Bactericidal Properties of Plants-Derived Metal and Metal Oxide Nanoparticles (NPs)
Abstract
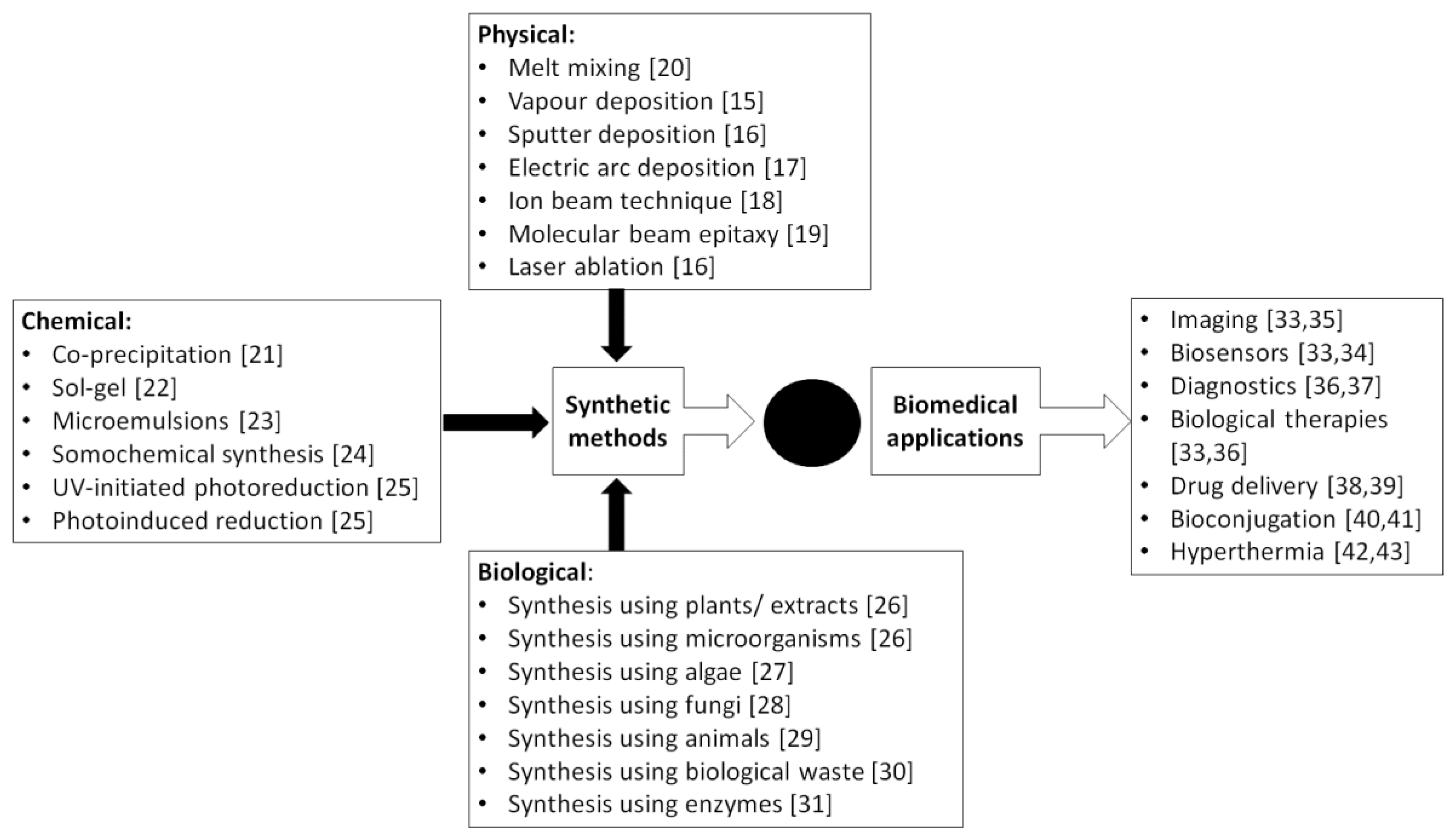
:1. Nanoparticles and Green Technology
2. Bactericidal Properties and Synergistic Enhancement of Common Antibiotics
3. Plant-derived Nanoparticles as Future Antibacterials
4. Conclusions and Future Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zare, Y.; Shabani, I. Polymer/metal nanocomposites for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Shriniwas, P.P.; Subhash, T.K. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017, 10, 76–81. [Google Scholar]
- Okafor, F.; Janen, A.; Kukhtareva, T.; Edwards, V.; Curley, M. Green synthesis of silver nanoparticles, their characterization, application and antibacterial activity. Int. J. Environ. Res. Public Health 2013, 10, 5221–5238. [Google Scholar] [CrossRef] [PubMed]
- Jiménez Pérez, Z.E.; Mathiyalagan, R.; Markus, J.; Kim, Y.; Kang, H.M.; Abbai, R.; Seo, K.H.; Wang, D.; Soshnikova, V.; Yang, D.C. Ginseng-berry-mediated gold and silver nanoparticle synthesis and evaluation of their in vitro antioxidant, antimicrobial, and cytotoxicity effects on human dermal fibroblast and murine melanoma skin cell lines. Int. J. Nanomed. 2017, 12, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Kelkawi, A.H.A.; Abbasi Kajani, A.; Bordbar, A.K. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnol. 2017, 11, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Naraginti, S.; Li, Y. Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol. B Biol. 2017, 170, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Wani, I.A.; Lone, I.H.; Ganguly, A.; Manzoor, N.; Ahmad, A.; Ahmed, J.; Al-Shihri, A.S. Antifungal activity of gold nanoparticles prepared by solvothermal method. Mater. Res. Bull. 2013, 48, 12–20. [Google Scholar] [CrossRef]
- Cagno, V.; Andreozzi, P.; D’Alicarnasso, M.; Silva, P.J.; Mueller, M.; Galloux, M.; Goffic, R.L.; Jones, S.T.; Vallino, M.; Hodek, J.; et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018, 17, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, H.H.; Kim, S.Y.; Kim, S.J.; Woo, K.; Ko, G. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl. Environ. Microbiol. 2014, 80, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Soflaei, S.; Dalimi, A.; Ghaffarifar, F.; Shakibaie, M.; Shahverdi, A.R.; Shafiepour, M. In vitro antiparasitic and apoptotic effects of antimony sulfide nanoparticles on Leishmania infantum. J. Parasitol. Res. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Bipinchandra, S.; Mouray, E.; Grellier, P.; Satish, P. In vitro antiparasitic activity of microbial pigments and their combination with phytosynthesized metal nanoparticles. Parasitol. Int. 2015, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Júnior, R.F.; de Araújo, A.A.; Pessoa, J.B.; Freire Neto, F.P.; da Silva, G.R.; Leitão Oliveira, A.L.; de Carvalho, T.G.; Silva, H.F.; Eugênio, M.; Sant’Anna, C.; et al. Anti-inflammatory, analgesic and anti-tumor properties of gold nanoparticles. Pharmacol. Rep. 2017, 69, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Laroui, H.; Sitaraman, S.V.; Merlin, D. Gastrointestinal delivery of anti-inflammatory nanoparticles. Methods Enzymol. 2012, 509, 101–125. [Google Scholar] [PubMed]
- Wang, E.C.; Wang, A.Z. Nanoparticles and their applications in cell and molecular biology. Integr. Biol. Quant. Biosci. Nano Macro 2014, 6, 9–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raula, J.; Kuivanen, A.; Lähde, A.; Jiang, H.; Antopolsky, M.; Kansikas, J.; Kauppinen, E.I. Synthesis of L-leucine nanoparticles via physical vapor deposition at varying saturation conditions. J. Aerosol Sci. 2007, 38, 1172–1184. [Google Scholar] [CrossRef]
- Ayyub, P.; Chandra, R.; Taneja, P.; Sharma, A.K.; Pinto, R. Synthesis of nanocrystalline material by sputtering and laser ablation at low temperatures. Appl. Phys. A 2001, 73, 67–73. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, A.K.; Sharma, V. Synthesis of carbon nanotubes by arc-discharge and chemical vapor deposition method with analysis of its morphology, dispersion and functionalization characteristics. Cogent Eng. 2015, 2, 1094017. [Google Scholar] [CrossRef]
- Perez-Rodriguez, A.; Garrido, B.; Bonafos, C.; Lopez, M.; Gonzalez-Varona, O.; Monrante, J.R.; Montserrat, J.; Rodriguez, R. Ion beam synthesis of compound nanoparticles in SiO2. J. Mater. Sci. Mater. Electron. 1999, 10, 385–391. [Google Scholar] [CrossRef]
- Wang, H.-C.; Liao, C.-H.; Chueh, Y.-L.; Lai, C.-C.; Chen, L.-H.; Tsiang, R.C.-C. Synthesis and characterization of ZnO/ZnMgO multiple quantum wells by molecular beam epitaxy. Opt. Mater. Express 2013, 3, 237–247. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Papageorgiou, G.Z.; Pavlidou, E.; Vouroutzis, N.; Palatzoglou, P.; Karayannidis, G.P. Preparation by melt mixing and characterization of isotactic polypropylene/SiO2 nanocomposites containing untreated and surface-treated nanoparticles. J. Appl. Polym. Sci. 2006, 100, 2684–2696. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Sui, R.; Charpentier, P. Synthesis of metal oxide nanostructures by direct Sol–Gel chemistry in supercritical fluids. Chem. Rev. 2012, 112, 3057–3082. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Wani, M.Y.; Hashim, M.A. Microemulsion method: A novel route to synthesize organic and inorganic nanomaterials: 1st nano update. Arab. J. Chem. 2012, 5, 397–417. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omrani, A.A.; Taghavinia, N. Photo-induced growth of silver nanoparticles using UV sensitivity of cellulose fibers. Appl. Surf. Sci. 2012, 258, 2373–2377. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Blázquez, M.L.; Muñoz, J.A.; González, F.; Ballester, A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 2013, 7, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, A.; Klimek-Ochab, M. Fungal synthesis of size-defined nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 043001. [Google Scholar] [CrossRef] [Green Version]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef]
- Ghosh, P.R.; Fawcett, D.; Sharma, S.B.; Poinern, G.E.J. Production of high-value nanoparticles via biogenic processes using aquacultural and horticultural food waste. Materials 2017, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S. Enzyme Nanoparticles: Preparation, Characterisation, Properties and Application, 1st ed.; William Andrew: Waltham, MA, USA, 2015; ISBN 978-0-323-38913-6. [Google Scholar]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Bogart, L.K.; Pourroy, G.; Murphy, C.J.; Puntes, V.; Pellegrino, T.; Rosenblum, D.; Peer, D.; Lévy, R. Nanoparticles for imaging, sensing, and therapeutic intervention. ACS Nano 2014, 8, 3107–3122. [Google Scholar] [CrossRef] [PubMed]
- Charbgoo, F.; Ramezani, M.; Darroudi, M. Bio-sensing applications of cerium oxide nanoparticles: Advantages and disadvantages. Biosens. Bioelectron. 2017, 96, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Nune, S.K.; Gunda, P.; Thallapally, P.K.; Lin, Y.Y.; Forrest, M.L.; Berkland, C.J. Nanoparticles for biomedical imaging. Expert Opin. Drug Deliv. 2009, 6, 1175–1194. [Google Scholar] [CrossRef] [PubMed]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef] [PubMed]
- Fortina, P.; Kricka, L.J.; Graves, D.J.; Park, J.; Hyslop, T.; Tam, F.; Halas, N.; Surrey, S.; Waldman, S.A. Applications of nanoparticles to diagnostics and therapeutics in colorectal cancer. Trends Biotechnol. 2007, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couto, C.; Vitorino, R.; Daniel-da-Silva, A.L. Gold nanoparticles and bioconjugation: A pathway for proteomic applications. Crit. Rev. Biotechnol. 2017, 37, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Surface Functionalization and Bioconjugation of Nanoparticles for Biomedical Applications. Ph.D. Dissertation, University of Western Ontario, London, ON, Canada, 2013. [Google Scholar]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Schrittwieser, S.; Reichinger, D.; Schotter, J. Applications, surface modification and functionalization of nickel nanorods. Materials 2017, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Shi, S.; Nikles, D.E.; Harrell, J.W. Easy control of the size and composition of FePt nanoparticles with improved synthesis. J. Appl. Phys. 2008, 103, 07D503. [Google Scholar] [CrossRef]
- Guerrero-Cázares, H.; Tzeng, S.Y.; Young, N.P.; Abutaleb, A.O.; Quiñones-Hinojosa, A.; Green, J.J. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano 2014, 8, 5141–5153. [Google Scholar] [CrossRef] [PubMed]
- Werengowska-Ciećwierz, K.; Wiśniewski, M.; Terzyk, A.P.; Furmaniak, S. The chemistry of bioconjugation in nanoparticles-based drug delivery system. Adv. Condens. Matter Phys. 2015, 2015. [Google Scholar] [CrossRef]
- Williams, H.M. The application of magnetic nanoparticles in the treatment and monitoring of cancer and infectious diseases. Biosci. Horiz. Int. J. Stud. Res. 2017, 10, hzx009. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Shetake, N.G.; Balla, M.M.S.; Kumar, A.; Pandey, B.N. Magnetic hyperthermia therapy: An emerging modality of cancer treatment in combination with radiotherapy. J. Radiat. Cancer Res. 2016, 7, 13–17. [Google Scholar]
- Park, H.; Park, H.; Kim, J.A.; Lee, S.H.; Kim, J.H.; Yoon, J.; Park, T.H. Inactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticles. J. Microbiol. Methods 2011, 84, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Barick, K.C.; Bahadur, D. Inactivation of bacterial pathogens under magnetic hyperthermia using Fe3O4–ZnO nanocomposite. Powder Technol. 2015, 269, 513–519. [Google Scholar] [CrossRef]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. AIP Conf. Proc. 2016, 1724, 020048. [Google Scholar]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. ‘Green’ nanotechnologies: Synthesis of metal nanoparticle using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar]
- Sabri, M.A.; Umer, A.; Awan, G.H.; Hassan, M.F.; Hasnain, A. Selection of suitable biological method for the synthesis of silver nanoparticles. Nanomater. Nanotechnol. 2016, 6, 29. [Google Scholar] [CrossRef]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. Influence of pH, precursor concentration, growth time, and temperature on the morphology of ZnO nanostructures grown by the hydrothermal method. J. Nanomater. 2011, 2011, 5. [Google Scholar] [CrossRef]
- Kumari, M.; Mishra, A.; Pandey, S.; Singh, S.P.; Chaudhry, V.; Mudiam, M.K.R.; Shukla, S.; Kakkar, P.; Nautiyal, C.S. Physico-chemical condition cptimization during biosynthesis lead to development of improved and catalytically efficient gold nano particles. Sci. Rep. 2016, 6, 27575. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Wang, J.; Wei, J. Effect of temperature on the size of biosynthesized silver nanoparticle: Deep insight into microscopic kinetics analysis. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Mansouri, S.S.; Ghader, S. Experimental study on effect of different parameters on size and shape of triangular silver nanoparticles prepared by a simple and rapid method in aqueous solution. Arab. J. Chem. 2009, 2, 47–53. [Google Scholar] [CrossRef]
- Thatoi, P.; Kerry, R.G.; Gouda, S.; Das, G.; Pramanik, K.; Thatoi, H.; Patra, J.K. Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. J. Photochem. Photobiol. B Biol. 2016, 163, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Tippayawat, P.; Phromviyo, N.; Boueroy, P.; Chompoosor, A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ 2016, 4, e2589. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Yuan, Q.; Wei, Y.; Khan, G.M.; Khan, Z.U.H.; Khan, S.; Ali, F.; Tahir, K.; Ahmad, A.; Khan, F.U. Photocatalytic and antibacterial response of biosynthesized gold nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Jafarirad, S.; Mehrabi, M.; Divband, B.; Kosari-Nasab, M. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Arokiyaraj, S.; Vincent, S.; Saravanan, M.; Lee, Y.; Oh, Y.K.; Kim, K.H. Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 2017, 45, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, V.; Sanjay, K.R. Green synthesis, characterization and bioactivity of plant-mediated silver nanoparticles using Decalepis hamiltonii root extract. IET Nanobiotechnol. 2017, 11, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kanjikar, A.P.; Hugar, A.L.; Londonkar, R.L. Characterization of phyto-nanoparticles from Ficus krishnae for their antibacterial and anticancer activities. Drug Dev. Ind. Pharm. 2018, 44, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Alsalhi, M.S.; Devanesan, S.; Alfuraydi, A.A.; Vishnubalaji, R.; Munusamy, M.A.; Murugan, K.; Nicoletti, M.; Benelli, G. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: Antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int. J. Nanomed. 2016, 11, 4439–4449. [Google Scholar] [CrossRef] [PubMed]
- Dhand, V.; Soumya, L.; Bharadwaj, S.; Chakra, S.; Bhatt, D.; Sreedhar, B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Chitra, K.; Manikandan, A.; Antony, S.A. Effect of poloxamer on Zingiber officinale extracted green synthesis and antibacterial studies of silver nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lim, J.M.; Velmurugan, P.; Park, Y.J.; Park, Y.J.; Bang, K.S.; Oh, B.T. Photobiologic-mediated fabrication of silver nanoparticles with antibacterial activity. J. Photochem. Photobiol. B Biol. 2016, 162, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Surendra, T.V.; Roopan, S.M.; Arasu, M.V.; Al-Dhabi, N.A.; Rayalu, G.M. RSM optimized Moringa oleifera peel extract for green synthesis of M. oleifera capped palladium nanoparticles with antibacterial and hemolytic property. J. Photochem. Photobiol. B Biol. 2016, 162, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Surendra, T.V.; Roopan, S.M. Photocatalytic and antibacterial properties of photosynthesized CeO2 NPs using Moringa oleifera peel extract. J. Photochem. Photobiol. B Biol. 2016, 161, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Gomathi, T.; Thiruvengadam, M.; Devi Rajeswari, V.; Kalpana, V.N.; Chung, I.M. Evaluation of anti-cholinesterase, antibacterial and cytotoxic activities of green synthesized silver nanoparticles using from Millettia pinnata flower extract. Microb. Pathog. 2017, 103, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.H.; Ma, Y.J.; Wang, J.W. Biosynthesis of silver nanoparticles using Taxus yunnanensis callus and their antibacterial activity and cytotoxicity in human cancer cells. Nanomaterials 2016, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.H.; Zheng, L.P.; Zhao, P.F.; Wang, J.W. Biosynthesis of silver nanoparticles using Artemisia annua callus for inhibiting stem-end bacteria in cut carnation flowers. IET Nanobiotechnol. 2017, 11, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Vilas, V.; Philip, D.; Mathew, J. Essential oil mediated synthesis of silver nanocrystals for environmental, anti-microbial and antioxidant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Hasan, S.H.; Banik, R.M. Photo-catalyzed and phyto-mediated rapid green synthesis of silver nanoparticles using herbal extract of Salvinia molesta and its antimicrobial efficacy. J. Photochem. Photobiol. B Biol. 2016, 155, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Ji, B.J.; Harper, S.L.; Yun, S.I. Plant extract-mediated biogenic synthesis of silver, manganese dioxide, silver-doped manganese dioxide nanoparticles and their antibacterial activity against food- and water-borne pathogens. Bioprocess Biosyst. Eng. 2016, 39, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.; Dhamecha, D.; Bhattacharya, D.; Patil, M. Green and ecofriendly synthesis of silver nanoparticles: Characterization, biocompatibility studies and gel formulation for treatment of infections in burns. J. Photochem. Photobiol. B Biol. 2016, 155, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Govarthanan, M.; Seo, Y.S.; Lee, K.J.; Jung, I.B.; Ju, H.J.; Kim, J.S.; Cho, M.; Kamala-Kannan, S.; Oh, B.T. Low-cost and eco-friendly synthesis of silver nanoparticles using coconut (Cocos nucifera) oil cake extract and its antibacterial activity. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1878–1882. [Google Scholar] [CrossRef] [PubMed]
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using chamomile terpenoids as a combined reducing and capping agent. Bioprocess Biosyst. Eng. 2016, 39, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.; Dorani, N.; Darroudi, M.; Sarani, M. Green synthesis of silver nanoparticles using Salvadora persica L. and its antibacterial activity. Cell. Mol. Biol. 2016, 62, 46–50. [Google Scholar] [PubMed]
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M.; et al. Green synthesis of silver, gold and silver/gold bimetallic nanoparticles using the Gloriosa superba leaf extract and their antibacterial and antibiofilm activities. Microb. Pathog. 2016, 101, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Bhuyan, B.; Purkayastha, D.D.; Dhar, S.S. Photocatalytic and antibacterial activities of gold and silver nanoparticles synthesized using biomass of Parkia roxburghii leaf. J. Photochem. Photobiol. B Biol. 2016, 154, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, S.; Sathya, P.; Palanisamy, P.K.; Gopalakrishnan, R. Synthesis of silver nanoparticles through green approach using Dioscorea alata and their characterization on antibacterial activities and optical limiting behavior. J. Photochem. Photobiol. B Biol. 2016, 159, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.R.; MacGregor-Ramiasa, M.; Zilm, P.; Majewski, P.; Vasilev, K. Chocolate’ silver nanoparticles: Synthesis, antibacterial activity and cytotoxicity. J. Colloid Interface Sci. 2016, 482, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Latha, M.; Priyanka, M.; Rajasekar, P.; Manikandan, R.; Prabhu, N.M. Biocompatibility and antibacterial activity of the Adathoda vasica Linn extract mediated silver nanoparticles. Microb. Pathog. 2016, 93, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Abbai, R.; Mathiyalagn, R.; Markus, J.; Kim, Y.; Wang, C.; Singh, P.; Ahn, S.; Farh, M.E.; Yang, D.C. Green synthesis of multifunctional silver and gold nanoparticles from the oriental herbal adaptogen: Siberian ginseng. Int. J. Nanomed. 2016, 11, 3131–3143. [Google Scholar] [Green Version]
- Otunola, G.A.; Afolayan, A.J.; Ajayi, E.O.; Odeyemi, S.W. Characterization, antibacterial and antioxidant properties of silver nanoparticles synthesized from aqueous extracts of Allium sativum, Zingiber officianale, and Capsicum frutscens. Pharmacogn. Mag. 2017, 13, S201–S208. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.R.; Albalawi, G.H.; Khan, S.T.; Khan, M.; Adil, S.F.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.R.; Alkhathlan, H.Z.; Khan, M. “Miswak” based green synthesis of silver nanoparticles: Evaluation and comparison of their microbicidal activities with the chemical synthesis. Molecules 2016, 21, E1478. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; El-Shibiny, A.; Salih, E. Photo-induced green synthesis and antimicrobial efficacy of poly (ε-caprolactone)/curcumin/grape leaf extract-silver hybrid nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 160, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Maji, A.; Mandal, A.K.; Das, S.; Aktara, M.N.; Jha, P.K.; Hossain, M. Green synthesis of silver nanoparticles using Pongamia pinnata seed: Characterization, antibacterial property, and spectroscopic investigation of interaction with human serum albumin. J. Mol. Recognit. 2016, 30. [Google Scholar] [CrossRef] [PubMed]
- Nakkala, J.R.; Mata, R.; Sandras, S.R. Green synthesized nano silver: Synthesis, physiochemical profiling, antibacterial, anticancer activities and biological in vivo toxicity. J. Colloid Interface Sci. 2017, 499, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Singh, H.; Yi, T.H. Antibacterial, anti-biofilm and anticancer potentials of green synthesized silver nanoparticles using benzoin gum (Styrax benzoin) extract. Bioprocess Biosyst. Eng. 2016, 39, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Baek, K.H. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front. Microbiol. 2017, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Tanomrod, N.; Rawdkuen, S.; Rhim, J.W. Preparation of pectin/silver nanoparticles composite films with UV-light barrier and properties. Int. J. Biol. Macromol. 2016, 92, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Preethi, J.; Vijayan, R.; Mohd Yusoff, A.R.; Ameen, F.; Suresh, S.; Balagurunathan, R.; Palvannan, T. Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesized silver nanoparticles using Coriandrum sativum leaf extract. J. Photochem. Photobiol. B Biol. 2016, 163, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Baghbani-Arani, F.; Movagharnia, R.; Sharifian, A.; Salehi, S.; Shandiz, S.A.S. Photo-catalytic, anti-bacterial, and anti-cancer properties of phyto-mediated synthesis of silver nanoparticles from Artemisia tournefortiana Rchb extract. J. Photochem. Photobiol. B Biol. 2017, 173, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Ammani, K.; Jobina, R.; Subhaswaraj, P.; Siddhardha, B. Photo-induced and phytomediated synthesis of silver nanoparticles using Derris trifoliata leaf extract and its larvicidal activity against Aedes aegypti. J. Photochem. Photobiol. B Biol. 2017, 171, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Chen, Z.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011. [Google Scholar] [CrossRef]
- Morales-Luckie, R.A.; Lopezfuentes-Ruiz, A.A.; Olea-Mejia, O.F.; Liliana, A.F.; Sanchez-Mendieta, V.; Brostow, W.; Hinestroza, J.P. Synthesis of silver nanoparticles using aqueous extracts of Heterotheca inuloides as reducing agent and natural fibers as templates: Agave lechuguilla and silk. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.; Gul, S.; Akhtar, J.; Haq, I.U.; Abbasi, B.H.; Hussain, A.; Naz, S.; Chaudhary, M.F. Green synthesis of silver nanoparticles from grape and tomato juices and evaluation of biological activities. IET Nanobiotechnol. 2017, 11, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.G.; Ansari, M.A.; Khan, H.M.; Jalal, M.; Mahdi, A.A.; Cameotra, S.S. Crataeva nurvala nanoparticles inhibit virulence factors and biofilm formation in clinical isolates of Pseudomonas aeruginosa. J. Basic Microbiol. 2017, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, N.; Vijaya, J.J.; Kaviyarasu, K.; Kombaiah, K.; Kennedy, L.J.; Ramalingam, R.J.; Munusamy, M.A.; Al-Lohedan, H.A. Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterial studies. J. Photochem. Photobiol. B Biol. 2017, 169, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Shkryl, Y.N.; Veremeichik, G.N.; Kamenev, D.G.; Gorpenchenko, T.Y.; Yugay, Y.A.; Mashtalyar, D.V.; Nepomnyaschiy, A.V.; Avramenko, T.V.; Karabtsov, A.A.; Ivanov, V.V.; et al. Green synthesis of silver nanoparticles using transgenic Nicotiana tabacum callus culture expressing silicatein gene from marine sponge Latrunculia oparinae. Artif. Cells Nanomed. Biotechnol. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Osibe, D.A.; Chiejina, N.V.; Ogawa, K.; Aoyagi, H. Stable antibacterial silver nanoparticles produced with seed-derived callus extract of Catharanthus roseus. Artif. Cells Nanomed. Biotechnol. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, S.; Shojaosadati, S.A.; Mohammadi, A. Evaluation of the catalytic, antibacterial and anti-biofilm activities of the Convolvulus arvensis extract functionalized silver nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 167, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Li, C. Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surf. B Biointerfaces 2017, 158, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Fayemi, O.E.; Ekennia, A.C.; Onwudiwe, D.C.; Ebenso, E.E. Silver nanoparticles mediated by Costus afer leaf extract: Synthesis, antibacterial, antioxidant and electrochemical properties. Molecules 2017, 22, E701. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Xavier, R.J.; Arumugam, M. Facile synthesis of multifunctional silver nanoparticles using mangrove plant Excoecaria agallocha L. for its antibacterial, antioxidant and cytotoxic effects. J. Parasit. Dis. Off. Organ Indian Soci. Parasitol. 2017, 41, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Dehghanizade, S.; Arasteh, J.; Mirzaie, A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: Characterization and in vitro biological activities. Artif. Cell Nanomed. Biotechnol. 2018, 46, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, N.; Dimopoulou, A.; Georgopoulou, A.; Gallios, N.; Papadopoulos, D.; Tsipas, D.; Theologidis, I.; Michailidis, N.; Chatzinikolaidou, M. The effect of silver nanoparticles size, produced using plant extract from Arbutus unedo on their antibacterial efficacy. Nanomaterials 2017, 7, E178. [Google Scholar] [CrossRef] [PubMed]
- Arya, G.; Sharma, N.; Ahmed, J.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Degradation of anthropogenic pollutant and organic dyes by biosynthesized silver nanocatalyst from Cicer arietinum leaves. J. Photochem. Photobiol. B Biol. 2017, 174, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. Int. 2018, 25, 10392–10406. [Google Scholar] [CrossRef] [PubMed]
- Jinu, U.; Gomathi, M.; Saiqa, I.; Geetha, N.; Benelli, G.; Venkatachalam, P. Green engineered biomolecule-capped silver and copper nanohybrids using Prosopis crineraria leaf extract: Enhanced antibacterial activity against microbial pathogens of public health relevance and cytotoxicity on human breast cancer cells (MCF-7). Microb. Pathog. 2017, 105, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mohan, S.; Singh, D.K.; Verma, D.K.; Singh, V.K.; Hasan, S.H. Photo-mediated optimized synthesis of silver nanoparticles for the selective detection of Iron(III), antibacterial and antioxidant activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Shim, J.; Bang, K.S.; Oh, B.T. Gold nanoparticles mediated coloring of fabrics and leather for antibacterial activity. J. Photochem. Photobiol. B Biol. 2016, 160, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Chahardoli, A.; Karimi, N.; Sadeghi, F.; Fattahi, A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Chen, S.M.; Elangovan, A.; Muthukrishnan, P.; Shanmugam, R.; Lou, B.S. Phyto mediated biogenic synthesis of gold nanoparticles using Cerasus serrulata and its utility in detecting hydrazine, microbial activity and DFT studies. J. Colloid Interface Sci. 2016, 468, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.G.; Huo, C.; Gui, B.; Cao, W.P. Green synthesis of gold nanoparticles using Citrus maxima peel extract and their catalytic/antibacterial activities. IET Nanobiotechnol. 2017, 11, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.U.; Chen, Y.; Khan, N.U.; Ahmad, A.; Tahir, K.; Khan, Z.U.; Khan, A.U.; Khan, S.U.; Raza, M.; Wan, P. Visible light inactivation of E. coli, cytotoxicity and ROS determination of biochemically capped gold nanoparticles. Microb. Pathog. 2017, 107, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.; Velmurugan, P.; Park, J.H.; Chang, W.S.; Park, Y.J.; Jayanthi, P.; Cho, M.; Oh, B.T. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Maaza, M.; Ayeshamariam, A.; Kennedy, L.J. Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: Cytotoxicity effect of nanoparticles against HT-29 cancer cells. J. Photochem. Photobiol. B Biol. 2016, 164, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Ahmed, B.; Khan, M.S.; Al-Shaeri, M.; Musarrat, J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb. Pathog. 2017, 111, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Park, J.H.; Lee, S.M.; Yi, Y.J.; Cho, M.; Jang, J.S.; Myung, H.; Bang, K.S.; Oh, B.T. Eco-friendly approach towards green synthesis of zinc oxide nanocrystals and its potential applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Shobiya, M. Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed. Pharmacother. 2016, 84, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Banumathi, B.; Vaseeharan, B.; Ishwarya, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Toxicity of herbal extracts used in ethno-veterinary medicine and green-encapsulated ZnO nanoparticles against Aedes aegypti and microbial pathogens. Parasitol. Res. 2017, 116, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Mohamad, R.; Mahdavi Shahri, M. Green microwave-assisted combustion synthesis of zinc oxide nanoparticles with Citrullus colocynthis L. schrad: Characterization and biomedical applications. Molecules 2017, 22, E301. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, L.; Rajini, N.; Nellaiah, H.; Kathiresan, T.; Jawaid, M.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite films with in situ generated copper nanoparticles using Terminalia catappa leaf extract. Int. J. Biol. Macromol. 2017, 95, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.B.; Montazer, M.; Rad, M.M. Photo and biocatalytic activities along with UV protection properties on polyester fabric through green in-situ synthesis of cauliflower-like CuO nanoparticles. J. Photochem. Photobiol. B Biol. 2017, 176, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Tahir, K.; Nazir, S.; Ahmad, A.; Li, B.; Khan, A.U.; Khan, Z.U.H.; Khan, F.U.; Khan, Q.U.; Khan, A.; Rahman, A.U. Facile and green synthesis of phytochemicals capped platinum nanoparticles and in vitro their superior antibacterial activity. J. Photochem. Photobiol. B Biol. 2017, 166, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Irshad, R.; Tahir, K.; Li, B.; Ahmad, A.; Siddiqui, R.A.; Nazir, S. Antibacterial activity of biochemically capped iron oxide nanoparticles: A view towards green chemistry. J. Photochem. Photobiol. B Biol. 2017, 170, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Tahir, K.; Nazir, S.; Li, B.; Ahmad, A.; Nasir, T.; Khan, A.U.; Shah, S.A.; Khan, Z.U.; Yasin, G.; Hameed, M.U. Sapium sebiferum leaf extract mediated synthesis of palladium nanoparticles and in vitro investigation of their bacterial and photocatalytic activities. J. Photochem. Photobiol. B Biol. 2016, 164, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, M.; Roopan, S.M.; Selvaraj, C.I.; Arunachalam, P. Phyllanthus emblica seed extract mediated synthesis of PdNPs against antibacterial, haemolytic and cytotoxic studies. J. Photochem. Photobiol. B Biol. 2017, 167, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, Q.; Nazar, M.; Naz, S.; Hussain, T.; Jabeen, N.; Kausar, R.; Anwaar, S.; Abbas, F.; Jan, T. Antimicrobial potential of green synthesized CeO2 nanoparticles from Olea europaea leaf extract. Int. J. Nanomed. 2016, 11, 5015–5025. [Google Scholar] [CrossRef] [PubMed]
- Nadaroglu, H.; Onem, H.; Alayli Gungor, A. Green synthesis of Ce2O3 NPs and determination of its antioxidant activity. IET Nanobiotechnol. 2017, 11, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Sonde, C.U.; Ehiri, R.C. Green synthesis of Ag/Ag2O nanoparticles using aqueous leaf extract of Eupatorium odoratum and its antimicrobial and mosquito larvicidal activities. Molecules 2017, 22, E674. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.; Salih, E.; Reicha, F. New trimethyl chitosan-based composite nanoparticles as promising antibacterial agents. Drug Dev. Ind. Pharm. 2016, 42, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Senthikumar, R.P.; Bhuvaneshwari, V.; Ranjithkumar, R.; Sathiyavimal, S.; Malayaman, V.; Chandarshekar, B. Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: As a bionanomaterials. Int. J. Biol. Macromol. 2017, 104, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Saleh, N.M.; Das, R.; Landis, R.F.; Bigdeli, A.; Motamedchaboki, K.; Campos, A.R.; Pomeroy, K.; Mahmoudi, M.; Rotello, V.M. Synergistic antimicrobial therapy using nanoparticles and antibiotics for the treatment of multidrug-resistant bacterial infection. Nano Futures 2017, 1, 015004. [Google Scholar] [CrossRef] [Green Version]
- National Center for Biotechnology Information (NCBI). Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 20 April 2018).
- Zgurskaya, H.I.; Lӧpez, C.A.; Gnanakaran, S. Permeability barrier of gram-negative cell envelopes and approaches to bypass it. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci. Rep. 2018, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef] [Green Version]
- Kalita, S.; Kandimalla, R.; Sharma, K.K.; Kataki, A.C.; Deka, M.; Kotoky, J. Amoxicilin functionalized gold nanoparticles reverts MRSA resistance. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; McShan, D.; Zhang, Y.; Sinha, S.S.; Arslan, Z.; Ray, P.C.; Yu, H. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ. Sci. Technol. 2016, 50, 8840–8848. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.S.; Hwang, J.H.; Choi, H.; Kim, K.J.; Lee, D.G. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J. Med. Microbiol. 2012, 61, 1719–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sudipta, K.M.; Jayanta, K.; Balasubramanya, S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulate leaf extract. Appl. Nanosci. 2015, 5, 73–81. [Google Scholar] [CrossRef]
- Ramseh, P.S.; Kokola, T.; Geetha, D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochimica Acta A Mol. Biomol. Spectrosc. 2015, 142, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Ovington, L.G. The truth about silver. Ostomy/Wound Manag. 2004, 50, 1S–10S. [Google Scholar]
- Raffi, M.; Hussain, F.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Hasan, M.M. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]
- Carnevale, J.; Ko, A.H. MM-398 (nanoliposomal irinotecan): Emergence of a novel therapy for the treatment of advanced pancreatic cancer. Future Oncol. 2016, 12, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Mori, K.; Corradini, N.; Redini, F.; Heymann, D. Mifamurtide for the treatment of nonmetastatic osteosarcoma. Expert Opin. Pharmacother. 2011, 12, 285–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovier, P.A. Epaxal: A virosomal vaccine to prevent hepatitis A infection. Expert Rev. Vaccines 2008, 7, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.; Hartmann, K.; Künzi, V.; Kürsteiner, O.; Mischler, R.; Lazar, H.; Glück, R. Eleven years of inflexal V—A virosomal adjuvanted influenza vaccine. Vaccine 2009, 27, 4381–4387. [Google Scholar] [CrossRef] [PubMed]
- Boswell, G.W.; Buell, D.; Bekersky, I. AmBisome (liposomal amphotericin B): A comparative review. J. Clin. Pharmacol. 1998, 38, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Grabrucker, A.M.; Ruozi, B.; Belletti, D.; Pederzoli, F.; Forni, F.; Vandelli, M.A.; Tosi, G. Nanoparticle transport across the blood brain barrier. Tissue Barriers 2016, 4, e1153568. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control Rel. 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanfins, E.; Augustsson, C.; Dahlbäck, B.; Linse, S.; Cedervall, T. Size-dependent effects of nanoparticles on enzymes in the blood coagulation cascade. Nano Lett. 2014, 14, 4736–4744. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Jaiswa, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2015. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Vega, A.; Gómez-Quintero, T.; Nuñez-Anita, R.; Acosta-Torres, L.; Castaño, V. Polymeric and ceramic nanoparticles in biomedical applications. J. Nanotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Woźniak, A.; Malankowska, A.; Nowaczyk, G.; Grześkowiak, B.F.; Tuśnio, K.; Slomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2017, 28, 92. [Google Scholar] [CrossRef] [PubMed]
- Yildrimer, L.; Thanh, N.T.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; Zbořil, R. Bacterial resistance to silver nanoparticles and how to overcome it. Nanotechnology 2018, 13, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed]
| Nanoparticles | Size (nm) | Source | Scientific Name | Common Name | Target Bacteria | References |
|---|---|---|---|---|---|---|
| Ag-NPs | 26–28 | Leaves | Coleus aromaticus | Cuban oregano | Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) | [78] |
| 12.46 | Leaves | Salvinia molesta | Kariba weed | E. coli, S. aureus | [79] | |
| 70.7–192.02 | Leaves | Aloe vera | Aloe | Pseudomonas aeruginosa (P. aeruginosa), Streptococcus epidermidis (S. epidermidis) | [63] | |
| 5–50 | Leaves | Mentha pulegium | Pennyroyal | E. coli, S. aureus, Streptococcus pyogenes (S. pyogenes) | [5] | |
| 5–40 | Leaves | Cucurbita pepo | Summer squash | E. coli, S. aureus, Bacillus cereus (B. cereus), Listeria monocytogenes (L. monocytogenes), Salmonella typhi (S. typhi), Salmonella enterica (S. enterica) | [80] | |
| 112.6 | Crude | Ammania baccifera | Monarch redstem | S. aureus, P. aeruginosa, MRSA | [81] | |
| 10–70 | Oil cake | Cocos nucifera | Coconut | Aeromonas sp., Acinetobacter sp., Citrobacter sp. | [82] | |
| 3.2–16 | Seeds | Pimpinella anisum | Aniseed | K. pneumonia, P. aeruginosa, S. typhi Streptococcus pyogenes (S. pyogenes), Acinetobacter baumannii (A. baumannii) | [69] | |
| 2–25 | Crude | Matricaria camomilia | Camomile | E. coli, S. aureus, Bacillus subtilis (B. subtilis), P. aeruginosa | [83] | |
| 50 | Crude | Salvadora persica L. | Toothbrush tree | E. coli, S. aureus | [84] | |
| 25 | Rhizomes | Zingiber officinale | Ginger | E. coli, S. aureus, K. pneumonia | [71] | |
| 20 | Leaves | Gloriosa superba | Flame lily | E. coli, B. subtilis | [85] | |
| 5–25 | Leaves | Parkia roxburghii | Tree bean | E. coli, S. aureus | [86] | |
| 10–20 | Tubers | Dioscorea alata | Yams | E. coli, Staphylococcus auricularis (S. auricularis) | [87] | |
| 35–42.5 | Powder | Theobroma cacao | Cacao | E. coli, S. aureus, Staphylococcus epidermidis (S. epidermidis), P. aeruginosa | [88] | |
| 10–50 | Leaves | Adathoda vasica Linn | Vasaka | Vibrio parahaemolyticus (V. parahaemolyticus) | [89] | |
| 14.63 | Crude | Eleutherococcus senticosus | Siberian ginseng | E. coli, S. aureus, V. parahaemolyticus Bacillus anthracis (B. anthracis) | [90] | |
| 20–30 | Seeds | Coffea arabica | Arabian coffee | E. coli, S. aureus | [70] | |
| 20–100 | Leaves | Sonneratia apetala | Sonneratia mangrove | Shigella flexneri (S. flexneri), E. coli, S. aureus, Vibrio cholera (V. cholera), S. epidermidis, B. subtilis | [62] | |
| 50–400 | Bark | Heritiera fomes | Sundari | E. coli, S. aureus, V. cholera, S. epidermidis, B. subtilis | [62] | |
| 3–6 | Crude | Allium sativum L. | Garlic | E. coli, E. faecalis, Bacillus cereus (B. cereus), S. flexneri | [91] | |
| 3–22 | Crude | Zingiber officinale Rosc. | Ginger | E. coli, E. faecalis, B. cereus, S. flexneri | [91] | |
| 3–18 | Crude | Capsicum frutescens L. | Cayenne pepper | E. coli, E. faecalis, B. cereus, S. flexneri | [91] | |
| 10–20 | Roots | Salvadora persica L. | Toothbrush tree | E. coli, S. aureus, P. aeruginosa, Micrococcus luteus (M. luteus) | [92] | |
| 5–30 | Crude | Rumex dentatus | Toothed dock | P. aeruginosa, Bacillus thuringiensis (B. thuringiensis) | [93] | |
| 49 | Flowers | Millettia pinnata | Karanja | E. coli, P. aeruginosa, Proteus vulgaris (P. vulgaris), S. aureus, K. pneumonia | [75] | |
| 16.4 | Seeds | Pongamia pinnata | Seashore Mempari | E. coli | [94] | |
| 5–60 | Rhizomes | Dryopteris crassirhizoma | Japanese fern | P. aeruginosa, B. cereus | [72] | |
| 1–69 | Leaves | Ficus religiosa | Peepul tree | E. coli, B. subtilis, S. typhi, Pseudomonas fluorescens (P. fluorescens) | [95] | |
| 12–38 | Powder | Styrax benzoin | Benzoin gum | E. coli, P. aeruginosa, S. aureus | [96] | |
| 6.4–27.2 | Callus | Taxus yunnanensis | Himalayan yew | E. coli, S. aureus, S. paratyphi, B. subtilis | [76] | |
| 10–20 | Ginseng berry | Panax ginseng | Meyer berries | E. coli, S. aureus | [4] | |
| 45.26 | Corn leaves | Zea mays L. | Maize | E. coli, S. aureus, S. typhimurium, L. monocytogenes, B. cereus | [97] | |
| 20–80 | Shoot tip | Caesalpinia mimosoides Lam. | Mimosa thorn | E. coli, L. monocytogenes | [98] | |
| 37 | Leaves | Coriandrum sativum | Coriander | Propionibacterium acnes (P. acnes) | [99] | |
| 22.89 | Aerial parts | Artemisia tournefortiana | - | E. coli, B. subtilis, S. pyogenes, P. aeruginosa | [100] | |
| 20 | Leaves | Derris trifoliata | Common derris | E. coli, S. aureus, S. enterica, Vibrio parahaemolyticus (V. parahaemolyticus) | [101] | |
| 121 | Roots | Rheum palmatum | Chinese Rhubarb | S. aureus, P. aeruginosa | [66] | |
| 12.46 | Leaves | Salvinia molesta | Giant salvinia | E. coli, S. aureus | [102] | |
| 32.5 | Roots | Decalepis hamiltonii | Indian Sarsaparilla | E. coli, S. aureus, P. aeruginosa, B. cereus, B. licheniformis | [67] | |
| 16 | Crude | Heterotheca inuloides | Mexican arnica | E. coli, S. aureus | [103] | |
| 10–30 | Fruit juices | Vitis vinifera and Solanum lycopersicum | Grape and tomato | Pseudomonas septica (P. septica), S. aureus, M. luteus, Enterobacter aerogenes (E. aerogenes), B. subtilis, S. typhi | [104] | |
| 2.1–45.2 | Callus | Artemisia annua | Sweet wormwood | Arthrobacter arilaitensis (A. arilaitensis), Staphylococcus equorum (S. equorum), Microbacterium oxydans (M. oxydans) | [77] | |
| 15.2 | Bark | Crataeva nurvala | Ayurveda | P. aeruginosa | [105] | |
| 6–8 | Fruit | Tamarindus indica | Tamarind | P. aeruginosa, S. aureus, M. luteus, Enterobacter aerogenes (E. aerogenes) B. subtilis, B. cereus, S. typhi | [106] | |
| 12–80 | Callus | Nicotiana tabacum | Tobacco | E. coli, Agrobacterium rhizogenes (A. rhizogenes) | [107] | |
| 410–450 | Leaves | Lantana camara | Verbanaceae | E. coli, S. aureus, P. aeruginosa | [2] | |
| 25–40 | Crude | Actinidia deliciosa | Kiwi fruit | P. aeruginosa | [6] | |
| 15–28 | Stem bark | Ficus krishnae | Krishna fig | E. coli, S. aureus, S. typhimurium | [68] | |
| 2–15 | Callus | Catharanthhus roseus | Madagascar periwinkle | E. coli | [108] | |
| 28 | Leaves | Convolvulus arvensis | Field bindweed | E. coli | [109] | |
| 25 | Leaves | Artemisia vulgaris | Common wormwood | E. coli, S. aureus, P. aeruginosa, K. pneumonia, Haemophilus influenza (H. influenza) | [110] | |
| 20 | Leaves | Costus afer | - | E. coli, S. aureus, P. aeruginosa, K. pneumonia, B. subtilis | [111] | |
| 23–42 | Leaves | Exocoecaria agallocha | Blinding tree | P. aeruginosa, S. aureus, S typhi, B. cereus | [112] | |
| 10–80 | Aerial parts | Anthemis atropatana | - | E. coli, S. aureus, P. aeruginosa, S. pyogenes | [113] | |
| 40–60 | Leaves | Arbutus unedo | Strawberry tree | E. coli, S. epidermis, B. subtilis, P. aeruginosa | [114] | |
| 88.8 | Leaves | Cicer arietinum | Chickpea | E. coli, P. aeruginosa | [115] | |
| 5–30 | Leaves | Taraxacum officinale | Dandelion | Xanthomonas axonopodis (X. axonopodis), Pseudomonas syringae (P. syringae) | [116] | |
| 20–44.49 | Leaves | Prosopis cinerraria | Khejri tree | E. coli, K. pneumonia, S. epidermidis | [117] | |
| 15–25 | Leaves | Croton bonplandianum | Bantulasi | E. coli, S. aureus | [118] | |
| Au-NPs | 5–10 | Ginseng berry | Panax ginseng | Meyer berries | E. coli, S. aureus | [4] |
| 5–25 | Leaves | Parkia roxburghii | Tree bean | E. coli, S. aureus | [71] | |
| 10–75 | Leaves | Ginkgo biloba Linn | Ginkgo tree | Brevibacterium linens (B. linens) | [119] | |
| 3–37 | Leaves | Nigella arvensis | Love-in-a-mist | E. coli, S. aureus, P. aeruginosa, Serratia marcescens (S. marcescens), B. subtilis, S. epidermidis | [120] | |
| 25 | Fruit | Dimocarpus longan | Longan | S. aureus, B. subtilis, E. coli | [64] | |
| 5–25 | Leaves | Cerasus serrulata | Japanese cherry | E. coli, S. aureus | [121] | |
| 7–20 | Crude | Actinidia deliciosa | Kiwi fruit | P. aeruginosa | [6] | |
| 8–25 | Peel | Citrus maxima | Pomelo | E. coli, S. aureus | [122] | |
| 20–30 | Crude | Coptis chinensis | Gold thread | Drug-resistant E. coli | [123] | |
| Ag2O-NPs | 42.7 | Roots | Ficus benghalensis | Banyan | Streptococcus mutans (S. mutans), Lactobacilli sp. | [124] |
| NiO-NPs | 9.69 | Crude | Moringa oleifera | Drumstick tree | S. aureus, S. pneumonia, Escherichia hermannii (E. hermannii), E. coli | [125] |
| 10–20 | Leaves | Eucalyptus globulus | Blue glum | E. coli, S. aureus, MRSA, P. aeruginosa | [126] | |
| ZnO-NPs | 20.06 | Leaves | Prunus x yedoensis Matsumura | Yoshino cherry | B. linens, S. epidermidis | [127] |
| 400–500 | Leaves | Sonneratia apetala | Sonneratia mangrove | S. flexneri | [62] | |
| 47.27 | Leaves | Laurus nobilis | Bay tree | S. aureus, P. aeruginosa | [128] | |
| 50 | Fruit | Rosa canina | Dog rose | E. coli, L. monocytogenes, P. aeruginosa | [65] | |
| - | Leaves | Lobelia leschenaultiana | Lobelia | P. aeruginosa, Shigella sonnei (S. sonnei), P. vulgaris, V. parahaemolyticus | [129] | |
| 27–85 | Fruit, seed, and pulp | Citrullus colocynthis L. | Schrad | MRSA, P. aeruginosa, E. coli, B. subtilis | [130] | |
| Cu-NPs | 21–30 | Leaves | Terminalia catappa | Tropical almond | E. coli | [131] |
| 18.9–32.09 | Leaves | Prosopis cineraria | Khejri tree | E. coli, K. pneumonia, S. epidermidis | [117] | |
| CuO-NPs | 30 –222.5 | Leaves | Seidlitzia rosmarinus | Keliab | E. coli, S. aureus | [132] |
| Pt-NPs | 2–7 | Crude | Taraxacum laevigatum | Red-seeded dandelion | P. aeruginosa, B. subtilis | [133] |
| FeO-NPs | - | Peel | Punica granatum | Pomegranate | P. aeruginosa | [134] |
| Pd-NPs | 5 | Leaves | Sapium sebiferum | Chinese tallow tree | S. aureus, P. aeruginosa Bacillus subtilis | [135] |
| 30 | Seeds | Phyllanthus emblica | Indian Gooseberry | S. aureus, P. aeruginosa, B subtilis Proteus mirabilis | [136] | |
| 27 | Peel | Moringa oleifera | Horseradish tree | E. coli, S. aureus | [73] | |
| CeO2-NPs | 45 | Peel | Moringa oleifera | Horseradish tree | E. coli, S. aureus | [74] |
| 24 | Leaves | Olea europaea | Olive | E. coli, S. aureus, K. pneumonia, P. aeruginosa | [137] | |
| Ce2O3-NPs | 8.6–10.5 | Crude | Euphorbia amygdaloides | Wood spurge | Pediococcus acidilactici (P. acidilactici) | [138] |
| Pectin/Ag-NPs | 20–80 | Shoot tip | Caesalpinia mimosoides Lam. | Mimosa thorn | E. coli, L. monocytogenes | [98] |
| Ag/Ag2O-NPs | 8.2–20.5 | Leaves | Eupatorium odoratum | Christmas bush | E. coli, S. typhi, S. aureus, B. subtilis | [139] |
| Ag/Au-NPs | 10 | Leaves | Gloriosa superba | Flame lily | E. coli, B. subtilis | [85] |
| Chitosan/Ag-NPs | 378–402 | Crude | Rumex dentatus | Toothed dock | P. aeruginosa, B. thuringiensis | [140] |
| Chitosan/CeO2-NPs | 3.61–24.4 | Leaves | Sida acuta | Common wireweed | E. coli, B. subtilis | [141] |
| PCL/Cur/GLE-Ag-NPs | 200 | Leaves | Vitis vinifera | Grape | E. coli, S. aureus, P. aeruginosa, B. subtilis, S. enterica | [93] |
| GLE-Ag-NPs | 30 | Leaves | Vitis vinifera | Grape | E. coli, S. aureus, P. aeruginosa, B. subtilis, S. enterica | [93] |
| Cellulose/Cu-NPs | 20–40 | Leaves | Terminalia catappa | Tropical almond | E. coli | [131] |
| Ag-MnO2-NPs | 5–40 | Leaves | Cucurbita pepo | Summer squash | E. coli, S. aureus, B. cereus, L. monocytogenes, S. typhi, S. enterica | [142] |
| Gram-Negative Species | Ag | Au | Cu | Pt | Pd | Ag2O | NiO | ZnO | CuO | FeO | CeO2 | Ce2O3 |
| E. coli | 46 | 6 | 2 | - | 1 | - | 2 | 2 | 1 | - | 2 | - |
| Drug-resistant E. coli | - | 1 | - | - | - | - | - | - | - | - | - | - |
| E. hermannii | - | - | - | - | - | - | 1 | - | - | - | - | - |
| P. fluorescens | 1 | - | - | - | - | - | - | - | - | - | - | - |
| P. aeruginosa | 23 | 2 | - | 1 | 2 | - | 1 | 4 | - | 1 | 1 | - |
| P. syringae | 1 | - | - | - | - | - | - | - | - | - | - | - |
| P. septica | 1 | - | - | - | - | - | - | - | - | - | - | - |
| K. pneumoniae | 6 | - | 1 | - | - | - | - | - | - | - | 1 | - |
| P. vulgaris | 1 | - | - | - | - | - | - | 1 | - | - | - | - |
| P. mirabilis | - | - | - | - | 1 | - | - | - | - | - | - | - |
| S. flexneri | 4 | - | - | - | - | - | - | 1 | - | - | - | - |
| S. sonnei | - | - | - | - | - | - | - | 1 | - | - | - | - |
| S. paratyphi | 1 | - | - | - | - | - | - | - | - | - | - | - |
| S. typhi | 6 | - | - | - | - | - | - | - | - | - | - | - |
| S. typhimurium | 2 | - | - | - | - | - | - | - | - | - | - | - |
| S. enterica | 2 | - | - | - | - | - | - | - | - | - | - | - |
| V. parahaemolyticus | 3 | - | - | - | - | - | - | 1 | - | - | - | - |
| V. cholera | 2 | - | - | - | - | - | - | - | - | - | - | - |
| Aeromonas sp. | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Acinetobacter sp. | 1 | - | - | - | - | - | - | - | - | - | - | - |
| A. baumannii | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Citrobacter sp. | 1 | - | - | - | - | - | - | - | - | - | - | - |
| E. aerogenes | 2 | - | - | - | - | - | - | - | - | - | - | - |
| A. rhizogenes | 1 | - | - | - | - | - | - | - | - | - | - | - |
| H. influenza | 1 | - | - | - | - | - | - | - | - | - | - | - |
| X. axonopodis | 1 | - | - | - | - | - | - | - | - | - | - | - |
| S. marcescens | - | 1 | - | - | - | - | - | - | - | - | - | - |
| Lactobacilli sp. | - | - | - | - | - | 1 | - | - | - | - | - | - |
| Gram-Positive Species | Ag | Au | Cu | Pt | Pd | Ag2O | NiO | ZnO | CuO | FeO | CeO2 | Ce2O3 |
| S. aureus | 35 | 6 | - | - | 3 | - | 2 | 1 | 1 | - | 2 | - |
| S. epidermidis | 5 | 1 | 1 | - | - | - | - | 1 | - | - | - | - |
| MRSA | 1 | - | - | - | - | - | 1 | 1 | - | - | - | - |
| S. pyogenes | 4 | - | - | - | - | - | - | - | - | - | - | - |
| S. mutans | - | - | - | - | - | 1 | - | - | - | - | - | - |
| S. pneumonia | - | - | - | - | - | - | 1 | - | - | - | - | - |
| B. thuringiensis | 1 | - | - | - | - | - | - | - | - | - | - | - |
| B. cereus | 9 | - | - | - | - | - | - | - | - | - | - | - |
| B. subtilis | 11 | 2 | - | 1 | 2 | - | - | 1 | - | - | - | - |
| B. licheniformis | 1 | - | - | - | - | - | - | - | - | - | - | - |
| B. anthracis | 1 | - | - | - | - | - | - | - | - | - | - | - |
| E. faecalis | 3 | - | - | - | - | - | - | - | - | - | - | - |
| L. monocytogenes | 3 | - | - | - | - | - | - | 1 | - | - | - | - |
| M. luteus | 3 | - | - | - | - | - | - | - | - | - | - | - |
| P. acnes | 1 | - | - | - | - | - | - | - | - | - | - | - |
| A. arilaitensis | 1 | - | - | - | - | - | - | - | - | - | - | - |
| M. oxydans | 1 | - | - | - | - | - | - | - | - | - | - | - |
| B. linens | - | 1 | - | - | - | - | - | 1 | - | - | - | - |
| P. acidilactici | - | - | - | - | - | - | - | - | - | - | - | 1 |
| Structural Challenge | |
| Size | Smaller size enhances the cell penetration, but may have decreased stability or bioavailability |
| Shape | Certain shape of NPs may improve the functionality due to total surface exposure area |
| Aggregate | NPs that form aggregate increase the overall particle size, hence limiting the cell permeation and may increase toxicity |
| Biological Challenge | |
| Biodistribution | Poor dispersion due to limited entry (e.g., skin barrier) |
| Bioavailability | Poor bioavailability results in rapid loss of function |
| Specificity | High specificity results in less off-target effects and more effective |
| Clearance | High retention rate ensures the high efficiency |
| Toxicity | Accumulation of toxic materials may damage the host |
| Technological Challenge | |
| Heterogeneity of human disease | Variation within disease may complicate treatment |
| Scale-up | Optimization of NPs synthesis and production with uniform size without aggregates in controlled and consistent fashion |
| Throughput | Synthesis of NP is multistep and laborious which does not allow high-throughput optimization |
| Prediction | Prediction using computer modelling on NP efficiency is extremely challenging |
| Industrial Challenge | |
| Quantity | Large scale production may result in inconsistent size and physicochemical properties of NPs |
| Processes | Reproducible and consistent manufacturing processes requires modern technology and instrumentation |
| Quality | Continuous production of high level uniformity and functionality of NPs |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teow, S.-Y.; Wong, M.M.-T.; Yap, H.-Y.; Peh, S.-C.; Shameli, K. Bactericidal Properties of Plants-Derived Metal and Metal Oxide Nanoparticles (NPs). Molecules 2018, 23, 1366. https://doi.org/10.3390/molecules23061366
Teow S-Y, Wong MM-T, Yap H-Y, Peh S-C, Shameli K. Bactericidal Properties of Plants-Derived Metal and Metal Oxide Nanoparticles (NPs). Molecules. 2018; 23(6):1366. https://doi.org/10.3390/molecules23061366
Chicago/Turabian StyleTeow, Sin-Yeang, Magdelyn Mei-Theng Wong, Hooi-Yeen Yap, Suat-Cheng Peh, and Kamyar Shameli. 2018. "Bactericidal Properties of Plants-Derived Metal and Metal Oxide Nanoparticles (NPs)" Molecules 23, no. 6: 1366. https://doi.org/10.3390/molecules23061366





