Protein Carriers for Glycoconjugate Vaccines: History, Selection Criteria, Characterization and New Trends
Abstract
:1. Introduction
2. The Concept of Hapten and Carrier
3. Polysaccharide Based Vaccines
4. From Polysaccharide Vaccines to Glycoconjugate Vaccines
5. Mechanism of Action of Glycoconjugate Vaccines
6. Traditional Protein Carriers
7. New Protein Carriers under Investigation
7.1. Proteins with Dual Role of Carrier and Antigen
7.2. Nanoparticle Carriers
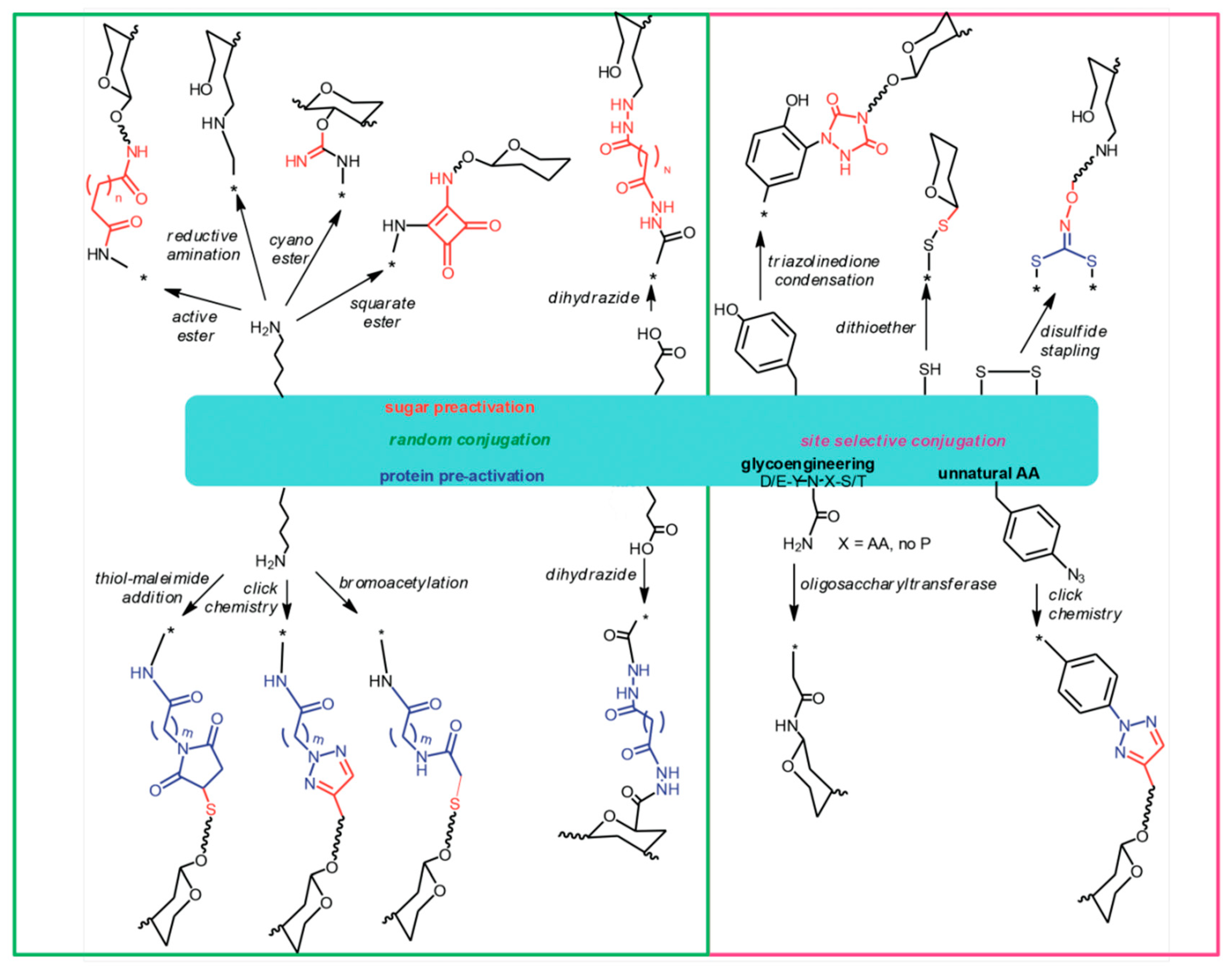
8. The Chemistry of Protein Carriers
9. Selection Criteria for Protein Carriers
10. Characterization of Protein Carriers
10.1. Characterization of Derivatized Protein Carriers
10.2. Characterization of the Conjugated Protein
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schneerson, R.; Barrera, O.; Sutton, A.; Robbins, J.B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980, 152, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Peltola, H.; Kayhty, H.; Sivonen, A.; Makela, H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: A double-blind field study of 100,000 vaccinees 3 months to 5 years of age in finland. Pediatrics 1977, 60, 730–737. [Google Scholar] [PubMed]
- Koskela, M.; Leinonen, M.; Haiva, V.M.; Timonen, M.; Makela, P.H. First and second dose antibody responses to pneumococcal polysaccharide vaccine in infants. Pediatr. Infect. Dis. 1986, 5, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.A.V. The response of infants to inoculation with type I pneumococcus carbohydrate 1. J. Immunol. 1937, 33, 1–7. [Google Scholar]
- Pollard, A.J.; Perrett, K.P.; Beverley, P.C. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 2009, 9, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.F.; Pollard, A.J.; Moxon, E.R. Immunological memory: The role of b cells in long-term protection against invasive bacterial pathogens. JAMA 2005, 294, 3019–3023. [Google Scholar] [CrossRef] [PubMed]
- Costantino, P.; Rappuoli, R.; Berti, F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin. Drug Discov. 2011, 6, 1045–1066. [Google Scholar] [CrossRef] [PubMed]
- Landsteiner, K. Experiments on anaphylaxis to azoproteins. J. Exp. Med. 1924, 39, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Avery, O.T.; Goebel, W.F. Chemo-immunological studies on conjugated carbohydrate-proteins: II. Immunological specificity of synthetic sugar-protein antigens. J. Exp. Med. 1929, 50, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Goebel, W.F.; Avery, O.T. Chemo-immunological studies on conjugated carbohydrate-proteins: IV. The synthesis of thep-aminobenzyl ether of the soluble specific substance of type III pneumococcus and its coupling with protein. J. Exp. Med. 1931, 54, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Finland, M.; Sutliff, W.D. Specific antibody response of human subjects to intracutaneous injection of pneumococcus products. J. Exp. Med. 1932, 55, 853–865. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, C.M.; Hodges, R.G. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J. Exp. Med. 1945, 82, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Costantino, P.; Adamo, R. Potential targets for next generation anti-microbial glycoconjugate vaccines. FEMS Microbiol. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.J. Human immune responses to polysaccharide antigens: An analysis of bacterial polysaccharide vaccines in infants. Adv. Pediatr. 1985, 32, 139–158. [Google Scholar] [PubMed]
- Weintraub, A. Immunology of bacterial polysaccharide antigens. Carbohydr. Res. 2003, 338, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallari, M.; Stallforth, P.; Kalinichenko, A.; Rathwell, D.C.; Gronewold, T.M.; Adibekian, A.; Mori, L.; Landmann, R.; Seeberger, P.H.; De Libero, G. A semisynthetic carbohydrate-lipid vaccine that protects against S. Pneumoniae in mice. Nat. Chem. Biol. 2014, 10, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Zhou, Z.; Suryawanshi, S.; Mondal, M.A.; Guo, Z. Fully synthetic self-adjuvanting alpha-2,9-oligosialic acid based conjugate vaccines against group C meningitis. ACS Cent. Sci. 2016, 2, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.J.; Deck, R.R.; Liu, M.A. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseria meningitidis outer membrane protein complex conjugate vaccine. J. Immunol. 1990, 145, 3071–3079. [Google Scholar] [PubMed]
- Kilpi, T.; Ahman, H.; Jokinen, J.; Lankinen, K.S.; Palmu, A.; Savolainen, H.; Gronholm, M.; Leinonen, M.; Hovi, T.; Eskola, J.; et al. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: Randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 2003, 37, 1155–1164. [Google Scholar] [PubMed]
- Forsgren, A.; Riesbeck, K.; Janson, H. Protein D of Haemophilus influenzae: A protective nontypeable H. Influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin. Infect. Dis. 2008, 46, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Prymula, R.; Peeters, P.; Chrobok, V.; Kriz, P.; Novakova, E.; Kaliskova, E.; Kohl, I.; Lommel, P.; Poolman, J.; Prieels, J.-P.; et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both streptococcus pneumoniae and non-typable Haemophilus influenzae: A randomised double-blind efficacy study. Lancet 2006, 367, 740–748. [Google Scholar] [CrossRef]
- Giannini, G.; Rappuoli, R.; Ratti, G. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res. 1984, 12, 4063–4069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malito, E.E.; Bursulaya, B.B.; Chen, C.C.; Lo Surdo, P.P.; Picchianti, M.M.; Balducci, E.E.; Biancucci, M.M.; Brock, A.A.; Berti, F.F.; Bottomley, M.J.M.; et al. Structural basis for lack of toxicity of the diphtheria toxin mutant CRM197. Proc. Natl. Acad. Sci. USA 2012, 109, 5229–5234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickey, J.M.; Toprani, V.M.; Kaur, K.; Mishra, R.P.N.; Goel, A.; Oganesyan, N.; Lees, A.; Sitrin, R.; Joshi, S.B.; Volkin, D.B. Analytical comparability assessments of five recombinant CRM197 proteins from different manufacturers and expression systems. J. Pharm. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bröker, M.; Costantino, P.; DeTora, L.; McIntosh, E.D.; Rappuoli, R. Biochemical and biological characteristics of cross-reacting material 197 (CRM197), a non-toxic mutant of diphtheria toxin: Use as a conjugation protein in vaccines and other potential clinical applications. Biologicals 2011, 39, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Shinefield, H.R. Overview of the development and current use of CRM197 conjugate vaccines for pediatric use. Vaccine 2010, 28, 4335–4339. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Ashkenazi, S.; Green, M.S.; Gdalevich, M.; Robin, G.; Slepon, R.; Yavzori, M.; Orr, N.; Block, C.; Ashkenazi, I.; et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet 1997, 349, 155–159. [Google Scholar] [CrossRef]
- Fattom, A.; Schneerson, R.; Watson, D.C.; Karakawa, W.W.; Fitzgerald, D.; Pastan, I.; Li, X.; Shiloach, J.; Bryla, D.A.; Robbins, J.B. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect. Immun. 1993, 61, 1023–1032. [Google Scholar] [PubMed]
- Kossaczka, Z.; Lin, F.Y.; Ho, V.A.; Thuy, N.T.; Van Bay, P.; Thanh, T.C.; Khiem, H.B.; Trach, D.D.; Karpas, A.; Hunt, S.; et al. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in vietnam. Infect. Immun. 1999, 67, 5806–5810. [Google Scholar] [PubMed]
- Szu, S.C.; Stone, A.L.; Robbins, J.D.; Schneerson, R.; Robbins, J.B. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J. Exp. Med. 1987, 166, 1510–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatz, C.F.R.; Bally, B.; Rohrer, S.; Steffen, R.; Kramme, S.; Siegrist, C.-A.; Wacker, M.; Alaimo, C.; Fonck, V.G. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: A single blind, partially randomized phase I study. Vaccine 2015, 33, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.S.; Kaminski, R.W.; Di Paolo, C.; Porter, C.K.; Gutierrez, R.L.; Clarkson, K.A.; Weerts, H.E.; Duplessis, C.; Castellano, A.; Alaimo, C.; et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: A single-blind, randomized phase I study. Clin. Vaccine Immunol. 2016, 23, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Hatz, C.; van den Dobbelsteen, G.; Abbanat, D.; Hornacek, A.; Frolich, R.; Dreyer, A.M.; Martin, P.; Davies, T.; Fae, K.; et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: A randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect. Dis. 2017, 17, 528–537. [Google Scholar] [CrossRef]
- Wacker, M.; Wang, L.; Kowarik, M.; Dowd, M.; Lipowsky, G.; Faridmoayer, A.; Shields, K.; Park, S.; Alaimo, C.; Kelley, K.A.; et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J. Infect. Dis. 2014, 209, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Tontini, M.; Romano, M.R.; Proietti, D.; Balducci, E.; Micoli, F.; Balocchi, C.; Santini, L.; Masignani, V.; Berti, F.; Costantino, P. Preclinical studies on new proteins as carrier for glycoconjugate vaccines. Vaccine 2016, 34, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Baraldo, K.; Mori, E.; Bartoloni, A.; Norelli, F.; Grandi, G.; Rappuoli, R.; Finco, O.; Del Giudice, G. Combined conjugate vaccines: Enhanced immunogenicity with the N19 polyepitope as a carrier protein. Infect. Immun. 2005, 73, 5835–5841. [Google Scholar] [CrossRef] [PubMed]
- Baraldo, K.; Mori, E.; Bartoloni, A.; Petracca, R.; Giannozzi, A.; Norelli, F.; Rappuoli, R.; Grandi, G.; Del Giudice, G. N19 polyepitope as a carrier for enhanced immunogenicity and protective efficacy of meningococcal conjugate vaccines. Infect. Immun. 2004, 72, 4884–4887. [Google Scholar] [CrossRef] [PubMed]
- Falugi, F.; Petracca, R.; Mariani, M.; Luzzi, E.; Mancianti, S.; Carinci, V.; Melli, M.L.; Finco, O.; Wack, A.; Di Tommaso, A.; et al. Rationally designed strings of promiscuous CD4(+) T cell epitopes provide help to Haemophilus influenzae type b oligosaccharide: A model for new conjugate vaccines. Eur. J. Immunol. 2001, 31, 3816–3824. [Google Scholar] [CrossRef]
- Bongat, A.F.G.; Saksena, R.; Adamo, R.; Fujimoto, Y.; Shiokawa, Z.; Peterson, D.C.; Fukase, K.; Vann, W.F.; Kováč, P. Multimeric bivalent immunogens from recombinant tetanus toxin Hc fragment, synthetic hexasaccharides, and a glycopeptide adjuvant. Glycoconj. J. 2010, 27, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Rothbard, J.B.; Taylor, W.R. A sequence pattern common to T cell epitopes. EMBO J. 1988, 7, 93–100. [Google Scholar] [PubMed]
- Bixler, G.S., Jr.; Eby, R.; Dermody, K.M.; Woods, R.M.; Seid, R.C.; Pillai, S. Synthetic peptide representing a T-cell epitope of CRM197 substitutes as carrier molecule in a Haemophilus influenzae type b (Hib) conjugate vaccine. Adv. Exp. Med. Biol. 1989, 251, 175–180. [Google Scholar] [PubMed]
- Alexander, J.; del Guercio, M.F.; Maewal, A.; Qiao, L.; Fikes, J.; Chesnut, R.W.; Paulson, J.; Bundle, D.R.; DeFrees, S.; Sette, A. Linear padre T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 2000, 164, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; del Guercio, M.F.; Frame, B.; Maewal, A.; Sette, A.; Nahm, M.H.; Newman, M.J. Development of experimental carbohydrate-conjugate vaccines composed of Streptococcus pneumoniae capsular polysaccharides and the universal helper T-lymphocyte epitope (PADRE). Vaccine 2004, 22, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Belot, F.; Guerreiro, C.; Baleux, F.; Mulard, L.A. Synthesis of two linear PADRE conjugates bearing a deca- or pentadecasaccharide B epitope as potential synthetic vaccines against Shigella flexneri serotype 2a infection. Chemistry 2005, 11, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Ingale, S.; Wolfert, M.A.; Gaekwad, J.; Buskas, T.; Boons, G.J. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat. Chem. Biol. 2007, 3, 663–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, H.; Dziadek, S.; Bundle, D.R.; Cutler, J.E. Synthetic glycopeptide vaccines combining β-mannan and peptide epitopes induce protection against candidiasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13526–13531. [Google Scholar] [CrossRef] [PubMed]
- Broker, M.; Berti, F.; Schneider, J.; Vojtek, I. Polysaccharide conjugate vaccine protein carriers as a “neglected valency”—Potential and limitations. Vaccine 2017, 35, 3286–3294. [Google Scholar] [CrossRef] [PubMed]
- Michon, F.; Fusco, P.C.; Minetti, C.A.; Laude-Sharp, M.; Uitz, C.; Huang, C.H.; D’Ambra, A.J.; Moore, S.; Remeta, D.P.; Heron, I.; et al. Multivalent pneumococcal capsular polysaccharide conjugate vaccines employing genetically detoxified pneumolysin as a carrier protein. Vaccine 1998, 16, 1732–1741. [Google Scholar] [CrossRef]
- Pozzi, C.; Wilk, K.; Lee, J.C.; Gening, M.; Nifantiev, N.; Pier, G.B. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS ONE 2012, 7, e46648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, V.B.; Burden, R.; Wagner, A.; Moran, E.E.; Lee, C.H. The development of an experimental multiple serogroups vaccine for Neisseria meningitidis. PLoS ONE 2013, 8, e79304. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Leuzzi, R.; Cappelletti, E.; Tontini, M.; Nilo, A.; Proietti, D.; Berti, F.; Costantino, P.; Adamo, R.; Scarselli, M. Recombinant Clostridium difficile toxin fragments as carrier protein for PSII surface polysaccharide preserve their neutralizing activity. Toxins 2014, 6, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Nilo, A.; Morelli, L.; Passalacqua, I.; Brogioni, B.; Allan, M.; Carboni, F.; Pezzicoli, A.; Zerbini, F.; Maione, D.; Fabbrini, M.; et al. Anti-group B Streptococcus glycan-conjugate vaccines using pilus protein GBS80 as carrier and antigen: Comparing lysine and tyrosine-directed conjugation. ACS Chem. Biol. 2015, 10, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Nilo, A.; Passalacqua, I.; Fabbrini, M.; Allan, M.; Usera, A.; Carboni, F.; Brogioni, B.; Pezzicoli, A.; Cobb, J.; Romano, M.R.; et al. Exploring the effect of conjugation site and chemistry on the immunogenicity of an anti-group B Streptococcus glycoconjugate vaccine based on GBS67 pilus protein and type V polysaccharide. Bioconjug. Chem. 2015, 26, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Tennant, S.M.; Wang, J.Y.; Schmidlein, P.J.; Lees, A.; Ernst, R.K.; Pasetti, M.F.; Galen, J.E.; Levine, M.M. Salmonella enterica serovar enteritidis core o polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. Enteritidis. Infect. Immun. 2011, 79, 4240–4249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X. Synthetic carbohydrate antigens for HIV vaccine design. Curr. Opin. Chem. Biol. 2013, 17, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamborrini, M.; Werz, D.B.; Frey, J.; Pluschke, G.; Seeberger, P.H. Anti-carbohydrate antibodies for the detection of Anthrax spores. Angew. Chem. Int. Ed. Engl. 2006, 45, 6581–6582. [Google Scholar] [CrossRef] [PubMed]
- Polonskaya, Z.; Deng, S.; Sarkar, A.; Kain, L.; Comellas-Aragones, M.; McKay, C.S.; Kaczanowska, K.; Holt, M.; McBride, R.; Palomo, V.; et al. T cells control the generation of nanomolar-affinity anti-glycan antibodies. J. Clin. Investig. 2017, 127, 1491–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, N.L.; Goyette-Desjardins, G.; Nothaft, H.; Valguarnera, E.; Szymanski, C.M.; Segura, M.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles: A novel platform for bacterial vaccines. Sci. Rep. 2016, 6, 24931. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Valentine, J.L.; Huang, C.J.; Endicott, C.E.; Moeller, T.D.; Rasmussen, J.A.; Fletcher, J.R.; Boll, J.M.; Rosenthal, J.A.; Dobruchowska, J.; et al. Outer Membrane Vesicles displaying engineered glycotopes elicit protective antibodies. Proc. Natl. Acad. Sci. USA 2016, 113, E3609–E3618. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.C.; Cywes-Bentley, C.; Moeller, T.D.; Weyant, K.B.; Putnam, D.; Chang, Y.F.; Jones, B.D.; Pier, G.B.; DeLisa, M.P. Immunization with Outer Membrane Vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc. Natl. Acad. Sci. USA 2018, 115, E3106–E3115. [Google Scholar] [CrossRef] [PubMed]
- Valguarnera, E.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles as a platform for vaccine development. Methods Enzymol. 2017, 597, 285–310. [Google Scholar] [PubMed]
- Said Hassane, F.; Phalipon, A.; Tanguy, M.; Guerreiro, C.; Belot, F.; Frisch, B.; Mulard, L.A.; Schuber, F. Rational design and immunogenicity of liposome-based diepitope constructs: Application to synthetic oligosaccharides mimicking the Shigella flexneri 2a o-antigen. Vaccine 2009, 27, 5419–5426. [Google Scholar] [CrossRef] [PubMed]
- Safari, D.; Marradi, M.; Chiodo, F.; Th Dekker, H.A.; Shan, Y.; Adamo, R.; Oscarson, S.; Rijkers, G.T.; Lahmann, M.; Kamerling, J.P.; et al. Gold nanoparticles as carriers for a synthetic Streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine 2012, 7, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Vetro, M.; Safari, D.; Fallarini, S.; Salsabila, K.; Lahmann, M.; Penadés, S.; Lay, L.; Marradi, M.; Compostella, F. Preparation and immunogenicity of gold glyco-nanoparticles as antipneumococcal vaccine model. Nanomedicine 2017, 12, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Kelly, M.; Vann, W.F.; Qadri, F.; Ryan, E.T.; Kovac, P. Conjugate vaccines from bacterial antigens by squaric acid chemistry: A closer look. ChemBioChem 2017, 18, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Marburg, S.; Jorn, D.; Tolman, R.L.; Arison, B.; McCauley, J.; Kinskern, P.J.; Hagopian, A.; Vella, P.P. Bimolecular chemistry of macromolecules: Synthesis of bacterial polysaccharide conjugates with Neissseria meningitis membrane protein. J. Am. Chem. Soc. 1986, 108, 5282–5287. [Google Scholar] [CrossRef]
- Lipinski, T.; Luu, T.; Kitov, P.I.; Szpacenko, A.; Bundle, D.R. A structurally diversified linker enhances the immune response to a small carbohydrate hapten. Glycoconj. J. 2011, 28, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Stefanetti, G.; Hu, Q.Y.; Usera, A.; Robinson, Z.; Allan, M.; Singh, A.; Imase, H.; Cobb, J.; Zhai, H.; Quinn, D.; et al. Sugar-protein connectivity impacts on the immunogenicity of site-selective Salmonella O-antigen glycoconjugate vaccines. Angew. Chem. Int. Ed. Engl. 2015, 54, 13198–13203. [Google Scholar] [CrossRef] [PubMed]
- Crotti, S.; Zhai, H.; Zhou, J.; Allan, M.; Proietti, D.; Pansegrau, W.; Hu, Q.-Y.; Berti, F.; Adamo, R. Defined conjugation of glycans to the lysines of CRM197 guided by their reactivity mapping. ChemBioChem 2014, 15, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Grayson, E.J.; Bernardes, G.J.; Chalker, J.M.; Boutureira, O.; Koeppe, J.R.; Davis, B.G. A coordinated synthesis and conjugation strategy for the preparation of homogeneous glycoconjugate vaccine candidates. Angew. Chem. Int. Ed. Engl. 2011, 50, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-Y.; Berti, F.; Adamo, R. Towards the next generation of biomedicines by site-selective conjugation. Chem. Soc. Rev. 2016, 45, 1691–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowarik, M.; Young, N.M.; Numao, S.; Schulz, B.L.; Hug, I.; Callewaert, N.; Mills, D.C.; Watson, D.C.; Hernandez, M.; Kelly, J.F.; et al. Definition of the bacterial N-glycosylation site consensus sequence. EMBO J. 2006, 25, 1957–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, M.F.; Wacker, M.; Hernandez, M.; Hitchen, P.G.; Marolda, C.L.; Kowarik, M.; Morris, H.R.; Dell, A.; Valvano, M.A.; Aebi, M. Engineering N-linked protein glycosylation with diverse o antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl. Acad. Sci. USA 2005, 102, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, J.; Kowarik, M.; Dilettoso, S.; Tanner, C.; Wacker, M.; Thöny-Meyer, L. Production of glycoprotein vaccines in Escherichia coli. Microb. Cell Fact. 2010, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Vanjak, I.; Rozzelle, J.; Berges, A.; Chan, W.; Yin, G.; Tran, C.; Sato, A.K.; Steiner, A.R.; Pham, T.P.; et al. Malaria derived glycosylphosphatidylinositol anchor enhances anti-Pfs25 functional antibodies that block malaria transmission. Biochemistry 2018, 57, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, Y.J.; Malley, R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 13564–13569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanawastien, A.; Cartee, R.T.; Griffin, I.; Thomas, J.; Killeen, K.P.; Mekalanos, J.J. Conjugate-like immunogens produced as protein capsular matrix vaccines. Proc. Natl. Acad. Sci. USA 2015, 112, E1143–E1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.H.; Zhang, G.; Nayerhoda, R.; Beitelshees, M.; Hill, A.; Rostami, P.; Li, Y.; Davidson, B.A.; Knight, P., 3rd; Pfeifer, B.A. Comprehensive vaccine design for commensal disease progression. Sci. Adv. 2017, 3, e1701797. [Google Scholar] [CrossRef] [PubMed]
- Frech, C.; Hilbert, A.K.; Hartmann, G.; Mayer, K.; Sauer, T.; Bolgiano, B. Physicochemical analysis of purified diphtheria toxoids: Is toxoided then purified the same as purified then toxoided? Dev. Biol. 2000, 103, 205–215. [Google Scholar]
- Donnarumma, D.; Faleri, A.; Costantino, P.; Rappuoli, R.; Norais, N. The role of structural proteomics in vaccine development: Recent advances and future prospects. Expert Rev. Proteom. 2016, 13, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Malito, E.; Carfi, A.; Bottomley, M. Protein crystallography in vaccine research and development. Int. J. Mol. Sci. 2015, 16, 13106–13140. [Google Scholar] [CrossRef] [PubMed]
- Malito, E.; Faleri, A.; Lo Surdo, P.; Veggi, D.; Maruggi, G.; Grassi, E.; Cartocci, E.; Bertoldi, I.; Genovese, A.; Santini, L.; et al. Defining a protective epitope on factor H binding protein, a key meningococcal virulence factor and vaccine antigen. Proc. Natl. Acad. Sci. USA 2013, 110, 3304–3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, X.Y.; Keyhani, N.O.; Lee, Y.C. Spectrophotometric determination of hydrazine, hydrazides, and their mixtures with trinitrobenzenesulfonic acid. Anal. Biochem. 1988, 175, 139–144. [Google Scholar] [CrossRef]
- Micoli, F.; Rondini, S.; Pisoni, I.; Proietti, D.; Berti, F.; Costantino, P.; Rappuoli, R.; Szu, S.; Saul, A.; Martin, L.B. Vi-CRM197 as a new conjugate vaccine against Salmonella typhi. Vaccine 2011, 29, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Kiick, K.L.; Saxon, E.; Tirrell, D.A.; Bertozzi, C.R. Incorporation of azides into recombinant proteins for chemoselective modification by the staudinger ligation. Proc. Natl. Acad. Sci. USA 2002, 99, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Seid, R.C.; Boykins, R.A.; Liu, D.F.; Kimbrough, K.W.; Hsieh, C.L.; Eby, R. Chemical evidence for covalent linkages of a semi-synthetic glycoconjugate vaccine for Haemophilus influenzae type b disease. Glycoconj. J. 1989, 6, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Barazzone, G.C.; Pinto, V.; Donnarumma, D.; Tanizaki, M.M.; Norais, N.; Berti, F. Identification of glycosylated regions in pneumococcal PspA conjugated to serotype 6B capsular polysaccharide. Glycoconj. J. 2014, 31, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Bardotti, A.; Averani, G.; Berti, F.; Berti, S.; Carinci, V.; D’Ascenzi, S.; Fabbri, B.; Giannini, S.; Giannozzi, A.; Magagnoli, C.; et al. Physicochemical characterisation of glycoconjugate vaccines for prevention of meningococcal diseases. Vaccine 2008, 26, 2284–2296. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, G.; Salvini, L.; Gotta, S.; Cescutti, P.; Micoli, F. Investigation on sugar-protein connectivity in Salmonella O-antigen glycoconjugate vaccines. Bioconjug. Chem. 2018, 29, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Poolman, J.; Siegrist, C. Glycoconjugate vaccines and immune interference: A review. Vaccine 2010, 28, 5513–5524. [Google Scholar] [CrossRef] [PubMed]
- Gerritzen, M.J.H.; Martens, D.E.; Wijffels, R.H.; van der Pol, L.; Stork, M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 2017, 35, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Adamo, R.; Hu, Q.-Y.; Torosantucci, A.; Crotti, S.; Brogioni, G.; Allan, M.; Chiani, P.; Bromuro, C.; Quinn, D.; Tontini, M.; et al. Deciphering the structure–immunogenicity relationship of anti-Candida glycoconjugate vaccines. Chem. Sci. 2014, 5, 4302–4311. [Google Scholar] [CrossRef]
- Pozsgay, V.; Chu, C.; Pannell, L.; Wolfe, J.; Robbins, J.B.; Schneerson, R. Protein conjugates of synthetic saccharides elicit higher levels of serum IgG lipopolysaccharide antibodies in mice than do those of the O-specific polysaccharide from Shigella dysenteriae type 1. Proc. Natl. Acad. Sci. USA 1999, 96, 5194–5197. [Google Scholar] [CrossRef] [PubMed]
| Category | Attribute Measured | Key Techniques | Comment | Impact on Glycoconjugate |
|---|---|---|---|---|
| Purity | % of target protein | SDS-PAGE, SEC-HPLC, RP-HPLC | Impact on consistency, potency and safety | Low purity might reflect in poor glycoconjugate purity |
| Truncated or degradation forms | SEC/RP-HPLC, MS, ELISA | Product-related substances or impurities (depending on retention of biological activity) | Heterogeneity of glycoconjugates which might impact on consistency and immunogenicity | |
| Aggregation | SEC-MALS, DLS, MS | Aggregation is source of heterogeneity: impact on manufacturing consistency, immunogenicity, and safety | Heterogeneity of glycoconjugates which might impact on consistency and immunogenicity | |
| Impurities | Host cell residual proteins | WB, ELISA, MS | Poor control of process related impurities might impact on consistency, potency and safety | Poor control of process related impurities might impact on consistency, potency and safety |
| DNA | Picogreen, Threshold | |||
| Chemical residuals | HPLC, Colorimetric assays, NMR | |||
| Contaminants | Endotoxins, Sterility or Bioburden | LAL, Microbial count, Compendial sterility test | Impact on safety | Impact on safety |
| Identity and Structure | Antibody binding | WB, ELISA | Immunochemical identity | Important if dual role of carrier and antigen is considered |
| Key Protein B cell Epitopes | Epitope mapping tools: X-ray, HDX-MS, SPR | Mapping of key functional protein epitopes | Important to precisely map key protein B cell epitopes if dual role of carrier and antigen is considered | |
| Primary structure, Intact Mass, Amino acidic composition | MS, Amino acids analysis | Classical physicochemical protein identification tests | Well defined protein moiety of glycoconjugate | |
| Structure | Secondary structure | CD | Structure knowledge important if dual role of carrier and antigen is considered | Important to compare structure before and after conjugation if dual role of carrier and antigen is considered |
| Tertiary structure | FLR, DSC, X-ray, EM | |||
| Lipidation, Deamidation, N-terminal methionine, Glycation | MS, SDS- PAGE, WB, ELISA, CE | Post-translational modifications | Lipidation might provide adjuvant effect; deamidation might results in additional conjugation sites (depending on the chemistry); glycation might interfere with carbohydrate analyses on conjugates | |
| Stability | Purity, Integrity, Identity, Sterility/Bioburden | SDS PAGE, SEC-HPLC, RP-HPLC, SEC-MALS, WB, DLS | Stability protocols at different temperatures are practices common to all biological products | Impact on glycoconjugate stability |
| Toxicity | Safety | In vitro tests, animal studies | Standard for vaccines | Standard for vaccines |
| Category | Attribute Measured | Key Techniques | Comment | Impact on Glycoconjugate |
|---|---|---|---|---|
| Structure | Extent of chemical derivatization | MS and/or colorimetric assays suitable for the kind of derivatization (e.g., hydrazide, thiol) | Level of chemical derivatization can inform conjugation stoichiometry. Can impact on protein folding, protein T and B cell epitope preservation and aggregate formation | Level of chemical derivatization dictates the maximum protein to carbohydrate ratio, and immunogenicity |
| Site of chemical derivatization | MS | Can impact on protein epitopes | Informs the conjugate structure and impact on immunogenicity | |
| Genetic derivatization | DNA Sequence, MS, in vitro labeling techniques | Glycosylation sequences or un-natural amino acids amenable for conjugation can be inserted into the carrier protein | Precisely inform the glycoconjugate structure | |
| Purity | Aggregation | SEC-MALS, DLS, MS, SDS-PAGE (covalent aggregates) | Chemical derivatization can cause covalent or non-covalent aggregation with consequent glycoconjugate heterogeneity | Heterogeneity of glycoconjugates might impact on consistency and immunogenicity |
| Identity | Antibody binding | WB, ELISA, SPR | Mapping of key functional protein epitopes | Important to preserve key protein B cell epitopes during conjugation if dual role of carrier and antigen is considered |
| Category | Attribute Measured | Technique | Comment |
|---|---|---|---|
| Proof of conjugation (potency) | Covalent linkage between protein and glycans | SDS PAGE, WB, MS, SEC-HPLC | Covalent linkage is the gold standard for glycoconjugate vaccines |
| Extent of conjugation | Protein to Carbohydrate ratio | Protein content in conjunction with sugar quantification: HPAEC-PAD, colorimetric assays, Mass Spec | Mass Spec is applicable only to conjugates with well-defined synthetic carbohydrates |
| Identity | Antibody binding | WB, ELISA, SPR | Mapping of key functional protein epitopes |
| Identity and Structure | Key protein B cell epitope | Epitope mapping tools, X-Ray, HDX-MS, SPR, in vitro potency assays | Key protein B cell epitopes should be preserved if dual role of carrier and antigen is considered |
| Structure | Location of saccharide chains | MS | Confirmatory for site selective conjugation methods and glycoengineering methods where attachment sites are predetermined |
| Structure | Secondary and tertiary structure | CD, FLR, DSC | Secondary structure can be modified as result of glycoconjugation |
| Potency | Free protein | SDS PAGE, SEC-HPLC, CE | Levels of un-conjugated protein might interfere with glycoconjugate immunogenicity |
| Purity | Aggregation and multimerization | SDS PAGE, HPLC, SEC-MALS, DLS | Heterogeneity of glycoconjugates might impact on consistency and immunogenicity |
| Stability | Purity, Integrity, Identity, Sterility/Bioburden, Free protein | SEC-HPLC, SEC-MALS, SDS PAGE, WB | Generally part of the stability program for the glycoconjugate antigen |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micoli, F.; Adamo, R.; Costantino, P. Protein Carriers for Glycoconjugate Vaccines: History, Selection Criteria, Characterization and New Trends. Molecules 2018, 23, 1451. https://doi.org/10.3390/molecules23061451
Micoli F, Adamo R, Costantino P. Protein Carriers for Glycoconjugate Vaccines: History, Selection Criteria, Characterization and New Trends. Molecules. 2018; 23(6):1451. https://doi.org/10.3390/molecules23061451
Chicago/Turabian StyleMicoli, Francesca, Roberto Adamo, and Paolo Costantino. 2018. "Protein Carriers for Glycoconjugate Vaccines: History, Selection Criteria, Characterization and New Trends" Molecules 23, no. 6: 1451. https://doi.org/10.3390/molecules23061451






