Insights into Resistance Mechanisms of Inhibitors to Mps1 C604Y Mutation via a Comprehensive Molecular Modeling Study
Abstract
:1. Introduction
2. Results and Discussion
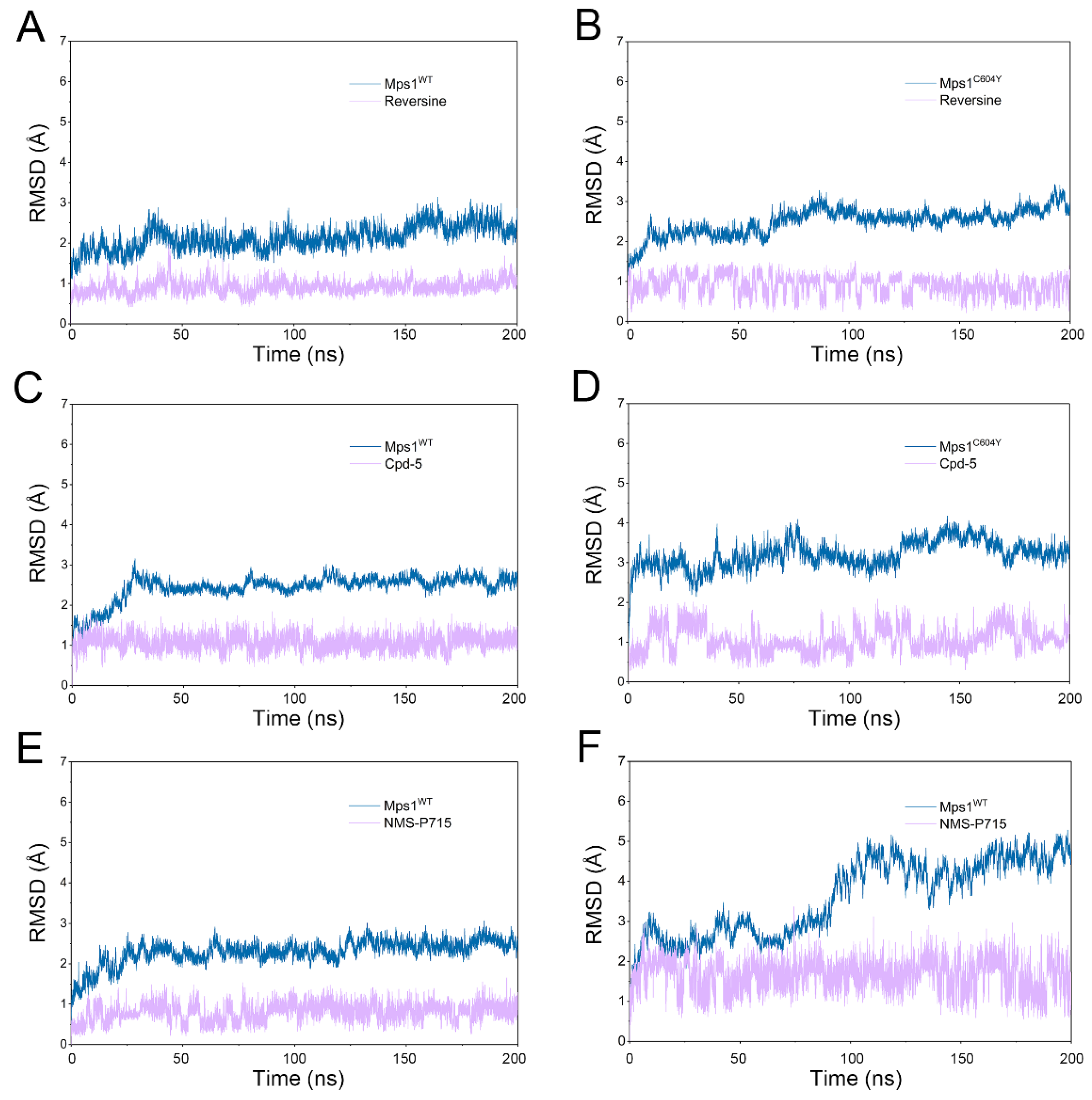
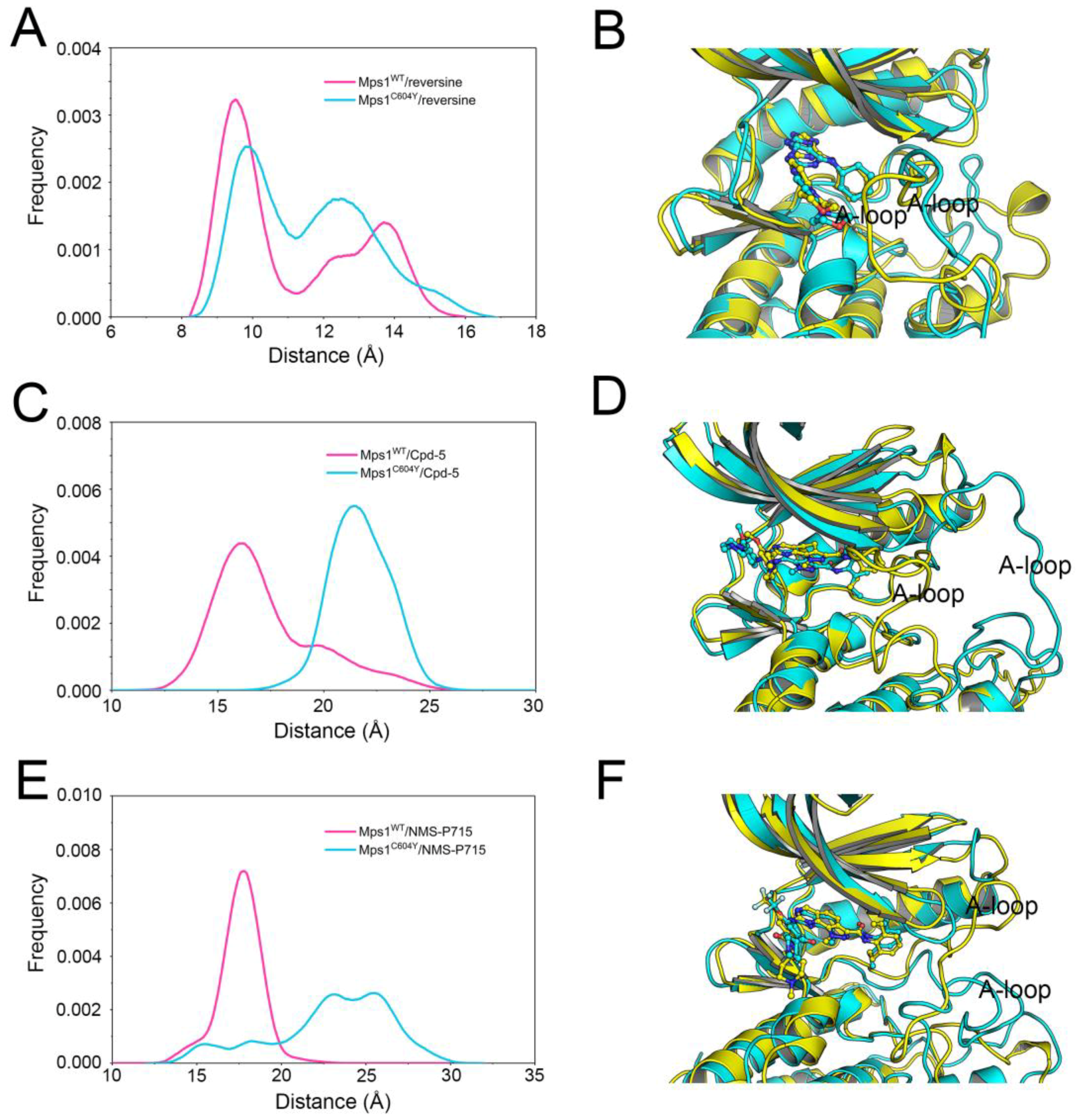
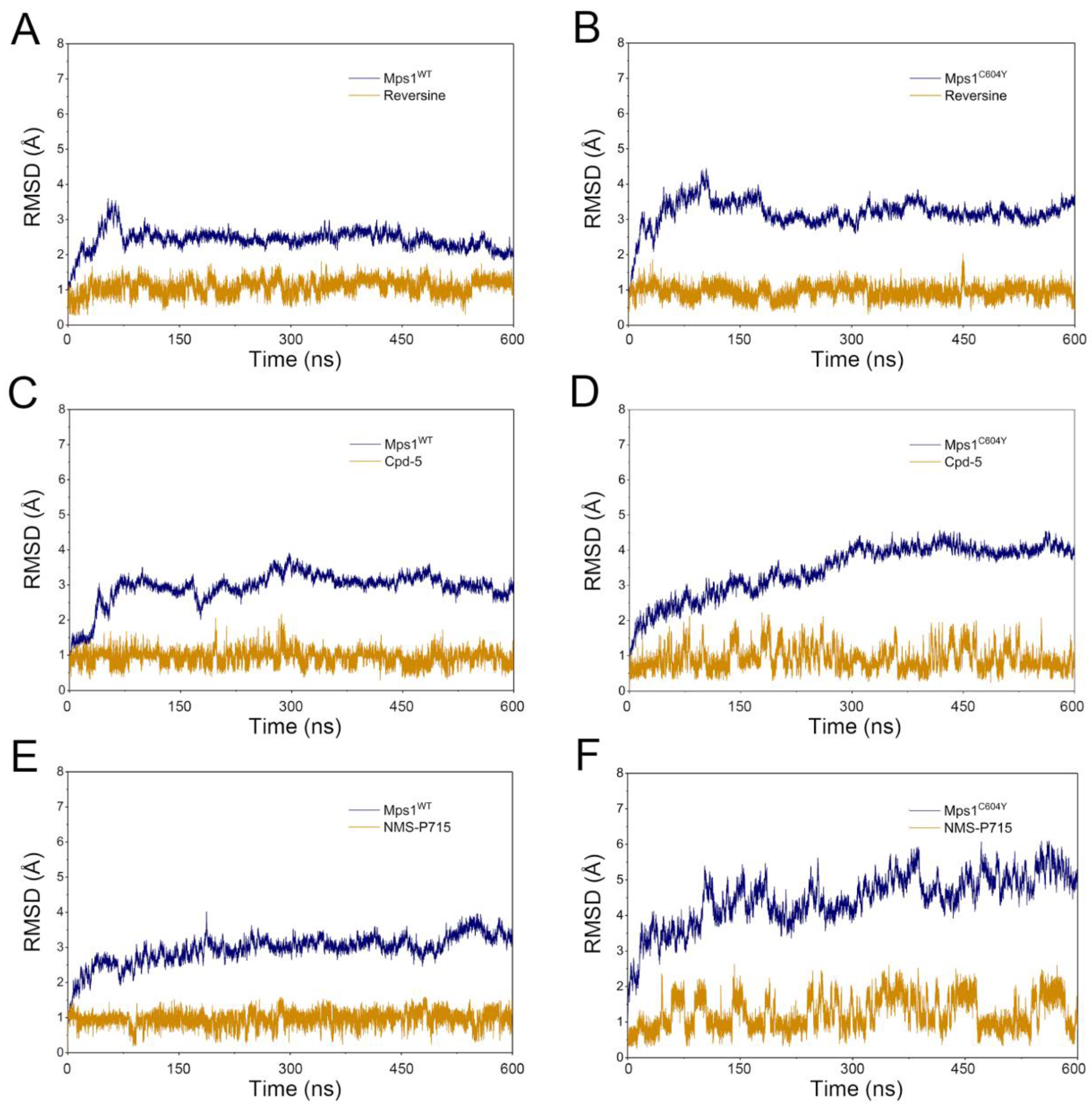
2.1. Structural Properties Studied by Classical MD Simulations
2.2. Binding Profiles Revealed Through the MM/GBSA Binding Free Energy Calculations and the Residue-Ligand Interaction Spectra
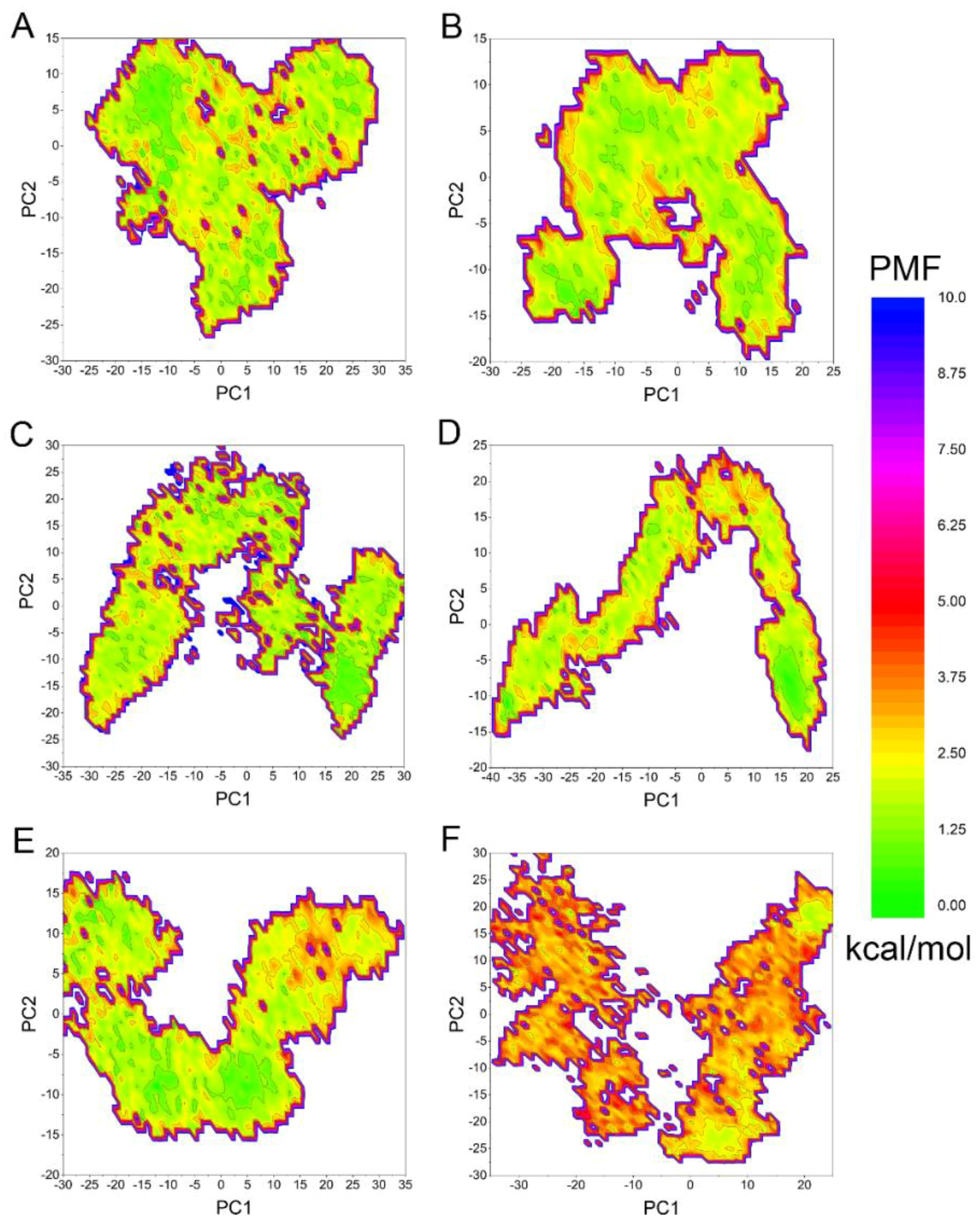
2.3. Accelerated MD (aMD) Simulations
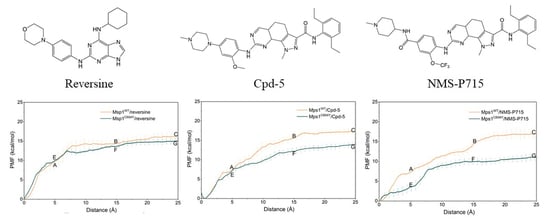
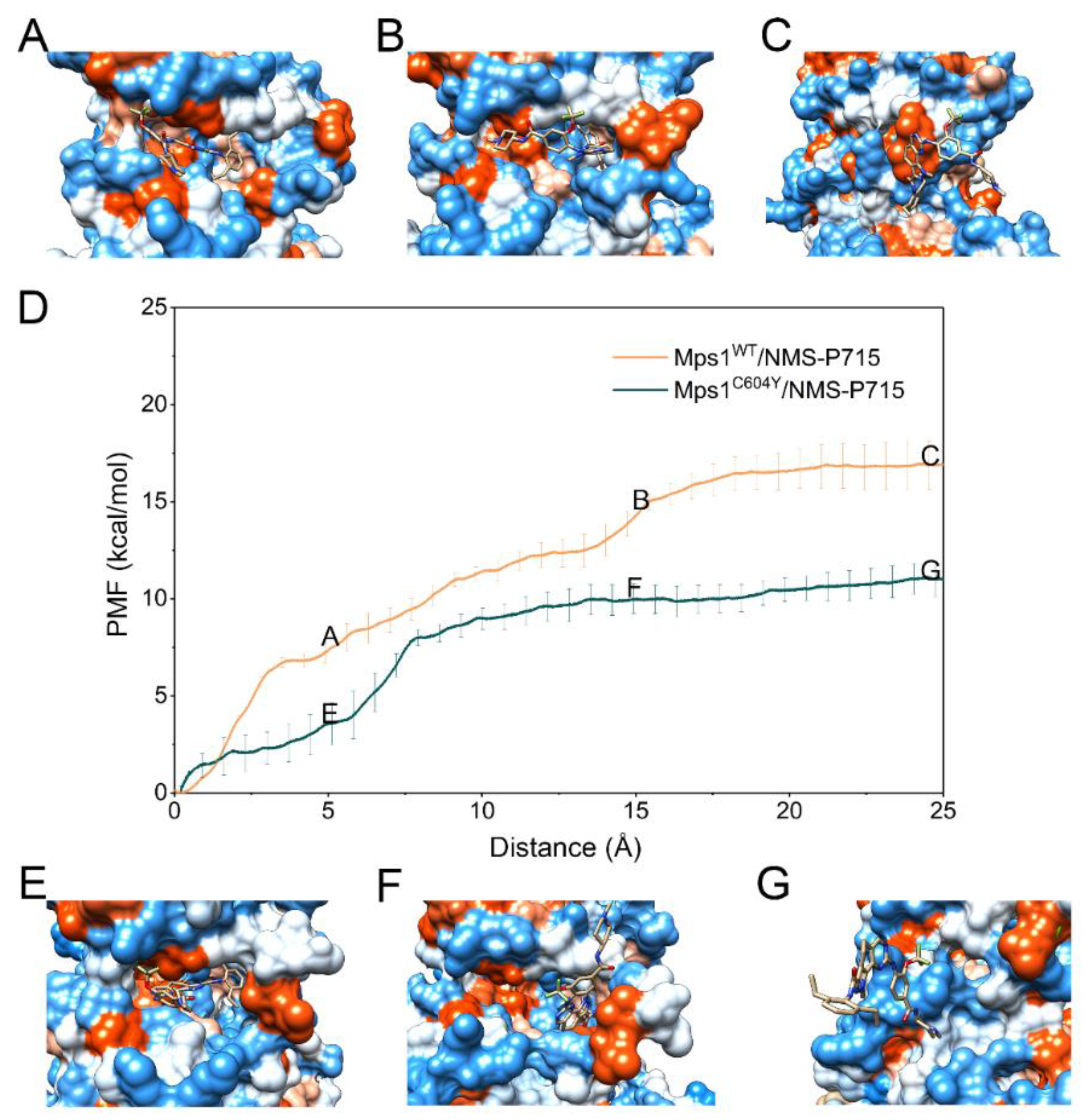
2.4. Dissociation Processes Revealed by US Simulations
3. Conclusions
4. Materials and Methods
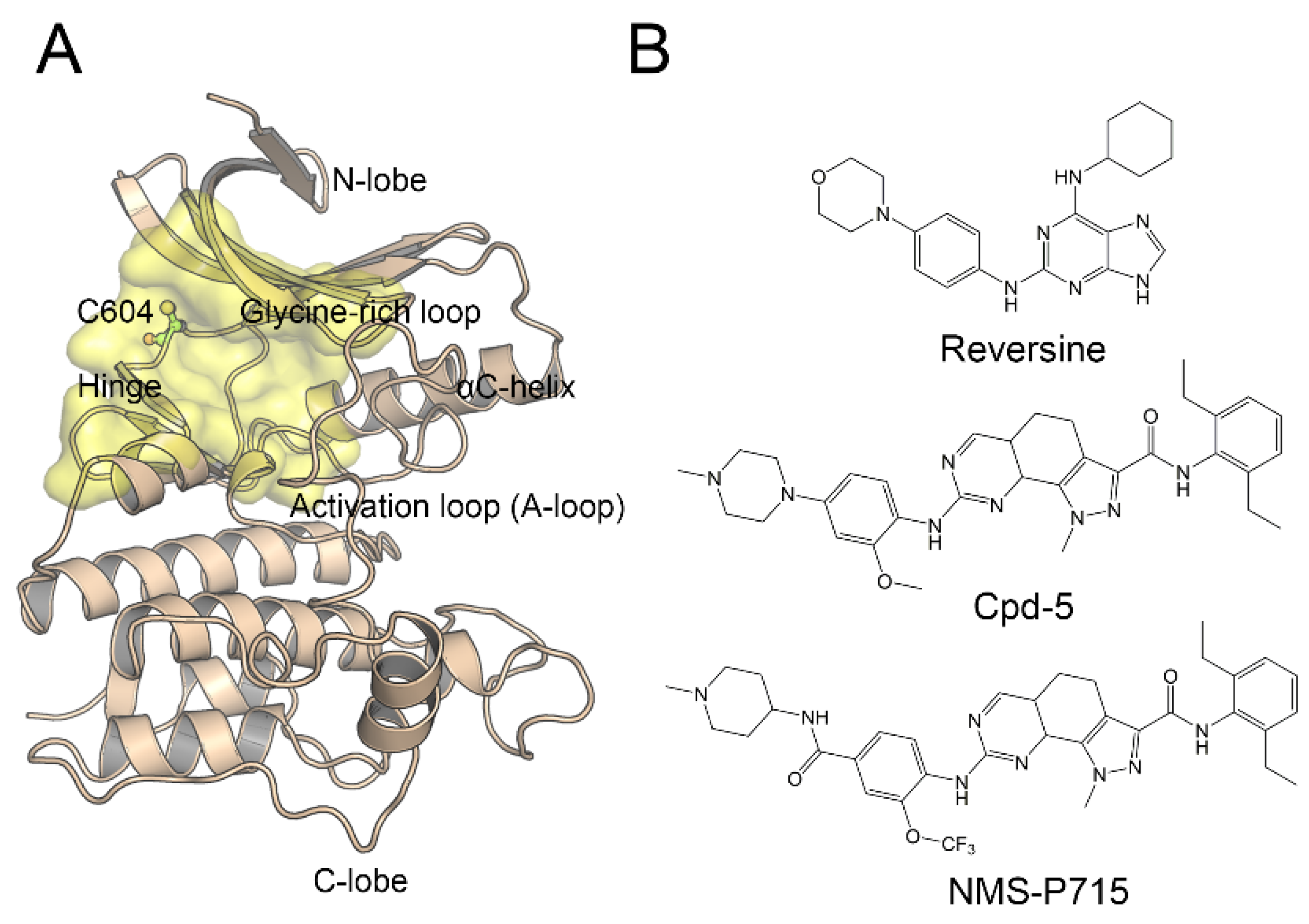
4.1. Initial Structures of Mps1WT/reversine, Mps1WT/Cpd-5, and Mps1WT/NMS-P715
4.2. Classical MD Simulations
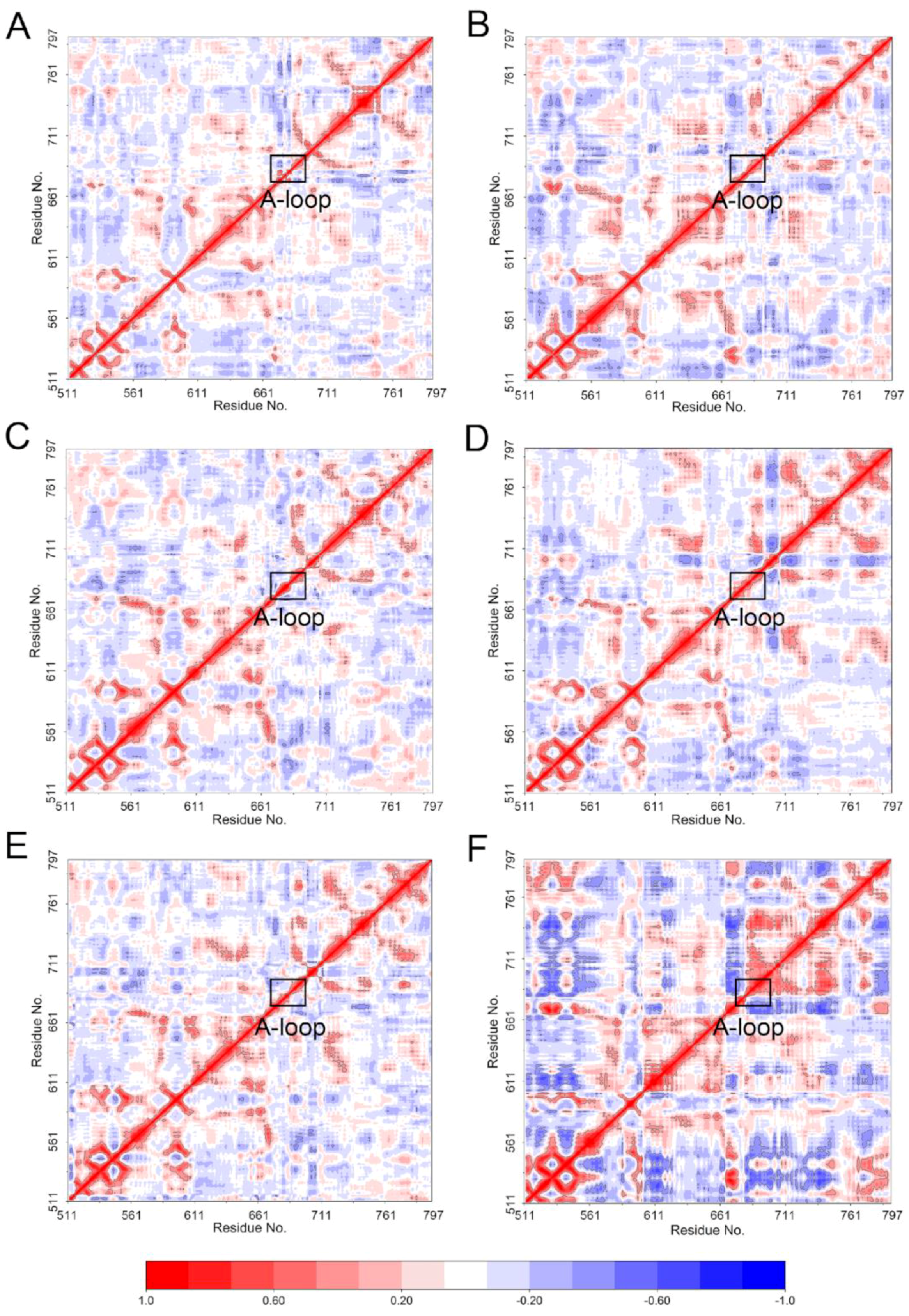
4.3. DCC Analysis
4.4. Binding Free Energy Calculations
4.5. Accelerated MD (aMD) Simulations
4.6. US Simulations
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Musacchio, A.; Salmon, E.D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007, 8, 379–393. [Google Scholar] [CrossRef] [PubMed]
- London, N.; Biggins, S. Signalling dynamics in the spindle checkpoint response. Nat. Rev. Mol. Cell Biol. 2014, 15, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.L.; Wassmann, K. Multiple duties for spindle assembly checkpoint kinases in meiosis. Front. Cell Dev. Biol. 2017, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ling, Y.; Guo, Y.; Bai, Y.; Shi, X.; Gong, F.; Tan, P.; Zhang, Y.; Wei, C.; He, X.; et al. Mps1 kinase regulates tumor cell viability via its novel role in mitochondria. Cell Death Dis. 2016, 7, e2292. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.; Coulter, J.; Woo, J.H.; Wilsbach, K.; Gabrielson, E. High levels of the Mps1 checkpoint protein are protective of aneuploidy in breast cancer cells. Proc. Natl. Acad. Sci. USA 2011, 108, 5384–5389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, R.; Luo, H.; Zhou, H.; Li, G.; Bu, D.; Yang, X.; Zhao, X.; Zhang, H.; Liu, S.; Zhong, Y.; et al. Identification of prognostic biomarkers in hepatitis B virus-related hepatocellular carcinoma and stratification by integrative multi-omics analysis. J. Hepatol. 2014, 61, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Zhang, X.; Bai, Y.; Li, P.; Wei, C.; Song, T.; Zheng, Z.; Guan, K.; Zhang, Y.; Zhang, B.; et al. Overexpression of Mps1 in colon cancer cells attenuates the spindle assembly checkpoint and increases aneuploidy. Biochem. Biophys. Res. Commun. 2014, 450, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Cleveland, D.W. A chemical tool box defines mitotic and interphase roles for Mps1 kinase. J. Cell Biol. 2010, 190, 21–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innocenti, P.; Woodward, H.L.; Solanki, S.; Naud, S.; Westwood, I.M.; Cronin, N.; Hayes, A.; Roberts, J.; Henley, A.T.; Baker, R.; et al. Rapid discovery of pyrido[3,4-D]pyrimidine inhibitors of monopolar spindle kinase 1 (Mps1) using a structure-based hybridization approach. J. Med. Chem. 2016, 59, 3671–3688. [Google Scholar] [CrossRef] [PubMed]
- Naud, S.; Westwood, I.M.; Faisal, A.; Sheldrake, P.; Bavetsias, V.; Atrash, B.; Cheung, K.M.; Liu, M.; Hayes, A.; Schmitt, J.; et al. Structure-based design of orally bioavailable 1h-pyrrolo[3,2-C]pyridine inhibitors of mitotic kinase monopolar spindle 1 (Mps1). J. Med. Chem. 2013, 56, 10045–10065. [Google Scholar] [CrossRef] [PubMed]
- Uitdehaag, J.C.M.; de Man, J.; Willemsen-Seegers, N.; Prinsen, M.B.W.; Libouban, M.A.A.; Sterrenburg, J.G.; de Wit, J.J.P.; de Vetter, J.R.F.; de Roos, J.; Buijsman, R.C.; et al. Target residence time-guided optimization on TTK kinase results in inhibitors with potent anti-proliferative activity. J. Mol. Biol. 2017, 429, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Mattison, C.P.; Old, W.M.; Steiner, E.; Huneycutt, B.J.; Resing, K.A.; Ahn, N.G.; Winey, M. Mps1 activation loop autophosphorylation enhances kinase activity. J. Biol. Chem. 2007, 282, 30553–30561. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, N.; Jelluma, N.; Filippakopoulos, P.; Soundararajan, M.; Manak, M.S.; Kwon, M.; Choi, H.G.; Sim, T.; Deveraux, Q.L.; Rottmann, S.; et al. Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nat. Chem. Biol. 2010, 6, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiruma, Y.; Koch, A.; Hazraty, N.; Tsakou, F.; Medema, R.H.; Joosten, R.P.; Perrakis, A. Understanding inhibitor resistance in Mps1 kinase through novel biophysical assays and structures. J. Biol. Chem. 2017, 292, 14496–14504. [Google Scholar] [CrossRef] [PubMed]
- Gurden, M.D.; Westwood, I.M.; Faisal, A.; Naud, S.; Cheung, K.M.; McAndrew, C.; Wood, A.; Schmitt, J.; Boxall, K.; Mak, G.; et al. Naturally occurring mutations in the Mps1 gene predispose cells to kinase inhibitor drug resistance. Cancer Res. 2015, 75, 3340–3354. [Google Scholar] [CrossRef] [PubMed]
- Alimova, I.; Ng, J.; Harris, P.; Birks, D.; Donson, A.; Taylor, M.D.; Foreman, N.K.; Venkataraman, S.; Vibhakar, R. Mps1 kinase as a potential therapeutic target in medulloblastoma. Oncol. Rep. 2016, 36, 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Caldarelli, M.; Mennecozzi, M.; Giorgini, M.L.; Sola, F.; Cappella, P.; Perrera, C.; Depaolini, S.R.; Rusconi, L.; Cucchi, U.; et al. Targeting the mitotic checkpoint for cancer therapy with nms-P715, an inhibitor of Mps1 kinase. Cancer Res. 2010, 70, 10255–10264. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Maia, A.; Janssen, A.; Medema, R.H. Molecular basis underlying resistance to Mps1/TTK inhibitors. Oncogene 2016, 35, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Perreira, M.; Jiang, J.K.; Klutz, A.M.; Gao, Z.G.; Shainberg, A.; Lu, C.; Thomas, C.J.; Jacobson, K.A. “Reversine” and its 2-substituted adenine derivatives as potent and selective A3 adenosine receptor antagonists. J. Med. Chem. 2005, 48, 4910–4918. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Takanashi, S.; Zhang, Q.; Xiong, W.; Zhu, S.; Peters, E.C.; Ding, S.; Schultz, P.G. Reversine increases the plasticity of lineage-committed mammalian cells. Proc. Natl. Acad. Sci. USA 2007, 104, 10482–10487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santaguida, S.; Tighe, A.; D’Alise, A.M.; Taylor, S.S.; Musacchio, A. Dissecting the role of Mps1 in chromosome biorientation and the spindle checkpoint through the small molecule iInhibitor reversine. J. Cell Biol. 2010, 190, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Libouban, M.A.A.; de Roos, J.A.D.M.; Uitdehaag, J.C.M.; Willemsen-Seegers, N.; Mainardi, S.; Dylus, J.; de Man, J.; Tops, B.; Meijerink, J.P.P.; Storchova, Z.; et al. Stable aneuploid tumors cells are more sensitive to TTK inhibition than chromosomally unstable cell lines. Oncotarget 2017, 8, 38309–38325. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The Mm/Pbsa and Mm/Gbsa methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Pan, P.; Li, D.; Tian, S.; Li, Y.; Hou, T. Importance of protein flexibility in ranking inhibitor affinities: Modeling the binding mechanisms of piperidine carboxamides as Type I1/2 ALK inhibitors. Phys. Chem. Chem. Phys. 2015, 17, 6098–6113. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.; Tian, J.; Li, J.; Liu, H. Molecular dynamics studies on the enzalutamide resistance mechanisms induced by androgen receptor mutations. J. Cell Biochem. 2017, 118, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Han, R.; Li, J.; Liu, H.; Zheng, L. Molecular mechanism of R-bicalutamide switching from androgen receptor antagonist to agonist induced by amino acid mutations using molecular dynamics simulations and free energy calculation. J. Comput. Aided Mol. Des. 2016, 30, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Markwick, P.R.; Pierce, L.C.; Goodin, D.B.; McCammon, J.A. Adaptive accelerated molecular dynamics (Ad-Amd) revealing the molecular plasticity of P450cam. J. Phys. Chem. Lett. 2011, 2, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Nichols, S.E.; Gasper, P.M.; Metzger, V.T.; McCammon, J.A. Activation and dynamic network of the M2 muscarinic receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 10982–10987. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Allen, T.W. Bennett’s acceptance ratio and histogram analysis methods enhanced by umbrella sampling along a reaction coordinate in configurational space. J. Chem. Phys. 2012, 136, 164103. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huang, W.; Wen, F.; Larson, R.G. Efficient estimation of binding free energies between peptides and an MHC class II molecule using coarse-grained molecular dynamics simulations with a weighted histogram analysis method. J. Comput. Chem. 2017, 38, 2007–2019. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, Y.; Koch, A.; Dharadhar, S.; Joosten, R.P.; Perrakis, A. Structural basis of reversine selectivity in inhibiting Mps1 more potently than aurora B kinase. Proteins 2016, 84, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. Ucsf Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. Ff14sb: Improving the accuracy of protein side chain and backbone parameters from Ff99sb. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2014, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Kräutler, V.; Van Gunsteren, W.F.; Hünenberger, P.H. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Loncharich, R.J.; Brooks, B.R.; Pastor, R.W. Langevin dynamics of peptides: The frictional dependence of isomerization rates of N-acetylalanyl-N’-methylamide. Biopolymers 1992, 32, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Izaguirre, J.A.; Catarello, D.P.; Wozniak, J.M.; Skeel, R.D. Langevin stabilization of molecular dynamics. J. Chem. Phys. 2001, 114, 2090–2098. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R., III; McGee, T.D., Jr.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA. py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, H.; Li, Y.; Wang, J.; Hou, T. Assessing the performance of MM/PBSA and MM/GBSA methods. 3. The impact of force fields and ligand charge models. J. Phys. Chem. B 2013, 117, 8408–8421. [Google Scholar] [CrossRef] [PubMed]
- Onufriev, A.; Bashford, D.; David, A. Modification of the generalized born model suitable for macromolecules. J. Phys. Chem. 2000, 104, 3712–3720. [Google Scholar] [CrossRef]
- Kongsted, J.; Ryde, U. An improved method to predict the entropy term with the Mm/Pbsa approach. J. Comput. Aided Mol. Des. 2009, 23, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hamelberg, D.; Mongan, J.; McCammon, J.A. Accelerated molecular dynamics: A promising and efficient simulation method for biomolecules. J. Chem. Phys. 2004, 120, 11919–11929. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Sinko, W.; Pierce, L.; Bucher, D.; Walker, R.C.; McCammon, J.A. Improved reweighting of accelerated molecular dynamics simulations for free energy calculation. J. Chem. Theory Comput. 2014, 10, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Kozlikova, B.; Sebestova, E.; Sustr, V.; Brezovsky, J.; Strnad, O.; Daniel, L.; Bednar, D.; Pavelka, A.; Manak, M.; Bezdeka, M.; et al. Caver analyst 1.0: Graphic tool for interactive visualization and analysis of tunnels and channels in protein structures. Bioinformatics 2014, 30, 2684–2685. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rosenberg, J.M.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A. The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J. Comput. Chem. 1992, 13, 1011–1021. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Name | Reversine | Cpd-5 | NMS-P715 | |||
|---|---|---|---|---|---|---|
| Mps1WT | Mps1C604Y | Mps1WT | Mps1C604Y | Mps1WT | Mps1C604Y | |
| ΔEvdW | 54.07 ± 2.93 | 51.87 ± 3.47 | 68.01 ± 4.41 | −66.45 ± 3.7 | 70.88 ± 3.18 | −71.32 ± 4.8 |
| ΔEelec | 26.01 ± 3.67 | 24.07 ± 3.59 | 28.42 ± 3.26 | 21.86 ± 6.77 | 29.57 ± 3.35 | −20.63 ± 6.7 |
| ΔGGB | 41.86 ± 3.05 | 39.58 ± 2.91 | 48.95 ± 3.63 | 51.61 ± 2.39 | 52.95 ± 7.66 | 54.11±5.94 |
| ΔGSA | −6.22 ± 0.27 | −6.02 ± 0.31 | −8.37 ± 0.39 | −8.21 ± 0.33 | −8.45 ± 0.40 | −8.78 ± 0.50 |
| TΔS | 10.02 ± 3.22 | 11.92 ± 4.41 | 14.02 ± 4.62 | 21.92 ± 5.58 | 13.02 ± 4.57 | 20.92 ± 4.34 |
| ΔGbind | 34.43 ± 3.02 | 31.39 ± 3.66 | 41.91 ± 4.22 | 32.99 ± 4.34 | 42.94 ± 4.40 | 25.72 ± 4.64 |
| ΔWPMF | 16.05 ± 0.16 | 14.88 ± 0.09 | 17.14 ± 0.09 | 13.97 ± 0.43 | 16.81 ± 0.08 | 10.89 ± 0.17 |
| KD (nM) | 41 ± 21 | 86 ± 24 | 1.6 ± 0.2 | 471 ± 50 | 4.7 ± 2.5 | 1764 ± 204 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yu, W.; Jiang, C.-c.; Zheng, J.-g. Insights into Resistance Mechanisms of Inhibitors to Mps1 C604Y Mutation via a Comprehensive Molecular Modeling Study. Molecules 2018, 23, 1488. https://doi.org/10.3390/molecules23061488
Chen Y, Yu W, Jiang C-c, Zheng J-g. Insights into Resistance Mechanisms of Inhibitors to Mps1 C604Y Mutation via a Comprehensive Molecular Modeling Study. Molecules. 2018; 23(6):1488. https://doi.org/10.3390/molecules23061488
Chicago/Turabian StyleChen, Yuan, Wenquan Yu, Cui-cui Jiang, and Jin-gui Zheng. 2018. "Insights into Resistance Mechanisms of Inhibitors to Mps1 C604Y Mutation via a Comprehensive Molecular Modeling Study" Molecules 23, no. 6: 1488. https://doi.org/10.3390/molecules23061488