Pentacyclic Triterpenes from Cecropia telenitida Can Function as Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material Collection
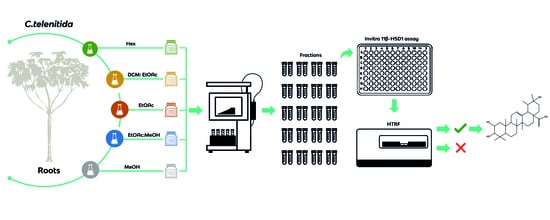
2.3. Extracts, Fractionation, and Fraction Library Obtained from Cecropia telenitida Roots
2.4. Fraction Solubility Test
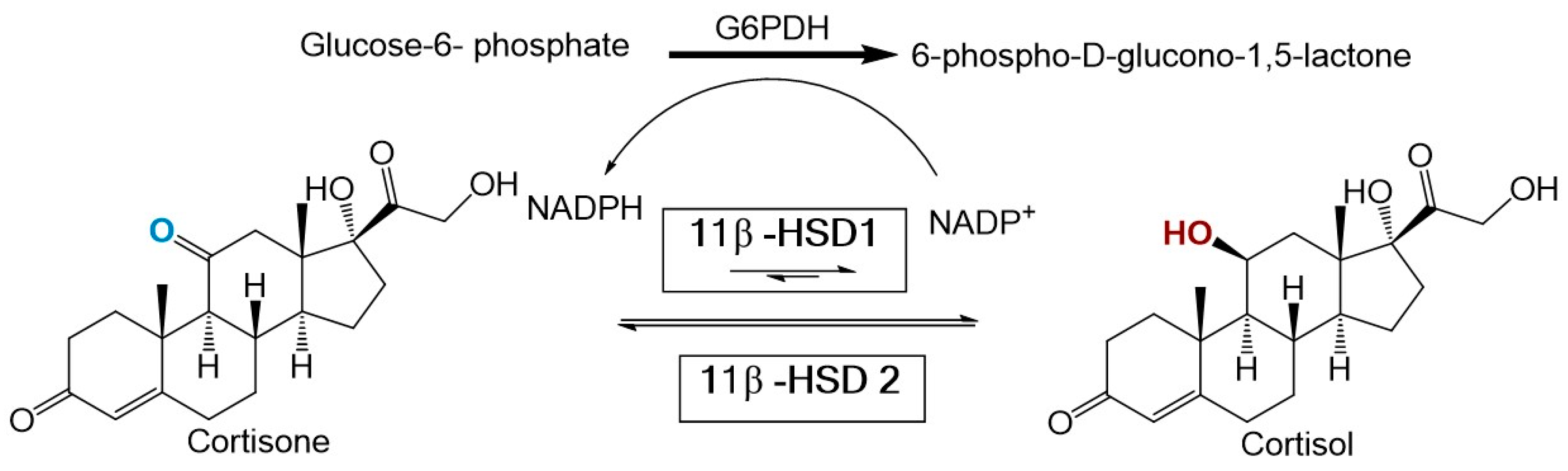
2.5. In Vitro 11β-HSD1 Assay
2.6. Chemical Library Screening
2.7. Structural Elucidation
3. Results and Discussion
3.1. Pre-Fractionated Library from C. telenitida Roots
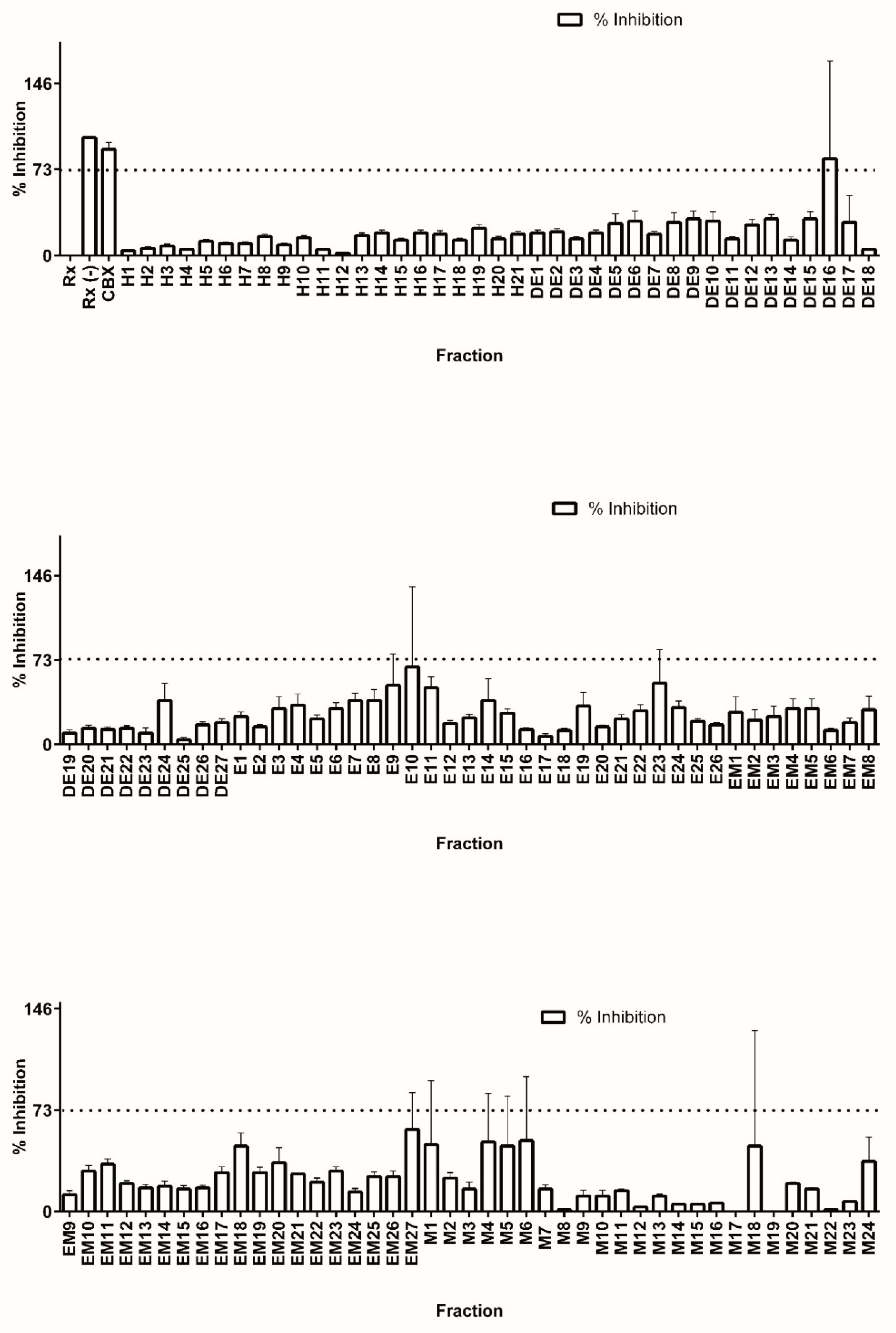
3.2. Library Screening for 11β-HSD1 Inhibitors
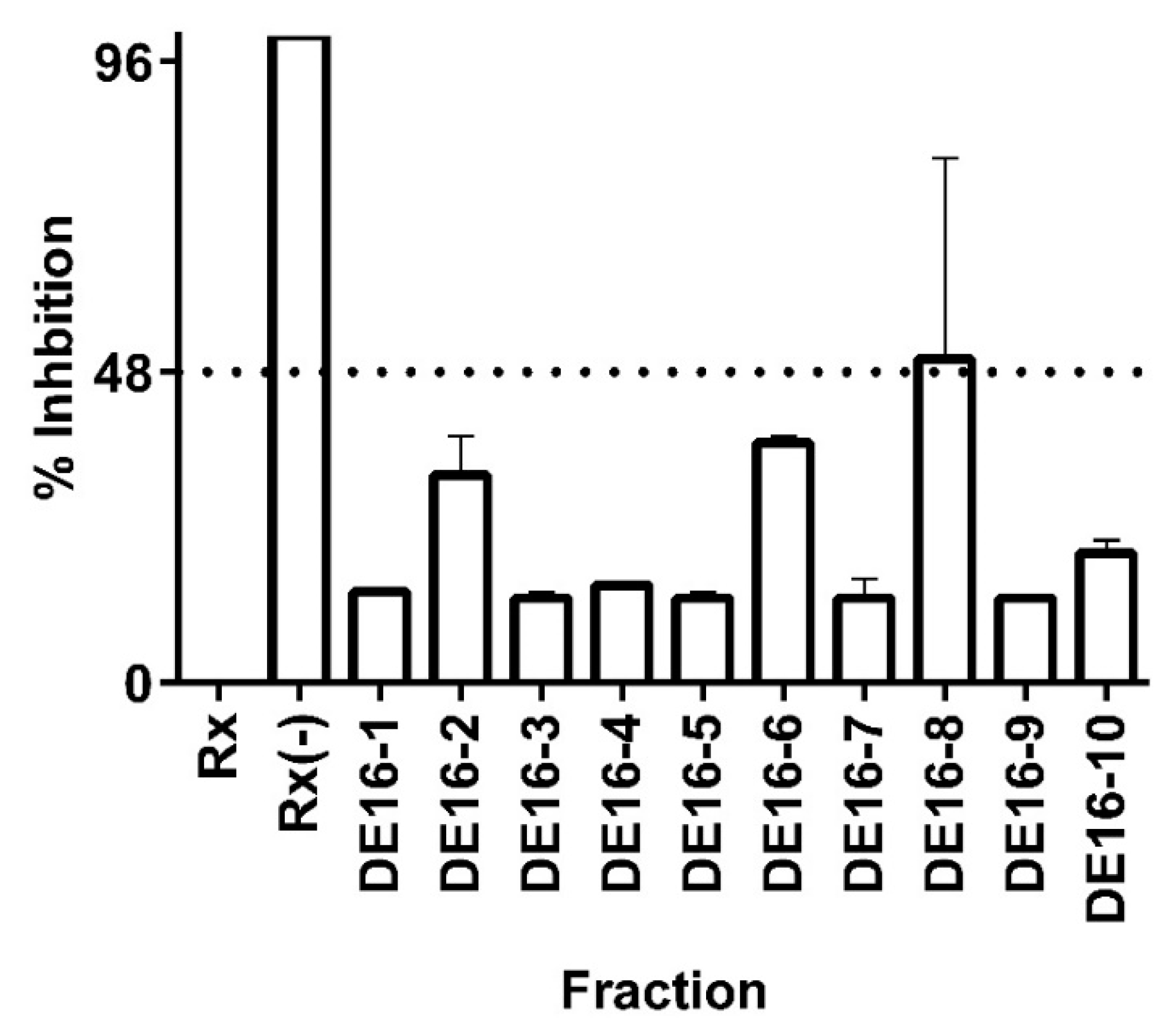
3.3. Bioassay-Guided Identification of 11β-HSD1 Inhibitors
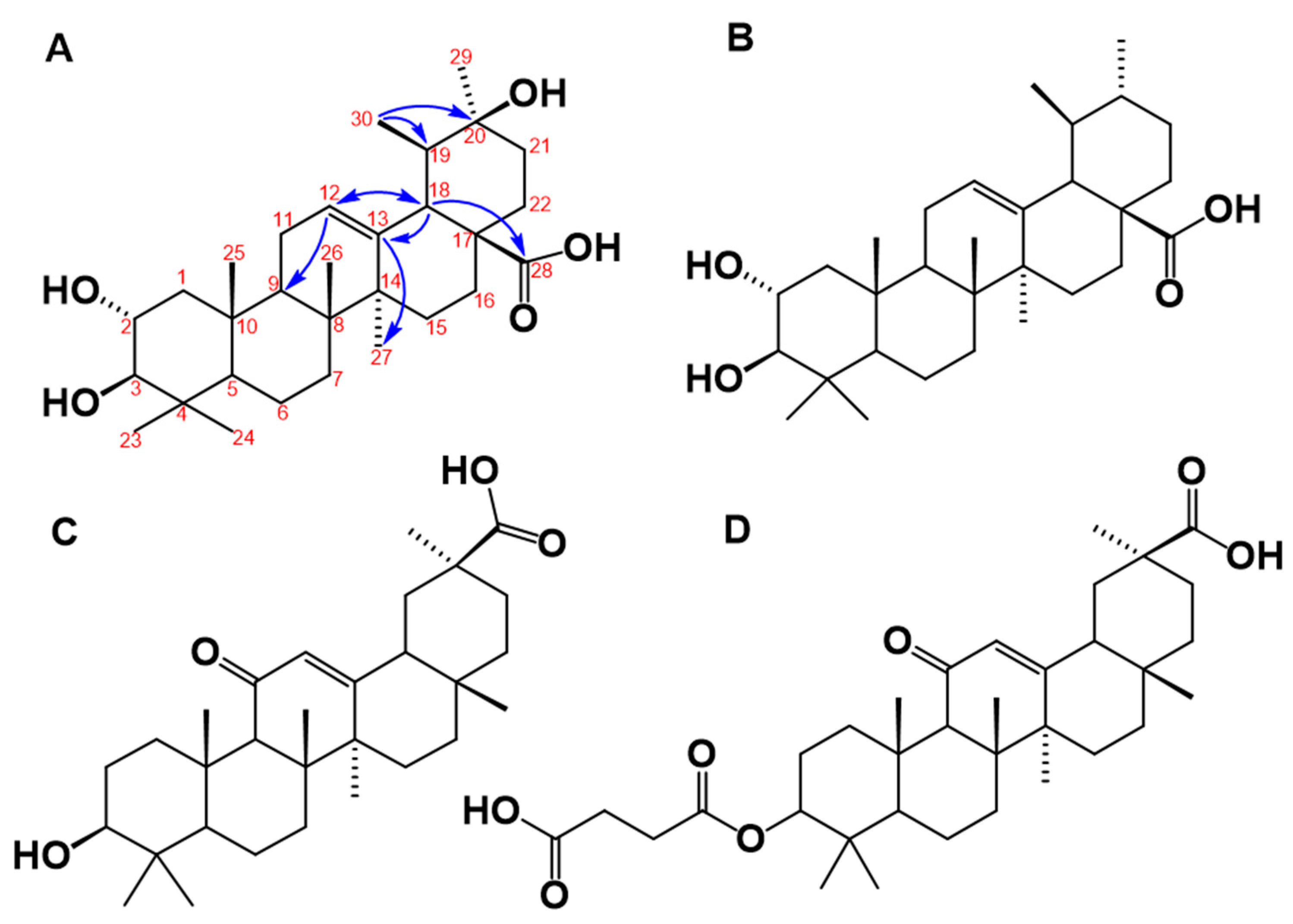
3.4. Structural Elucidation of the 11β-HSD1 Inhibitors Isolated from C. telenitida
3.5. Structural Analysis of C. telenitida Triterpene Inhibitors of 11β-HSD1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seidell, J.C. Obesity, insulin resistance and diabetes-A worldwide epidemic. Br. J. Nutr. 2000, 83, 8–11. [Google Scholar] [CrossRef]
- Uauy, R.; Albala, C.; Kain, J. Symposium: Obesity in Developing Countries: Biological and Ecological Factors Obesity Trends in Latin America: Transiting from Under- to Overweight 1. J. Nutr. 2001, 893–899. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.D.; Azevedo, I.; Monteiro, R.; Martins, M.J. 11β-Hydroxysteroid dehydrogenase type 1: Relevance of its modulation in the pathophysiology of obesity, the metabolic syndrome and type 2 diabetes mellitus. Diabetes Obes. Metab. 2012, 14, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.W.; Walker, E.A.; Bujalska, I.J.; Draper, N.; Lavery, G.G.; Cooper, M.S.; Hewison, M.; Stewart, P.M. 11β-Hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocr. Rev. 2004, 25, 831–866. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.M.; Krozowskit, Z.S. 11 beta-Hydroxysteroid Dehydrogenase. Vitam. Horm. 1997, 57, 249–324. [Google Scholar]
- Morton, N.M.; Paterson, J.M.; Masuzaki, H.; Holmes, M.C.; Staels, B.; Fievet, C.; Walker, B.R.; Flier, J.S.; Mullins, J.J.; Seckl, J.R. Novel Adipose Tissue–Mediated Resistance to Diet-Induced Visceral Obesity in 11β-Hydroxysteroid Dehydrogenase Type 1–Deficient Mice. Diabetes 2004, 53, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Morton, N.M.; Holmes, M.C.; Fiévet, C.; Staels, B.; Tailleux, A.; Mullins, J.J.; Seckl, J.R. Improved Lipid and Lipoprotein Profile, Hepatic Insulin Sensitivity, and Glucose Tolerance in 11β-Hydroxysteroid Dehydrogenase Type 1 Null Mice. J. Biol. Chem. 2001, 276, 41293–41300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuzaki, H.; Paterson, J.; Shinyama, H.; Morton, N.M.; Mullins, J.J.; Seckl, J.R.; Flier, J.S. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001, 294, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Masuzaki, H.; Yamamoto, H.; Kenyon, C.J.; Elmquist, J.K.; Morton, N.M.; Paterson, J.M.; Shinyama, H.; Sharp, M.G.F.; Fleming, S.; Mullins, J.J.; et al. Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J. Clin. Investig. 2003, 112, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, J.M.; Morton, N.M.; Fievet, C.; Kenyon, C.J.; Holmes, M.C.; Staels, B.; Seckl, J.R.; Mullins, J.J. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc. Natl. Acad. Sci. USA 2004, 101, 7088–7093. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.S.; Goldberg, F.W.; Turnbull, A.V. Medicinal chemistry of inhibitors of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). J. Med. Chem. 2013, 57, 4466–4486. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Rollinger, J.M.; Kratschmar, D.V.; Schuster, D.; Pfisterer, P.H.; Gumy, C.; Aubry, E.M.; Brandstötter, S.; Stuppner, H.; Wolber, G.; Odermatt, A. 11β-Hydroxysteroid dehydrogenase 1 inhibiting constituents from Eriobotrya japonica revealed by bioactivity-guided isolation and computational approaches. Bioorg. Med. Chem. 2010, 18, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, P.; Howes, M.J.R.; Edwards, S.E. Medicinal plants used in the traditional management of diabetes and its sequelae in Central America: A review. J. Ethnopharmacol. 2016, 184, 58–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duke, J.A.; Ottesen, A.R.; Bogenschutz-Godwin, M.J. Duke’s Handbook of Medicinal Plants of Latin Americle; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- Revilla-Monsalve, M.C.; Andrade-Cetto, A.; Palomino-Garibay, M.A.; Wiedenfeld, H.; Islas-Andrade, S. Hypoglycemic effect of Cecropia obtusifolia Bertol aqueous extracts on type 2 diabetic patients. J. Ethnopharmacol. 2007, 111, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Arellano, A.; Aguilar-Santamaría, L.; García-Hernández, B.; Nicasio-Torres, P.; Tortoriello, J. Clinical trial of Cecropia obtusifolia and Marrubium vulgare leaf extracts on blood glucose and serum lipids in type 2 diabetics. Phytomedicine 2004, 11, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Aragão, D.M.O.; Guarize, L.; Lanini, J.; da Costa, J.C.; Garcia, R.M.G.; Scio, E. Hypoglycemic effects of Cecropia pachystachya in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2010, 128, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.D.; Florentino, D.; Ortmann, C.F.; Martins, F.A.; Danielski, L.G.; Michels, M.; De Souza Constantino, L.; Petronilho, F.; Reginatto, F.H. Anti-inflammatory and antioxidant activities of aqueous extract of Cecropia glaziovii leaves. J. Ethnopharmacol. 2016, 185, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Montoya Peláez, G.L.; Sierra, J.A.; Alzate, F.; Holzgrabe, U.; Ramirez-Pineda, J.R. Pentacyclic triterpenes from Cecropia telenitida with immunomodulatory activity on dendritic cells. Braz. J. Pharmacogn. 2013, 23, 754–761. [Google Scholar] [CrossRef]
- Balcazar, N.; Tabares-Guevara, J.H.; Gonzalez, P.; Ramirez-Pineda, J.R.; Montoya, G. Hypoglycemic activity of serjanic acid obtained from Cecropia telenitida: Preliminary data. In Proceedings of the 23° SILAE Congress, Sicilia, Italy, 7–12 September 2014; PharmacologyOnLine: Marsala, Italy, 2014; p. 183. [Google Scholar]
- Chandran, S.; Gesenberg, C.; Levons, J.; Hubert, M.; Raghavan, K. A high-throughput spectrophotometric approach for evaluation of precipitation resistance. J. Pharm. Biomed. Anal. 2011, 56, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Kim, C.H.; Cheon, H.G. Cell-based assay for screening 11beta-hydroxysteroid dehydrogenase 1 inhibitors. Anal. Biochem. 2009, 392, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Stenken, J.A.; Yang, A.Y.; Zhao, J.J.; Musson, D.G. An in vitro microdialysis methodology to study 11beta-hydroxysteroid dehydrogenase type 1 enzyme activity in liver microsomes. Anal. Biochem. 2007, 370, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.S.; Himmelsbach, F.; Nar, H.; Schuler-Metz, A.; Krosky, P.; Guo, J.; Guo, R.; Meng, S.; Zhao, Y.; Lala, D.S.; et al. Pharmacological characterization of the selective 11β-hydroxysteroid dehydrogenase 1 inhibitor, BI 135585, a clinical candidate for the treatment of type 2 diabetes. Eur. J. Pharmacol. 2015, 746, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, Y.; Hirabayashi, K.; Matsumoto, H.; Sato, T.; Shibata, S.; Inoue, H. Effects of glycyrrhetinic acid derivatives on hepatic and renal 11beta-hydroxysteroid dehydrogenase activities in rats. J. Pharm. Pharmacol. 2003, 55, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A. Evaluation of Enzyme Inhibitors in Drug Discovery; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Zhang, J.-H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Iversen, P.W.; Eastwood, B.J.; Sittampalam, G.S.; Cox, K.L. A comparison of assay performance measures in screening assays: Signal window, Z′ factor, and assay variability ratio. J. Biomol. Screen. 2006, 11, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Fontaine, F.; Cooper, M.A. Natural product libraries: Assembly, maintenance, and screening. Planta Med. 2014, 80, 1161–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, A.; Favia, A.D.; Maser, E. 11beta-Hydroxysteroid dehydrogenase type 1 inhibitors with oleanan and ursan scaffolds. Mol. Cell. Endocrinol. 2009, 301, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Nazaruk, J.; Borzym-Kluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.; Yunes, A.; Malheiros, A.; Gomez, E. Two new 11α,12α-epoxy-ursan-28,13β-olides and other triterpenes from Cecropia catharinensis. Nat. Prod. Res. 2008, 22, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Li, E.N.; Luo, J.G.; Kong, L.Y. Qualitative and quantitative determination of seven triterpene acids in Eriobotrya japonica Lindl. by high-performance liquid chromatography with photodiode array detection and mass spectrometry. Phytochem. Anal. 2009, 20, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.Z.; Aquino, R.; De Feo, V.; De Simone, F.; Pizza, C. Polyhydroxylated triterpenes from Eriobotrya japonica. Planta Med. 1990, 56, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Degorce, F. HTRF: A Technology Tailored for Drug Discovery—A Review of Theoretical Aspects and Recent Applications. Curr. Chem. Genom. 2009, 3, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Janzen, W.P. Screening Technologies for Small Molecule Discovery: The State of the Art. Chem. Biol. 2014, 21, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy-directed fractionation of botanical medicines: A case study with goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Tan, K.L.; Zhang, C.L.; Tan, C.Y.; Chen, Y.Z.; Jiang, Y.Y. What Does It Take to Synergistically Combine Sub-Potent Natural Products into Drug-Level Potent Combinations? PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O.; Hamburger, M. Concepts and technologies for tracking bioactive compounds in natural product extracts: Generation of libraries, and hyphenation of analytical processes with bioassays. Nat. Prod. Rep. 2013, 30, 546–564. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.C.; Kumar, R.; Davis, R.A. The use of isolated natural products as scaffolds for the generation of chemically diverse screening libraries for drug discovery. Nat. Prod. Rep. 2016, 33, 372–381. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds serjanic acid and spergulagenic acid A are available from the authors. |
| Extract | Yield (g) | Number of Fractions | Fraction Identifier * |
|---|---|---|---|
| n-Hexane | 37.5 | 21 | H |
| Dichloromethane/Ethyl acetate | 53.7 | 27 | DE |
| Ethyl acetate | 58.3 | 26 | E |
| Ethyl acetate/Methanol | 65.6 | 27 | EM |
| Methanol | 53.6 | 24 | M |
| 1H-NMR (600 MHz) | 13C-NMR (151 MHz) | Carbon Type | |
|---|---|---|---|
| H/C | δH | δC | |
| 1 | 1.41 | 42.05 | secondary |
| 2 | 3.77 | 65.15 | tertiary |
| 3 | 3.16 | 78.38 | tertiary |
| 4 | 38.27 | quaternary | |
| 5 | 1.14 | 48.12 | tertiary |
| 6 | 1.35 | 18.17 | secondary |
| 7 | 1.58–1.51 | 37.74 | secondary |
| 8 | 1.67 | 47.00 | tertiary |
| 9 | 47.34 | quaternary | |
| 10 | 38.47 | quaternary | |
| 11 | 1.20–1.49 | 33.09 | secondary |
| 12 | 5.18 t (3.8) | 127.26 | tertiary |
| 13 | 139.15 | quaternary | |
| 14 | 41.63 | quaternary | |
| 15 | 0.91–1.65 | 28.45 | secondary |
| 16 | 1.9 | 23.63 | secondary |
| 17 | 40.50 | quaternary | |
| 18 | 2.37 | 53.63 | tertiary |
| 19 | 1.24 | 41.86 | tertiary |
| 20 | 72.10 | quaternary | |
| 21 | 1.61 | 26.40 | secondary |
| 22 | 1.36–2.52 | 25.61 | secondary |
| 23 | 0.79 | 22.32 | primary |
| 24 | 0.88 | 29.37 | primary |
| 25 | 0.89 | 16.59 | primary |
| 26 | 0.69 | 17.10 | primary |
| 27 | 1.3 | 24.55 | primary |
| 28 | 179.43 | quaternary | |
| 29 | 1.09 | 26.88 | primary |
| 30 | 0.84 d (6.7) | 16.76 | primary |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosquera, C.; Panay, A.J.; Montoya, G. Pentacyclic Triterpenes from Cecropia telenitida Can Function as Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1. Molecules 2018, 23, 1444. https://doi.org/10.3390/molecules23061444
Mosquera C, Panay AJ, Montoya G. Pentacyclic Triterpenes from Cecropia telenitida Can Function as Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1. Molecules. 2018; 23(6):1444. https://doi.org/10.3390/molecules23061444
Chicago/Turabian StyleMosquera, Catalina, Aram J. Panay, and Guillermo Montoya. 2018. "Pentacyclic Triterpenes from Cecropia telenitida Can Function as Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1" Molecules 23, no. 6: 1444. https://doi.org/10.3390/molecules23061444