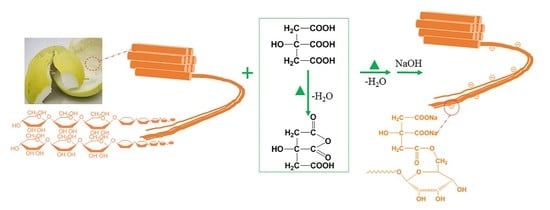
Pomelo Peel Modified with Citrate as a Sustainable Adsorbent for Removal of Methylene Blue from Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
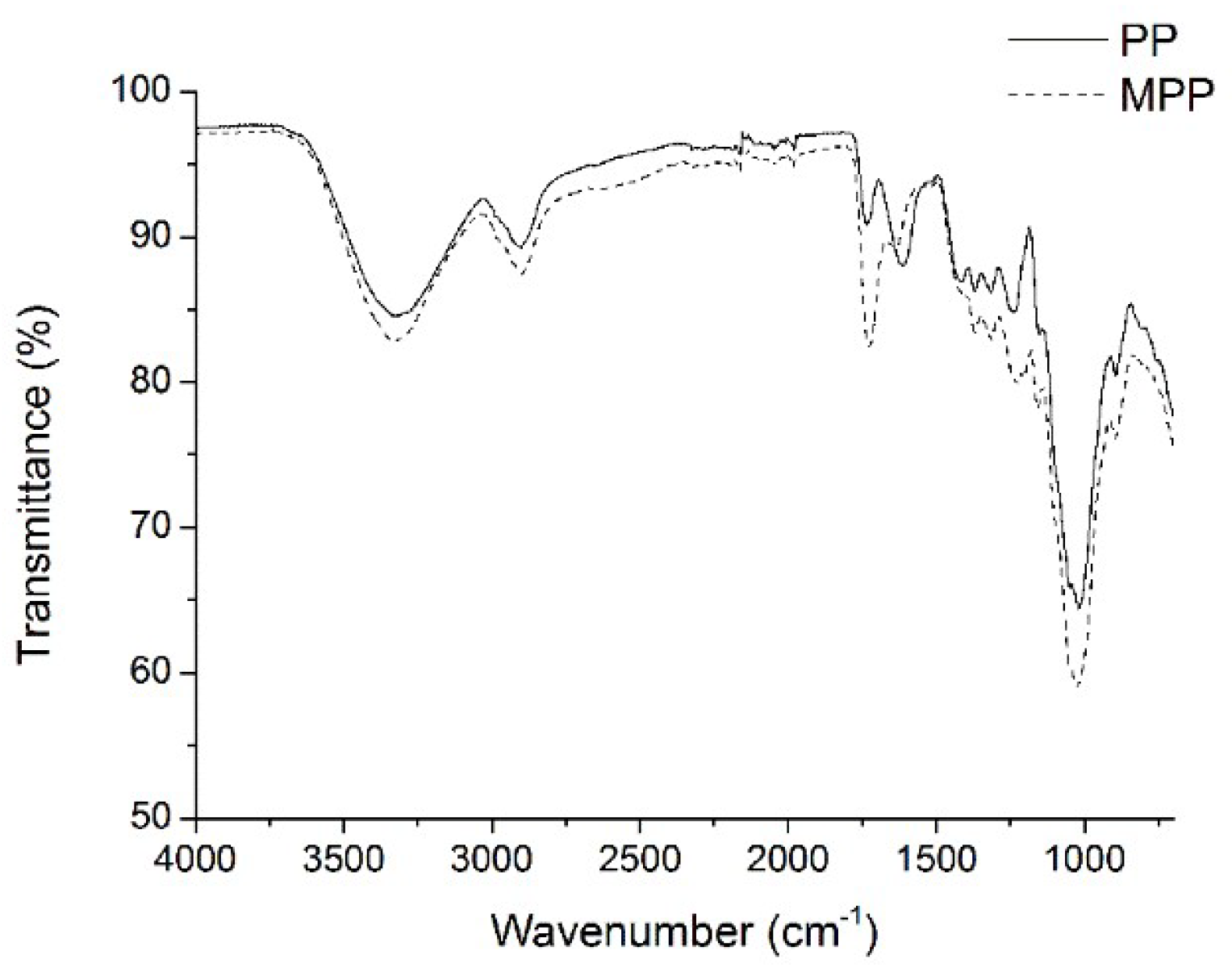
2.1. FTIR Analysis
2.2. SEM Observations
2.3. Comparison of MB Adsorption on PPL and MPPL
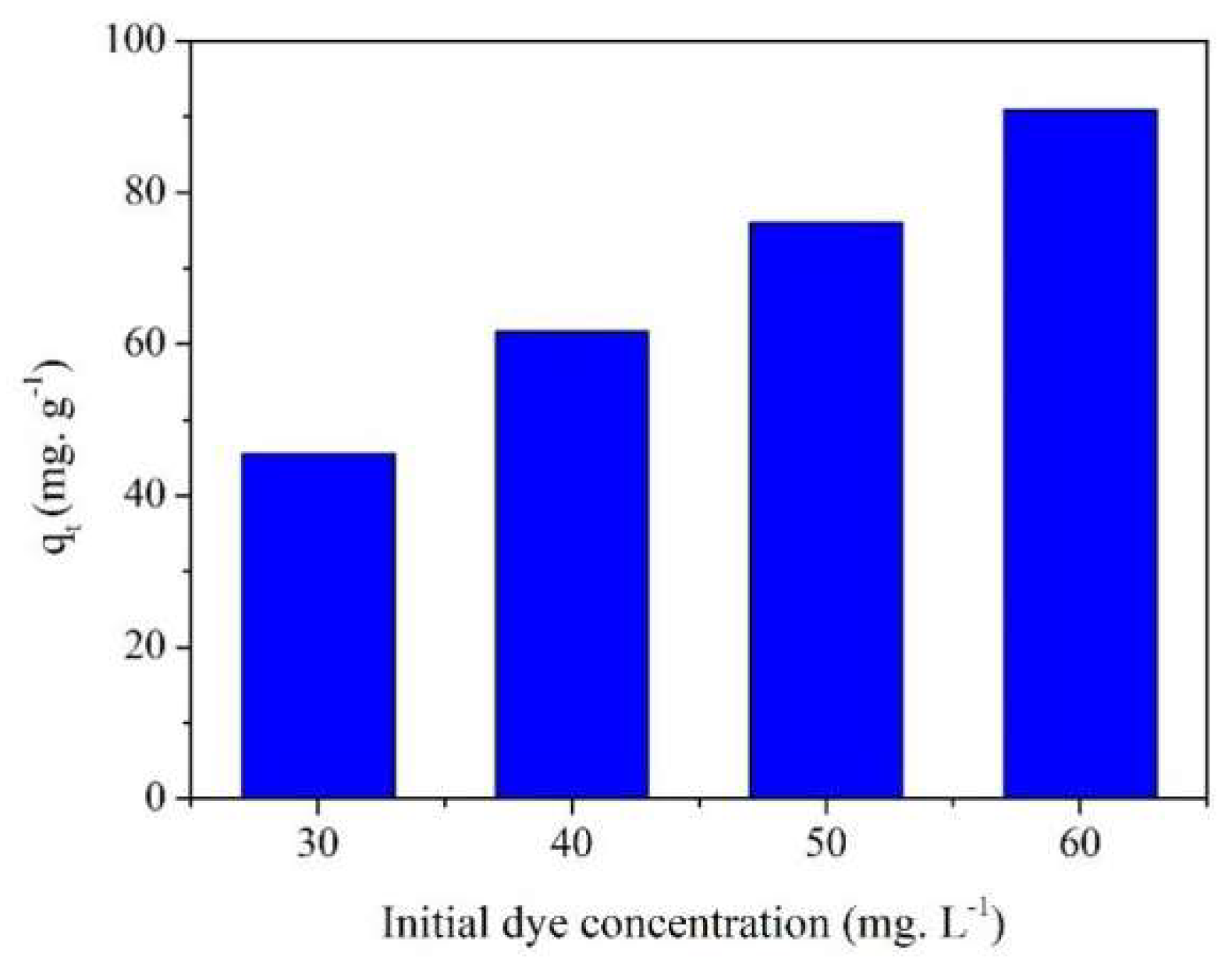
2.4. Effect of Initial Concentration on MB Adsorption
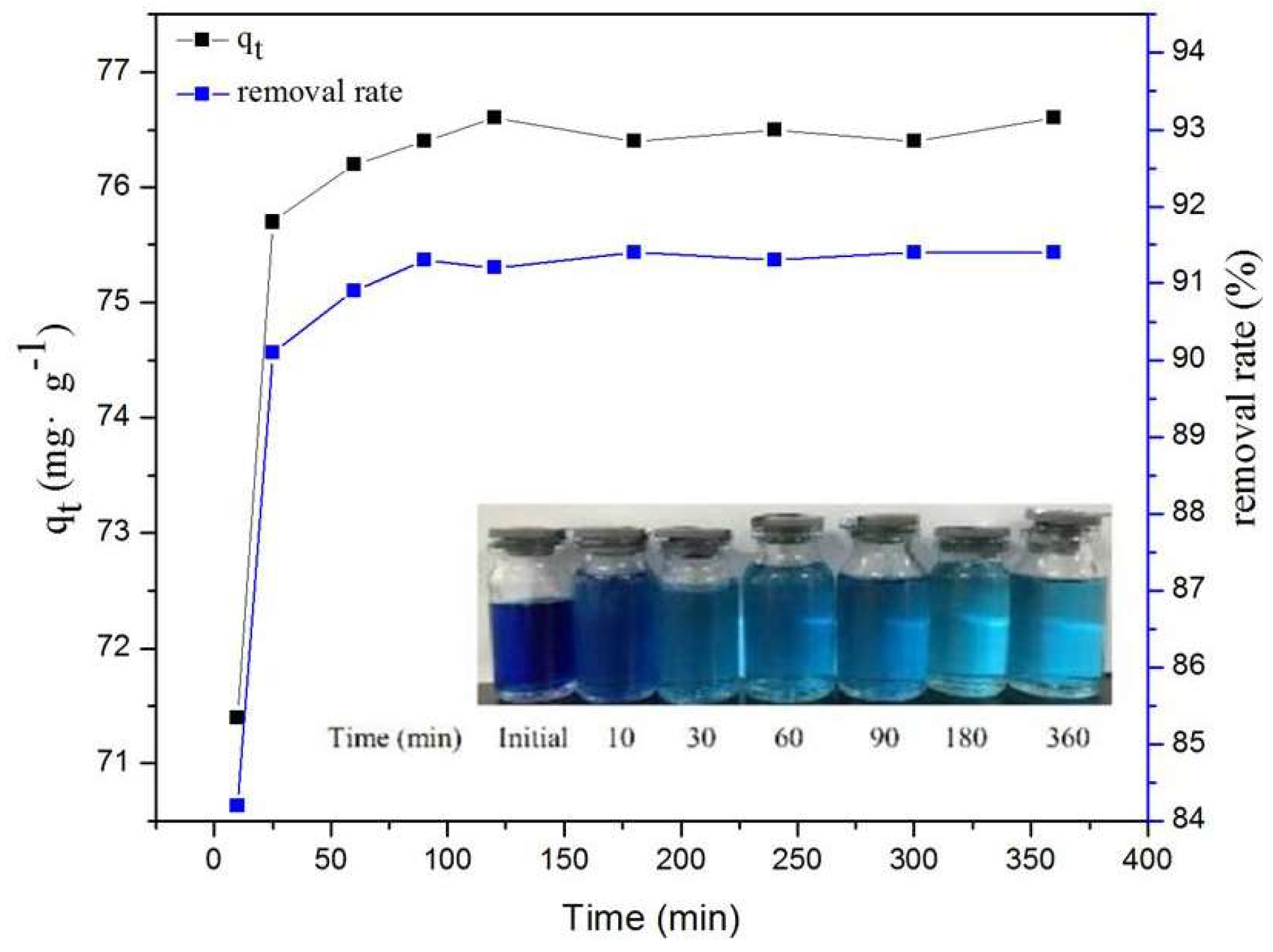
2.5. Effect of Contact Time on MB Adsorption
2.6. Effect of Initial pH on MB Adsorption
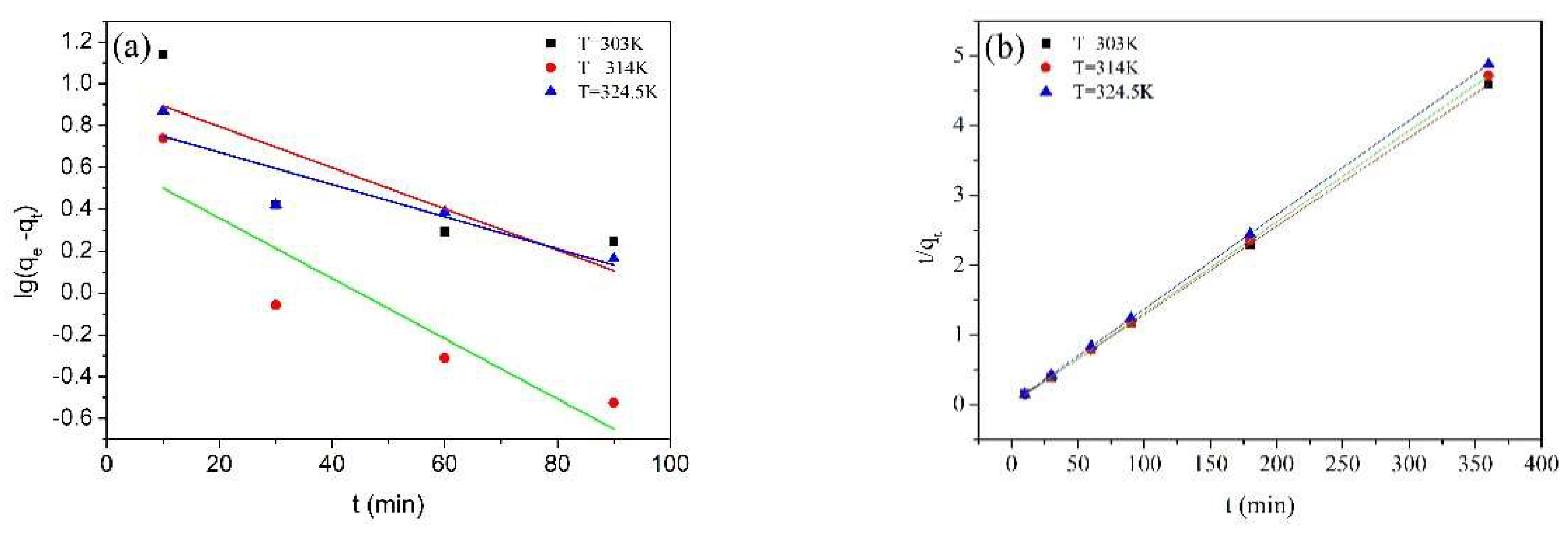
2.7. Adsorption Kinetics
2.8. Adsorption Isotherms
2.9. Thermodynamic Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of Adsorbents
3.3. Characterization
3.4. Batch Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lim, L.B.L.; Priyantha, N.; Tennakoon, D.T.B.; Chieng, H.I.; Dahri, M.K.; Suklueng, M. Breadnut peel as a highly effective low-cost biosorbent for methylene blue: Equilibrium, thermodynamic and kinetic studies. Arab. J. Chem. 2017, 10, S3216–S3228. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Carvalho, J.; Araujo, J.; Castro, F. Alternative Low-cost Adsorbent for Water and Wastewater Decontamination Derived from Eggshell Waste: An Overview. Waste Biomass Valoriz. 2011, 2, 157–167. [Google Scholar] [CrossRef]
- Dahri, M.K.; Kooh, M.R.R.; Lim, L.B.L. Water remediation using low cost adsorbent walnut shell for removal of malachite green: Equilibrium, kinetics, thermodynamic and regeneration studies. J. Environ. Chem. Eng. 2014, 2, 1434–1444. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab. J. Chem. 2016, 9, S707–S716. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Lim, L.B.L.; Priyantha, N.; Mansor, N.H.M. Artocarpus altilis (breadfruit) skin as a potential low-cost biosorbent for the removal of crystal violet dye: Equilibrium, thermodynamics and kinetics studies. Environ. Earth Sci. 2015, 73, 3239–3247. [Google Scholar] [CrossRef]
- Suemitsu, R.; Uenishi, R.; Akashi, I.; Nakano, M. The use of dyestuff-treated rice hulls for removal of heavy metals from waste water. J. Appl. Polym. Sci. 1986, 31, 75–83. [Google Scholar] [CrossRef]
- Kowalik, P.J.; Randerson, P.F. Nitrogen and phosphorus removal by willow stands irrigated with municipal waste water—A review of the Polish experience. Biomass Bioenerg 1994, 6, 133–139. [Google Scholar] [CrossRef]
- Periasamy, K.; Namasivayam, C. Removal of copper(II) by adsorption onto peanut hull carbon from water and copper plating industry wastewater. Chemosphere 1996, 32, 769–789. [Google Scholar] [CrossRef]
- Amin, M.N.; Mustafa, A.I.; Khalil, M.I.; Rahman, M.; Nahid, I. Adsorption of phenol onto rice straw biowaste for water purification. Clean Technol. Environ. Policy 2012, 14, 837–844. [Google Scholar] [CrossRef]
- Taha, G.M. Utilization of Low-Cost Waste Material Bagasse Fly Ash in Removing of Cu2+, Ni2+, Zn2+, and Cr3+ from Industrial Waste Water. Groundw. Monit. 2006, 26, 137–141. [Google Scholar] [CrossRef]
- Pacaphol, K.; Aht-Ong, D. Preparation of hemp nanofibers from agricultural waste by mechanical defibrillation in water. J. Clean. Prod. 2017, 142, 1283–1295. [Google Scholar] [CrossRef]
- Khaskheli, M.I.; Memon, S.Q.; Siyal, A.N.; Khuhawar, M.Y. Use of Orange Peel Waste for Arsenic Remediation of Drinking Water. Waste Biomass Valoriz. 2011, 2, 423–433. [Google Scholar] [CrossRef]
- Adel, A.M.; Abd El-Wahab, Z.H.; Ibrahim, A.A.; Al-Shemy, M.T. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part II: Physicochemical properties. Carbohydr. Polym. 2011, 83, 676–687. [Google Scholar] [CrossRef]
- Jong, M.-C.; Su, J.-Q.; Bunce, J.T.; Harwood, C.R.; Snape, J.R.; Zhu, Y.-G.; Graham, D.W. Co-Optimization of sponge-core bioreactors for removing total nitrogen and antibiotic resistance genes from domestic wastewater. Sci. Total Environ. 2018, 634, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Liu, Y.; Wang, J.; Zeng, G.; Deng, Y.; Dong, H.; Feng, H.; Wang, J.; Peng, B. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl. Catal. B Environ. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Duan, P.; Xu, X.; Shang, Y.; Gao, B.; Li, F. Amine-crosslinked Shaddock Peel embedded with hydrous zirconium oxide nano-particles for selective phosphate removal in competitive condition. J. Taiwan Inst. Chem. E 2017, 80, 650–662. [Google Scholar] [CrossRef]
- Chai, W.; Liu, X.; Zou, J.; Zhang, X.; Li, B.; Yin, T. Pomelo peel modified with acetic anhydride and styrene as new sorbents for removal of oil pollution. Carbohydr. Polym. 2015, 132, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Argun, M.E.; Güclü, D.; Karatas, M. Adsorption of Reactive Blue 114 dye by using a new adsorbent: Pomelo peel. J. Ind. Eng. Chem. 2014, 20, 1079–1084. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Microwave assisted preparation of activated carbon from pomelo skin for the removal of anionic and cationic dyes. Chem. Eng. J. 2011, 173, 385–390. [Google Scholar] [CrossRef]
- Li, F.; Tang, Y.; Wang, H.; Yang, J.; Li, S.; Liu, J.; Tu, H.; Liao, J.; Yang, Y.; Liu, N. Functionalized hydrothermal carbon derived from waste pomelo peel as solid-phase extractant for the removal of uranyl from aqueous solution. Environ. Sci. Pollut. Res. Int. 2017, 24, 22321–22331. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cha, L.; Fan, Y.; Fang, P.; Ming, Z.; Sha, H. Activated Biochar Prepared by Pomelo Peel Using H3PO4 for the Adsorption of Hexavalent Chromium: Performance and Mechanism. Water Air Soil Pollut. 2017, 228, 405. [Google Scholar] [CrossRef]
- Hameed, B.H.; Mahmoud, D.K.; Ahmad, A.L. Sorption of basic dye from aqueous solution by pomelo (Citrus grandis) peel in a batch system. Colloids Surf. Physicochem. Eng. Aspects 2008, 316, 78–84. [Google Scholar] [CrossRef]
- Widsten, P.; Dooley, N.; Parr, R.; Capricho, J.; Suckling, I. Citric acid crosslinking of paper products for improved high-humidity performance. Carbohydr. Polym. 2014, 101, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Márquez, O.P.; Márquez, J.; Gutiérrez, C. FTIR spectroscopy study of the electrochemical reduction of CO2 on various metal electrodes in methanol. J. Electroanal. Chem. 1995, 390, 99–107. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bandyopadhyay, A. Adsorption of methylene blue onto citric acid treated carbonized bamboo leaves powder: Equilibrium, kinetics, thermodynamics analyses. J. Mol. Liq. 2017, 248, 413–424. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Du, Q.; Wang, Z.; Xia, Y.; Yedinak, E.; Lou, J.; Ci, L. High performance agar/graphene oxide composite aerogel for methylene blue removal. Carbohydr. Polym. 2017, 155, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Vasanth Kumar, K.; Sivanesan, S. Isotherms for Malachite Green onto rubber wood (Hevea brasiliensis) sawdust: Comparison of linear and non-linear methods. Dyes Pigment. 2007, 72, 124–129. [Google Scholar] [CrossRef]
- Li, Y.; Gao, B.; Wu, T.; Wang, B.; Li, X. Adsorption properties of aluminum magnesium mixed hydroxide for the model anionic dye Reactive Brilliant Red K-2BP. J. Hazard. Mater. 2009, 164, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Buvaneswari, N.; Kannan, C. Plant toxic and non-toxic nature of organic dyes through adsorption mechanism on cellulose surface. J. Hazard. Mater. 2011, 189, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Q.; Wang, X.; Kang, I.-S. Ester Crosslinking of Cotton Fabric by Polymeric Carboxylic Acids and Citric Acid. Text. Res. J. 1997, 67, 334–342. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P. Sea shell powder as a new adsorbent to remove Basic Green 4 (Malachite Green) from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010, 164, 168–177. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mishra, R.; Saha, P.; Kushwaha, P. Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 2011, 265, 159–168. [Google Scholar] [CrossRef]
Sample Availability: Samples of the modified pomelo peels are available from the authors. |




| Adsorbent | Dose (mg) | MB Concentration (g/L) | Solution Volume (mL) | Adsorption Capacity (mg/g) |
|---|---|---|---|---|
| PPL | 10 | 50 | 50 | 81.71 |
| MPPL | 10 | 50 | 50 | 199.29 |
| Temperature (K) | 303 | 314 | 324.5 |
|---|---|---|---|
| qe experimental (mg/g) | 78.45 | 76.50 | 73.76 |
| pseudo-first-order model | |||
| k1 (1/min) | 0.029 | 0.016 | 0.009 |
| qe calculated (mg/g) | 1.64 | 2.31 | 2.36 |
| R2 | 0.9747 | 0.9913 | 0.9255 |
| pseudo-second-order model | |||
| k2 (mg/(g•min)) | 0.014 | 0.019 | 0.018 |
| qe calculated (mg/g) | 78.74 | 76.34 | 74.07 |
| R2 | 1.0000 | 1.0000 | 0.9999 |
| Isotherms | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|
| b | qm | R2 | RL | kf | 1/n | R2 | |
| Parameters | 0.066 | 104.17 | 0.998 | 0.233 | 3.289 | 1.708 | 0.990 |
| ΔH° (kJ mol−1) | ΔS° (J mol−1K−1) | ΔG° (kJ mol−1) | ||
|---|---|---|---|---|
| 303 K | 314 K | 324.5 K | ||
| −27.91 | −64.70 | −8.30 | −7.59 | −6.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Cui, C.; Wang, P. Pomelo Peel Modified with Citrate as a Sustainable Adsorbent for Removal of Methylene Blue from Aqueous Solution. Molecules 2018, 23, 1342. https://doi.org/10.3390/molecules23061342
Ren Y, Cui C, Wang P. Pomelo Peel Modified with Citrate as a Sustainable Adsorbent for Removal of Methylene Blue from Aqueous Solution. Molecules. 2018; 23(6):1342. https://doi.org/10.3390/molecules23061342
Chicago/Turabian StyleRen, Yimei, Chang Cui, and Pengjie Wang. 2018. "Pomelo Peel Modified with Citrate as a Sustainable Adsorbent for Removal of Methylene Blue from Aqueous Solution" Molecules 23, no. 6: 1342. https://doi.org/10.3390/molecules23061342