Four New Lignans from Kadsura Interior and Their Bioactivity
Abstract
:1. Introduction
2. Results and Discussion
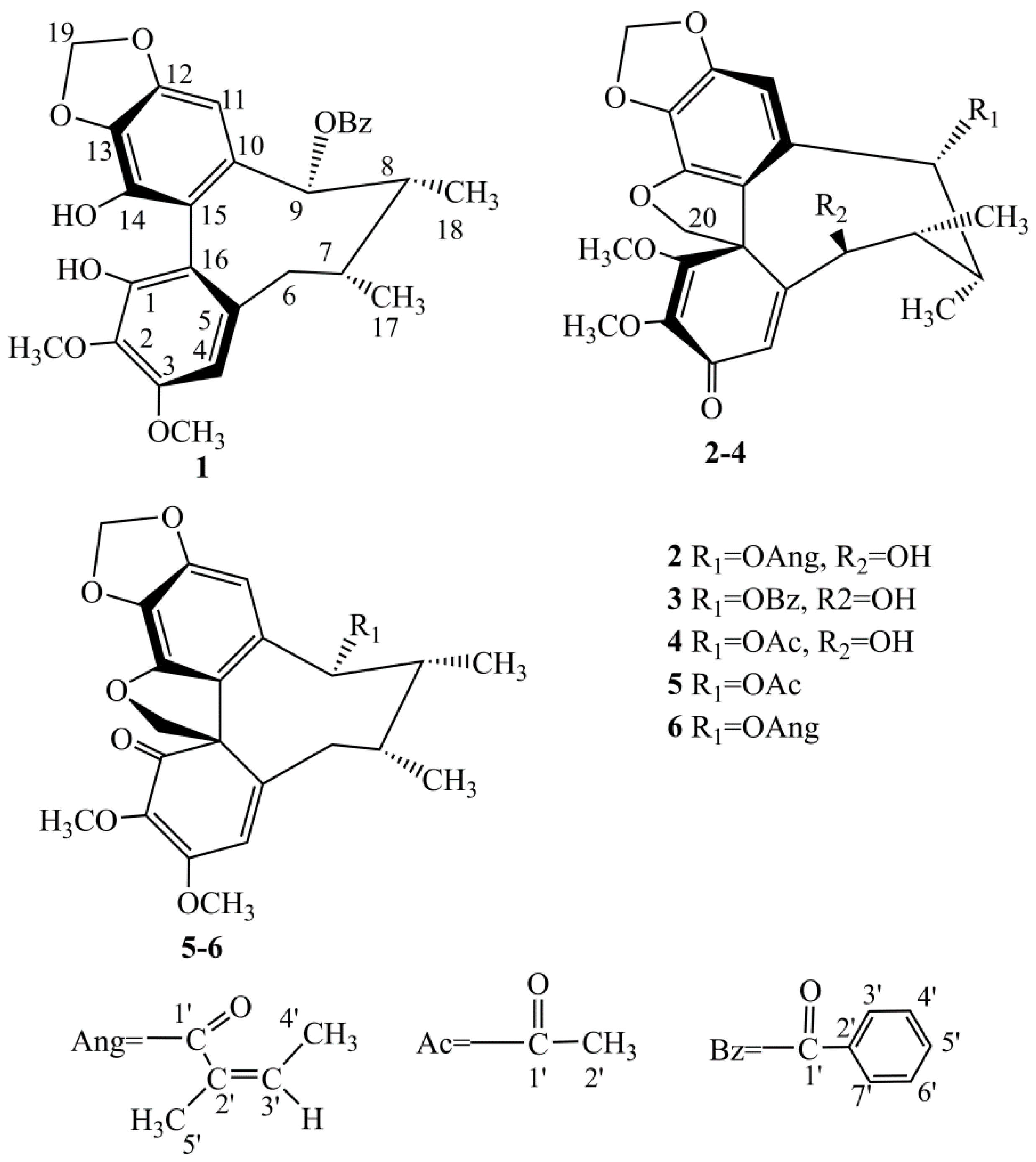
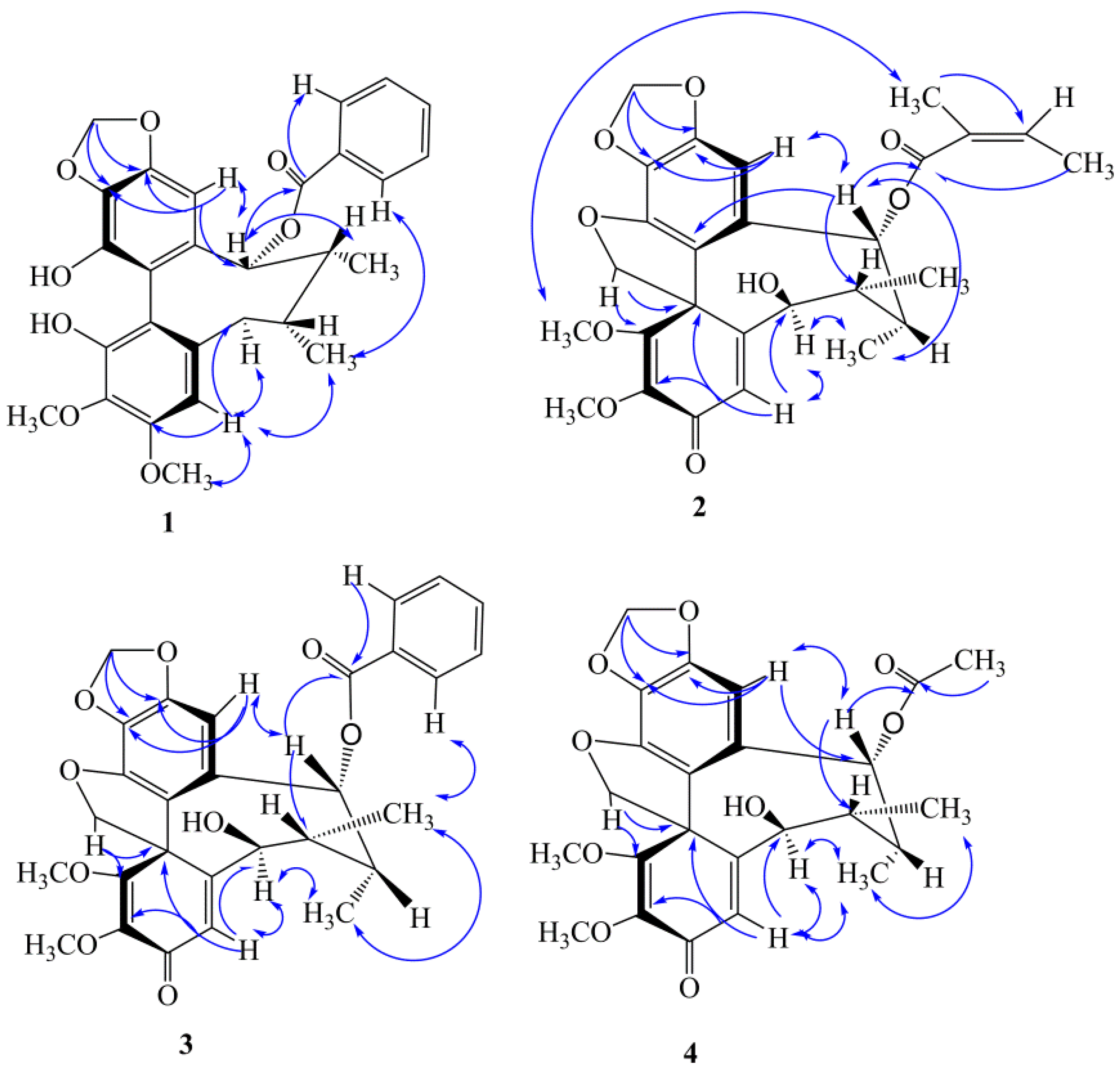
2.1. Four New Identified Compounds (1–4)
2.2. Anti-Platelet Effects of Compounds 1–6
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Kadsutherin E (1)
3.3.2. Kadsutherin F (2)
3.3.3. Kadsutherin G (3)
3.3.4. Kadsutherin H (4)
3.4. Anti-Platelet Aggregation Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pharmacopoeia Commission of PRC. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015; Volume 1, pp. 522–523. [Google Scholar]
- Chen, D.F.; Zhang, S.X.; Xie, L.; Xie, J.X.; Chen, K.; Kashiwada, Y.; Zhou, B.N.; Wang, P.; Cosentino, L.M.; Lee, K.H. Anti-AIDS agents-XXVI. Structure-activity correlations of gomisin G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorg. Med. Chem. 1997, 5, 1715–1723. [Google Scholar] [CrossRef]
- Chen, D.F.; Zhang, S.X.; Kozuka, M.; Sun, Q.Z.; Feng, J.; Wang, Q.; Mukainaka, T.; Nobukuni, Y.; Tokuda, H.; Nishino, H.; et al. Interiotherins C and D, two new lignans from Kadsura interior and antitumor-promoting effects of related neolignans on epstein-barr virus activation. J. Nat. Prod. 2002, 65, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.H.; Luo, S.D. Study on new lignans from Kadsura interior A. C. Smith. Acta Chim. Sin. 1990, 48, 1075–1079. [Google Scholar]
- Xu, L.J.; Ma, P.; Li, L.; Wang, W.Y.; Xiao, W.; Peng, Y.; Xiao, P.G. New collection of crude drugs in Chinese Pharmacopoeia 2010 III. Chin. Herb. Med. 2012, 4, 177–182. [Google Scholar]
- Yang, X.W.; Hattori, M.; Namba, T.; Chen, D.F.; Xu, G.J. Anti-lipid peroxidative effect of an extract of the stems of Kadsura heteroclita and its major constituent, kadsurin, in mice. Chem. Pharm. Bull. 1992, 40, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Miyashiro, H.; Hattori, M.; Namba, T.; Tezuka, Y.; Kikuchi, T.; Chen, D.F.; Xu, G.J. Isolation of novel lignans, heteroclitins F and G, from the stems of Kadsura heteroclite, and Anti-lipid peroxidative actions of heteroclitins A-G and related componds in the in vitro rat liver homogenate system. Chem. Pharm. Bull. 1992, 40, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.L.; Gu, Z.; Hu, T.X.; Wang, M.H.; Chen, D.F. Effects of lignan gomisin J from Kadsura interior on liver mitochondria lipid peroxidation and on the superoxide anion radical. Chin. Pharmacol. Bull. 2000, 16, 26–28. [Google Scholar]
- Chen, D.F.; Zhang, S.X.; Chen, K.; Zhou, B.N.; Wang, P.; Cosentino, L.M.; Lee, K.H. Two new lignans, interiotherins A and B, as anti-HIV principles from Kadsura interior. China J. Nat. Prod. 1996, 59, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.L.; Zhang, Y.Y.; Chen, D.F. Effects of heteroclitin D, schisanhenol and (+)-anwulignan on platelet aggregation. Fudan Univ. J. Med. Sci. 2005, 32, 467–470. [Google Scholar]
- Pu, J.X.; Yang, L.M.; Xiao, W.L.; Li, R.T.; Lei, M.; Gao, X.M.; Huang, S.X.; Li, S.H.; Zheng, Y.T.; Huang, H.; et al. Compounds from Kadsura heteroclita and related anti-HIV activity. Phytochemistry 2008, 69, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Luo, Y.P.; Zou, Y.L.; Lang, L.H.; Chen, D.F. Heteroclitins R-S: New dibenzocylooctadiene lignans from Kadsura heteroclita. Chin. J. Nat. Med. 2014, 12, 689–692. [Google Scholar] [CrossRef]
- Li, H.R.; Feng, Y.L.; Yang, Z.G.; Wang, J.; Akihiro, D.; Susumu, K.; Xu, L.Z.; Yang, S.L. New Lignans from Kadsura coccinea and Their Nitric Oxide Inhibitory Activities. Chem. Pharm. Bull. 2006, 54, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, D.F. Kadsutherin D, a new dibenzocyclooctadiene lignan from Kadsura species. Nat. Prod. Res. 2008, 22, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Li, L.N.; Xue, H. Dibenzocyclooctadiene lignans possessing a spirobenzofuranoid skeleton from Kadsura coccinea. Phytochemistry 1990, 29, 2730–2732. [Google Scholar] [CrossRef]
- Chen, D.F.; Xu, G.J.; Yang, X.W.; Hattori, M.; Tezuka, Y.; Kikuchi, T.; Namba, T. Dibenzocyclo-octadiene lignans from Kadsura heteroclita. Phytochemistry 1992, 31, 629–632. [Google Scholar] [CrossRef]
- Fuly, A.L.; Machado, O.L.; Alves, C.R.; Carlini, C.R. Mechanism of inhibitory action on platelet activation of a phospholipase A2 isolated from Lachesis muta (Bushmaster) snake venom. J. Thromb. Haemost. 1997, 78, 1372–1380. [Google Scholar]
- Chen, J.J.; Tsai, T.H.; Liao, H.R.; Chen, L.C.; Kuo, Y.H.; Sung, P.J.; Chen, C.L.; Wei, C.S. New sesquiterpenoids and anti-platelet aggregation constituents from the rhizomes of Curcuma zedoaria. Molecules 2016, 21, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not Available. |
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 4 | 6.60 (1H, s) | 6.31 (1H, s) | 6.49 (1H, s) | 6.34 (1H, s) |
| 6a | 2.72 (1H, d, 13.8) | 4.13 (1H, d, 10.8) | 4.26 (1H, d, 10.2) | 4.11 (1H, d, 10.2) |
| 6b | 2.77 (1H, dd, 13.8, 6.6) | - | - | - |
| 7 | 2.20 (1H, overlap) | 1.56 (1H, m) | 1.66 (1H, m) | 1.49 (1H, m) |
| 8 | 2.20 (1H, overlap) | 2.11 (1H, m) | 2.18 (1H, m) | 2.05 (1H, m) |
| 9 | 5.92 (1H, d, 7.2) | 5.61 (1H, d, 7.8) | 5.72 (1H, d, 7.2) | 5.86 (1H, d, 7.8) |
| 11 | 6.49 (1H, s) | 6.39 (1H, s) | 6.48 (1H, s) | 6.34 (1H, s) |
| 17 | 1.11 (3H, d, 7.2) | 1.02 (3H, d, 7.2) | 1.08 (3H, d, 6.6) | 0.92 (3H, d, 7.2) |
| 18 | 1.15 (3H, d, 7.2) | 1.04 (3H, d, 7.2) | 1.17 (3H, d, 7.8) | 1.02 (3H, d, 7.2) |
| 19 | 5.97 (2H, d, 1.2) | 5.99 (2H, s) | 6.02 (2H, s) | 6.00 (2H, s) |
| 20 | - | 4.46, 5.57 (2H, ABq, 8.4) | 4.40, 5.54 (2H, ABq, 8.4) | 4.57, 5.62 (2H, ABq, 8.4) |
| 1-OMe | - | 3.85 (3H, s) | 3.60 (3H, s) | 3.89 (3H, s) |
| 2-OMe | 3.40 (3H, s) | 3.69 (3H, s) | 2.86 (3H, s) | 3.76 (3H, s) |
| 3-OMe | 3.96 (3H, s) | - | - | - |
| 1-OH | 5,74 (1H, s) | - | - | - |
| 6-OH | - | 4.85 (1H, br s) | 4.68 (1H, br s) | 4.67 (1H, br s) |
| 14-OH | 5.26 (1H, s) | - | - | - |
| Acetoxy | ||||
| 2′ | 1.79 (3H, s) | |||
| Angeloyl | ||||
| 3′ | 5.87 (1H, m) | |||
| 4′ | 1.77 (3H, dd, 7.2, 1.2) | |||
| 5′ | 1.66 (3H, t, 4.8) | |||
| Benzoyl | ||||
| 3′,7′ | 7.43 (2H, dd,7.2, 1.2) | 7.76 (2H, dd, 7.8, 1.2) | ||
| 4′,6′ | 7.30 (2H, dd, 7.2, 7.2) | 7.40 (2H, dd, 7.8, 7.8) | ||
| 5′ | 7.48 (1H, dd, 7.2, 7.2) | 7.57 (1H, dd, 7.8, 7.8) |
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 138.2 | 168.3 | 169.5 | 169.8 |
| 2 | 134.2 | 134.8 | 136.0 | 136.2 |
| 3 | 151.2 | 185.5 | 186.6 | 187.0 |
| 4 | 107.0 | 131.8 | 134.6 | 156.8 |
| 5 | 133.8 | 155.3 | 131.6 | 131.5 |
| 6 | 38.3 | 80.8 | 81.8 | 78.9 |
| 7 | 34.8 | 38.7 | 44.0 | 40.4 |
| 8 | 42.2 | 42.3 | 39.8 | 43.3 |
| 9 | 83.5 | 79.2 | 82.1 | 82.2 |
| 10 | 117.8 | 130.1 | 122.0 | 122.2 |
| 11 | 101.1 | 100.2 | 101.7 | 101.3 |
| 12 | 147.9 | 150.6 | 152.0 | 156.8 |
| 13 | 135.2 | 129.8 | 131.3 | 130.0 |
| 14 | 134.4 | 145.3 | 146.5 | 156.8 |
| 15 | 147.6 | 120.4 | 156.4 | 146.6 |
| 16 | 117.3 | 57.9 | 59.2 | 59.0 |
| 17 | 18.5 | 18.2 | 19.6 | 19.4 |
| 18 | 14.3 | 9.11 | 10.4 | 9.1 |
| 19 | 100.2 | 102.0 | 103.4 | 103.2 |
| 20 | - | 83.7 | 85.1 | 84.2 |
| 1-OMe | - | 60.6 | 61.4 | 61.7 |
| 2-OMe | 55.2 | 59.4 | 59.4 | 60.7 |
| 3-OMe | 59.3 | - | - | - |
| 1′ | 167.3 | 168.1 | 168.5 | 171.9 |
| 2′ | 129.7 | 136.8 | 130.9 | 20.8 |
| 3′ | 129.3 | 127.7 | 130.8 | - |
| 4′ | 127.8 | 14.6 | 130.0 | - |
| 5′ | 132.4 | 19.8 | 132.8 | - |
| 6′ | 127.8 | - | 130.0 | - |
| 7′ | 129.3 | - | 130.8 | - |
| Compounds | Inhibition% |
|---|---|
| Kadsutherin E (1) | 23.64 ± 1.12 |
| Kadsutherin F (2) | 49.47 ± 2.93 |
| Kadsutherin G (3) | 33.10 ± 2.67 |
| Kadsutherin H (4) | 21.75 ± 2.37 |
| Acetoxyl oxokadsurane (5) | 34.31 ± 0.73 |
| Heteroclitin D (6) | 11.77 ± 2.30 |
| Aspirin | 59.94 ± 2.44 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.-S.; Zhang, J.; Qi, Y.-D.; Jia, X.-G.; Zhang, B.-G.; Liu, H.-T. Four New Lignans from Kadsura Interior and Their Bioactivity. Molecules 2018, 23, 1279. https://doi.org/10.3390/molecules23061279
Liu J-S, Zhang J, Qi Y-D, Jia X-G, Zhang B-G, Liu H-T. Four New Lignans from Kadsura Interior and Their Bioactivity. Molecules. 2018; 23(6):1279. https://doi.org/10.3390/molecules23061279
Chicago/Turabian StyleLiu, Jiu-Shi, Jin Zhang, Yao-Dong Qi, Xiao-Guang Jia, Ben-Gang Zhang, and Hai-Tao Liu. 2018. "Four New Lignans from Kadsura Interior and Their Bioactivity" Molecules 23, no. 6: 1279. https://doi.org/10.3390/molecules23061279





