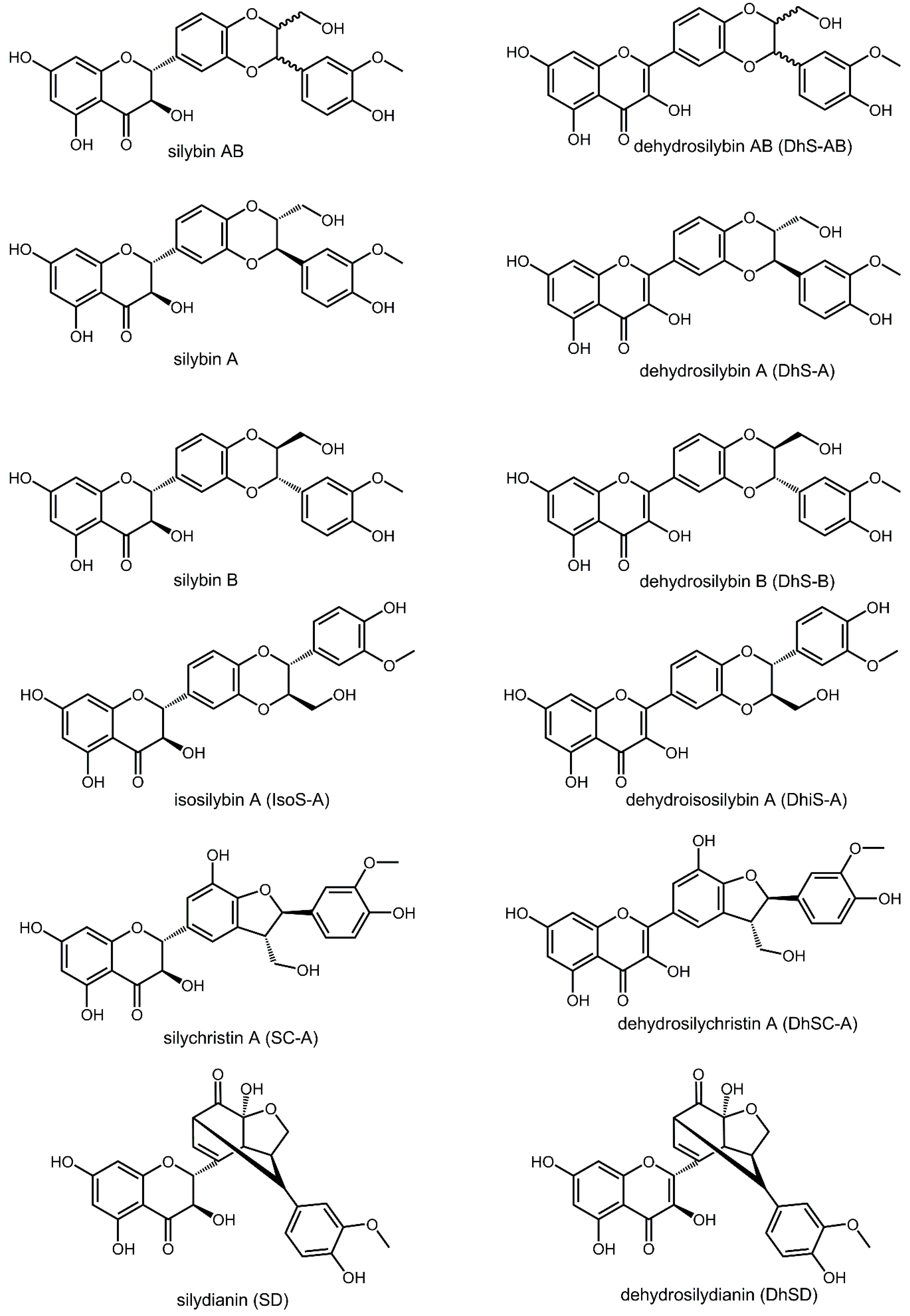
2. Results and Discussion
2.1. In-Vitro Activity against Li and Ld Promastigotes
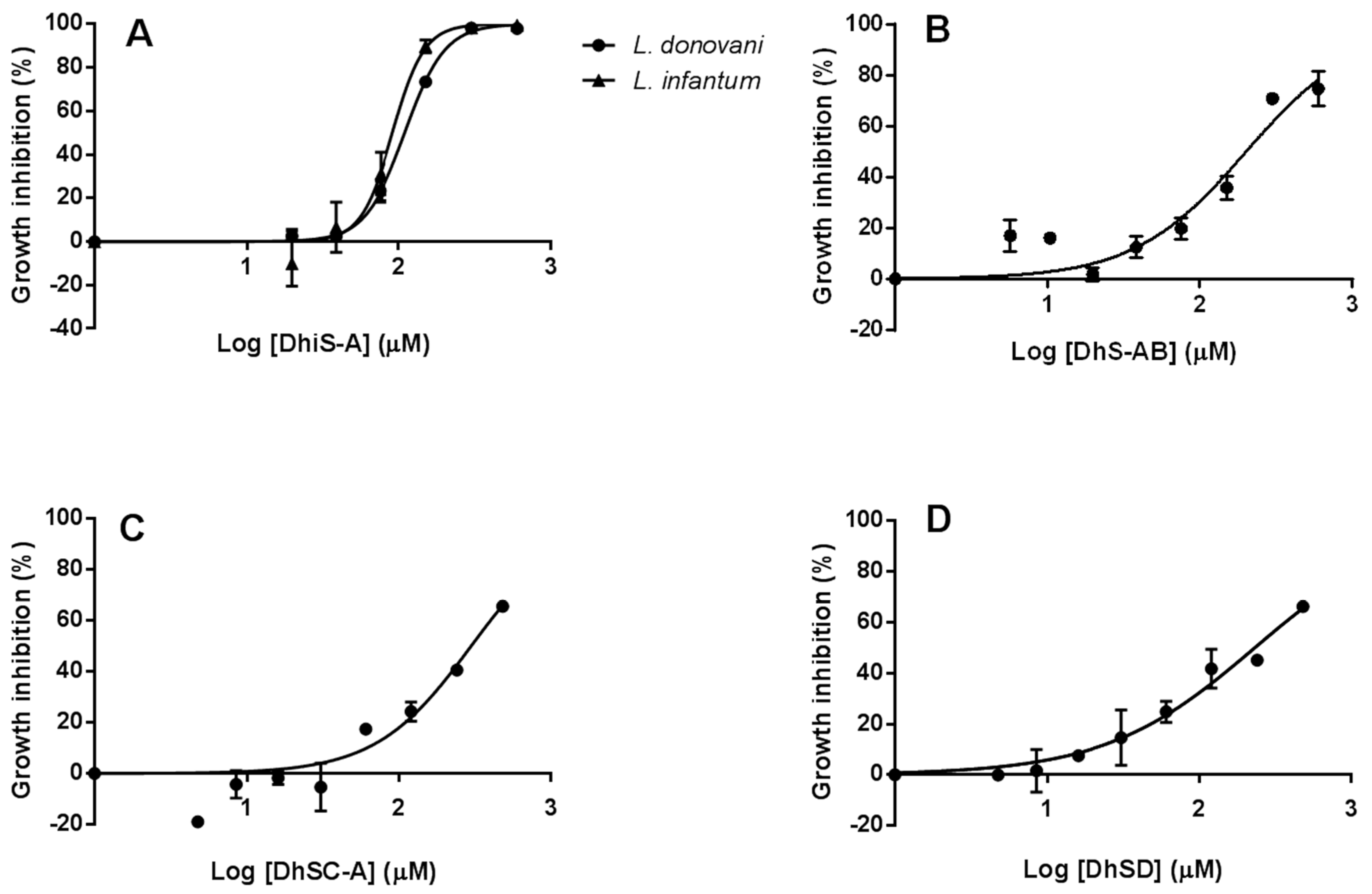
2.2. Synergistic Effect of Dehydroisosilybin A and AmB
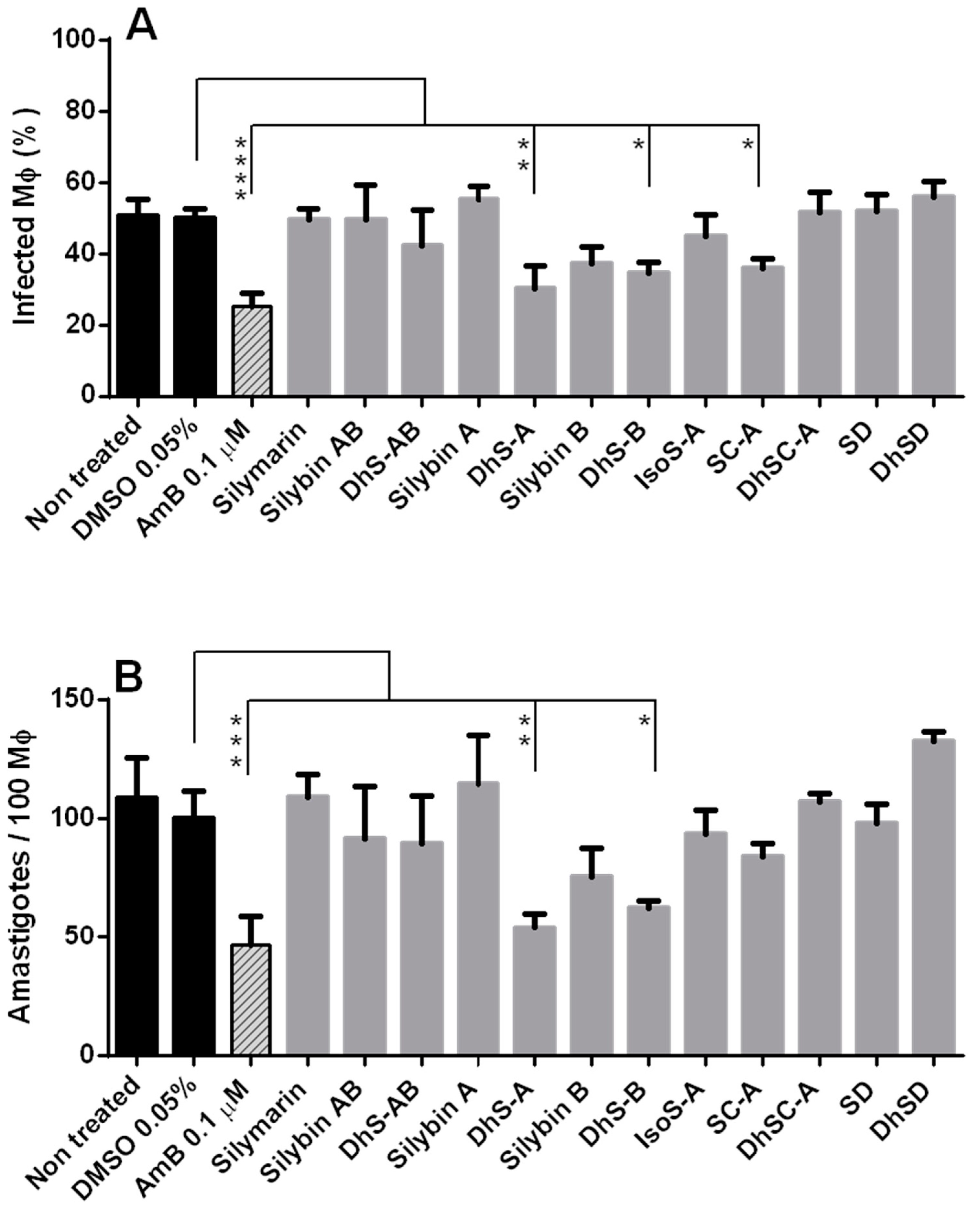
2.3. Efficacy of Flavonolignans against Intracellular Amastigotes
2.3.1. Toxicity of Flavonolignans for Macrophages (Mφ)
2.3.2. Efficacy of Flavonolignans against Intracellular Amastigotes
3. Materials and Methods
3.1. Leishmania Culture Media, Drugs and Mice Mφ
3.2. In-Vitro Activity against Li and Ld Promastigotes
3.3. Determination of IC50
3.4. Synergistic Effect between Dehydroisosilybin A, and AmB, SbIII and PMM
3.5. Toxicity of Flavonolignans for Macrophages (Mφ)
3.6. Activity of Flavonolignans against Intracellular Amastigotes
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DNDi. Available online: https://www.dndi.org/diseases-projects/leishmaniasis/ (accessed on 4 April 2018).
- Savoia, D. Recent updates and perspectives on leishmaniasis. J. Infect. Dev. Ctries 2015, 9, 588–596. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Available online: http://www.who.int/mediacentre/factsheets/fs375/en/2015 (accessed on 4 April 2018).[Green Version]
- Pavli, A.; Maltezou, H.C. Leishmaniasis, an emerging infection in travelers. Int. J. Infect. Dis. 2010, 14, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.G. Leishmaniosis of companion animals in Europe: An update. Vet. Parasitol. 2015, 218, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Alvar, J. Canine leishmaniasis: Epidemiological risk and the experimental model. Trends Parasitol. 2002, 18, 399–405. [Google Scholar] [CrossRef]
- Pace, D. Leishmaniasis. J. Infect. 2014, 69, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drugs resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Ready, P.D. Epidemiology of visceral leishmaniasis. Clin. Epidem. 2014, 6, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lehane, A.M.; Saliba, K.J. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Res. Notes 2008, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Mittra, B.; Saha, A.; Chowdhury, A.R.; Pal, C.; Mandal, S.; Mukhopadhyay, S.; Bandyopadhyay, S.; Majumder, H.K. Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol. Med. 2000, 6, 527–541. [Google Scholar] [PubMed]
- Lewin, G.; Cojean, S.; Gupta, S.; Verma, A.; Puri, S.K.; Loiseau, P.M. In vitro antileishmanial properties of new flavonoids against Leishmania donovani. Biomed. Prev. Nutr. 2011, 1, 168–171. [Google Scholar] [CrossRef]
- Borsari, C.; Luciani, R.; Pozzi, C.; Poehner, I.; Henrich, S.; Trande, M.; Cordeiro-da-Silva, A.; Santarem, N.; Baptista, C.; Tait, A.; et al. Profiling of flavonol derivatives for the development of antitrypanosomatidic drugs. J. Med. Chem. 2016, 59, 7598–7616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrba, J.; Papoušková, B.; Roubalová, L.K.; Zatloukalová, M.; Biedermann, D.; Křen, V.; Valentová, K.; Ulrichová, J.; Vacek, J. Metabolism of flavonolignans in human hepatocytes. J. Pharm. Biomed. Anal. 2018, 152, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Křen, V.; Walterová, D. Silybin and silymarin-new effects and applications. Biomed. Pap. 2005, 149, 29–41. [Google Scholar] [CrossRef]
- Radko, L.; Cybulski, W. Application of silymarin in human and animal medicine. J. Preclin. Clin. Res. 2007, 1, 22–26. [Google Scholar]
- Lovelace, E.S.; Wagoner, J.; MacDonald, J.; Bammler, T.; Bruckner, J.; Brownell, J.; Beyer, R.; Zink, E.M.; Kim, Y.-M.; Kyle, J.E.; et al. Silymarin suppresses cellular inflammation by inducing reparative stress signaling. J. Nat. Prod. 2015, 78, 1990–2000. [Google Scholar] [CrossRef] [PubMed]
- Polyak, S.J.; Morishima, C.; Lohmann, V.; Pal, S.; Lee, D.Y.W.; Liu, Y.; Graf, T.N.; Oberlies, N.H. Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad. Sci. USA 2010, 107, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Faridnia, R.; Kalani, H.; Fakhar, M.; Akhtari, J. Investigating in vitro anti-leishmanial effects of silibinin and silymarin on Leishmania major. Ann. Parasitol. 2018, 64, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Jabini, R.; Jaafari, M.R.; Vahdati Hasani, F.; Ghazizadeh, F.; Khamesipour, A.; Karimi, G. Effects of combined therapy with silymarin and glucantime on leishmaniasis induced by Leishmania major in BALB/c mice. Drug Res. 2015, 65, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The silymarin composition… and why does it matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Denny, B.J.; West, P.W.; Mathews, T.C. Antagonistic interactions between the flavonoids hesperetin and naringenin and beta-lactam antibiotics against Staphylococcus aureus. Br. J. Biomed. Sci. 2008, 65, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Fumarola, L.; Spinelli, R.; Brandonisio, O. In vitro assays for evaluation of drug activity against Leishmania spp. Res. Microbiol. 2004, 155, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: Recent progress and challenges in assay development. Drug Discov. Today 2017, 22, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Vermeersch, M.; Inocêncio da Luz, R.; Toté, K.; Timmermans, J.-P.; Cos, P.; Maes, L. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: Practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef] [PubMed]
- De Muylder, G.; Ang, K.K.H.; Chen, S.; Arkin, M.R.; Engel, J.C.; McKerrow, J.H. A screen against Leishmania intracellular amastigotes: Comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Neglec. Trop. Dis. 2011, 5, E1253. [Google Scholar] [CrossRef] [PubMed]
- Davis-Searles, P.R.; Nakanishi, Y.; Kim, N.C.; Graf, T.N.; Oberlies, N.H.; Wani, M.C.; Wall, M.E.; Argarwal, R.; Kroll, D.J. Milk thistle and prostate cancer: Differential effects of pure flavolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005, 65, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Victoria, J.M.; Pérez-Victoria, F.J.; Conseil, G.; Maitrejean, M.; Comte, G.; Barron, D.; Di Pietro, A.; Castanys, S.; Gamarro, F. High-affinity binding of silybin derivatives to the nucleotide-binding domain of a Leishmania tropica P-glycoprotein-like transporter and chemosensitization of a multidrug-resistant parasite to daunomycin. Antimicrob. Agents Chemother. 2001, 45, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.C.; Sivakumar, N.; Jose, J.; Costi, M.P.; Pozzi, C.; Schmidt, T.J. In silico identification and in vitro evaluation of natural inhibitors of Leishmania major pteridine reductase I. Molecules 2017, 22, 2166. [Google Scholar] [CrossRef] [PubMed]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojović, M.; Popović-Bijelić, A.; et al. Flavonolignan 2,3-dehydroderivatives: Preparation, antiradical and cytoprotective activity. Free Radic. Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Dumas, T.E.; Schrieber, S.J.; Hawke, R.L.; Fried, M.W.; Smith, P.C. Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab. Dispos. 2008, 36, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Křenek, K.; Marhol, P.; Peikerová, Z.; Křen, V.; Biedermann, D. Preparatory separation of the silymarin flavonolignans by Sephadex LH-20 gel. Food Res. Int. 2014, 65, 115–120. [Google Scholar] [CrossRef]
- Monti, D.; Gažák, R.; Marhol, P.; Biedermann, D.; Purchartová, K.; Fedrigo, M.; Riva, S.; Křen, V. Enzymatic kinetic resolution of silybin diastereoisomers. J. Nat. Prod. 2010, 73, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Gažák, R.; Marhol, P.; Purchartová, K.; Monti, D.; Biedermann, D.; Riva, S.; Cvak, L.; Křen, V. Large-SC-Ale separation of silybin diastereoisomers using lipases. Process Biochem. 2010, 45, 1657–1663. [Google Scholar] [CrossRef]
- Gažák, R.; Trouillas, P.; Biedermann, D.; Fuksová, K.; Marhol, P.; Kuzma, M.; Křen, V. Base-catalyzed oxidation of silybin and isosilybin into 2,3-dehydro derivatives. Tetrahedron Lett. 2013, 54, 315–317. [Google Scholar] [CrossRef]
- Maitrejean, M.; Comte, G.; Barron, D.; El Kirat, K.; Conseil, G.; Di Pietro, A. The flavonolignan silybin and its hemisynthetic derivatives, a novel series of potential modulators of P-glycoprotein. Bioorg. Med. Chem. Lett. 2000, 10, 157–160. [Google Scholar] [CrossRef]
- Méndez, S.; Nell, M.; Alunda, J.M. Leishmania infantum: Infection of macrophages in vitro with promastigotes. Int. J. Parasitol. 1996, 26, 619–622. [Google Scholar] [CrossRef]
- Corral-Caridad, M.J.; Moreno, I.; Toraño, A.; Domínguez, M.; Alunda, J.M. Effect of allicin on promastigotes and intracellular amastigotes of Leishmania donovani and L. infantum. Exp. Parasitol. 2012, 132, 475–482. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from D. Biedermann. |

| Drug Combination Dn + DhiS-A | % Growth Inhibition/EDn | CI Values | DRI | Dose Required (µM) | ||
|---|---|---|---|---|---|---|
| Dn | DhiS-A | Dn | DhiS-A | |||
| AmB | 10 | 1.49 (antagonism) | 2.85 | 0.88 | 0.023 | 34.79 |
| (0.01:15) | 25 | 1.24 (moderate antagonism) | 2.74 | 1.13 | 0.030 | 44.61 |
| 50 | 1.06 (additive effect) | 2.64 | 1.47 | 0.038 | 57.21 | |
| 75 | 0.92 (additive effect) | 2.54 | 1.90 | 0.048 | 73.36 | |
| 90 | 0.81 (moderate synergism) | 2.39 | 2.91 | 0.062 | 94.08 | |
| 95 | 0.76 (moderate synergism) | 2.26 | 4.28 | 0.074 | 111.42 | |
| SbIII | 50 | 1.89 (antagonism) | 0.82 | 1.50 | 56 | 56 |
| (1:1) | 75 | 2.09 (antagonism) | 0.92 | 0.99 | 141.12 | 141.12 |
| 90 | 2.50 (antagonism) | 1.04 | 0.65 | 355.63 | 355.63 | |
| 95 | 2.93 (antagonism) | 1.13 | 0.49 | 666.84 | 666.84 | |
| PMM | 50 | 1.98 (antagonism) | 39.54 | 0.51 | 822 | 164 |
| (5:1) | 75 | 1.65 (antagonism) | 182.9 | 0.60 | 1145 | 229 |
| 90 | 1.38 (moderate antagonism) | 845.2 | 0.72 | 1594 | 318 | |
| 95 | 1.23 (moderate antagonism) | 2393 | 0.81 | 1997 | 399 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olías-Molero, A.I.; Jiménez-Antón, M.D.; Biedermann, D.; Corral, M.J.; Alunda, J.M. In-Vitro Activity of Silybin and Related Flavonolignans against Leishmania infantum and L. donovani. Molecules 2018, 23, 1560. https://doi.org/10.3390/molecules23071560
Olías-Molero AI, Jiménez-Antón MD, Biedermann D, Corral MJ, Alunda JM. In-Vitro Activity of Silybin and Related Flavonolignans against Leishmania infantum and L. donovani. Molecules. 2018; 23(7):1560. https://doi.org/10.3390/molecules23071560
Chicago/Turabian StyleOlías-Molero, Ana Isabel, María Dolores Jiménez-Antón, David Biedermann, María J. Corral, and José María Alunda. 2018. "In-Vitro Activity of Silybin and Related Flavonolignans against Leishmania infantum and L. donovani" Molecules 23, no. 7: 1560. https://doi.org/10.3390/molecules23071560







